使用Saccadometry与深部脑刺激来研究正常和病理脑功能
Summary
This paper describes the use of quantitative measurement of eye movements in conjunction with stimulation of focal areas of the deep brain in order to study physiology, pathophysiology, and the mechanisms of deep brain stimulation.
Abstract
The oculomotor system involves a large number of brain areas including parts of the basal ganglia, and various neurodegenerative diseases including Parkinson’s and Huntington’s can disrupt it. People with Parkinson’s disease, for example, tend to have increased saccadic latencies. Consequently, the quantitative measurement of saccadic eye movements has received considerable attention as a potential biomarker for neurodegenerative conditions. A lot more can be learned about the brain in both health and disease by observing what happens to eye movements when the function of specific brain areas is perturbed. Deep brain stimulation is a surgical intervention used for the management of a range of neurological conditions including Parkinson’s disease, in which stimulating electrodes are placed in specific brain areas including several sites in the basal ganglia. Eye movement measurements can then be made with the stimulator systems both off and on and the results compared. With suitable experimental design, this approach can be used to study the pathophysiology of the disease being treated, the mechanism by which DBS exerts it beneficial effects, and even aspects of normal neurophysiology.
Introduction
近年来出现了在使用的反应时间的测量增加兴趣获得有关使1神经决定的高一级的机制的信息的数量和非侵入性的方式。已被广泛研究的反应时间的一种类型是启动一个视觉刺激,被称为扫视延迟的呈现的扫视所花费的时间。扫视是当我们快速,我们的目光从一个地方转移到另一个发生在快速眼球运动。他们是最常见的类型眼球运动的我们做,一般在两三每秒的频率出现。每个扫视实际上是看一个提示的视觉世界,而不是另一个2的决定。
神经通路控制眼球运动已被广泛研究,并相当有据可查3。使用敏感的电子设备,动眼神经功能的各方面可以精确和objectivelÿ量化。这有利于眼睛运动本身的详细研究,但也允许它们被用来作为一种工具来研究神经生理学和病理生理学的其他领域。
眼球运动的测量可以提供关于疾病状态的有用信息。眼跳最近,例如,受到很大关注作为神经变性疾病的潜在生物标志物,包括亨廷顿氏4,5和帕金森氏病6,7,而且它是公认的扫视的反应时间往往比在这些条件下正常慢。扫视测量的潜在用途包括艾滋病诊断和病情跟踪。扫视任务(如快速寻找尽可能朝向突然出现的视觉刺激,以左或右)的范围从简单的prosaccade到更复杂的任务,如antisaccade(尽快看向相反一侧视觉刺激)或内存 – 引导扫视(看朝着一个目标就是不再有)的记忆位置。
深部脑刺激是一种有效的治疗几种神经系统疾病。它最常用于治疗帕金森氏病,包括震颤,强直,运动迟缓,和运动障碍的运动症状。它也可用于其他的运动障碍,包括肌张力障碍和原发性震颤,和不太常用为神经性疼痛,癫痫,和精神病症如强迫症。它是在其中科学家必须在体内的人脑的深层结构的直接电连接,从而提供了实验神经学宝贵的机会的唯一设置。各种目标有刺激取决于所治疗的病症,包括在基底神经节的几个位置,其中许多都参与动眼神经通路。这意味着,一个广泛的研究,可以使用DBS系统递送刺激进行给定的大脑位置和眼睛跟踪装置,记录并分析其影响。根据不同的实验范例,这样的研究可以产生被刺激有关区域的生理信息,该疾病,或者这一机制的DBS是工作在该特定设置的影响。本文介绍了在深部脑刺激患者眼跳运动检测的通用方法。
几种不同类型的眼动追踪设备可用。对于本协议描述的研究使用了便携式saccadometer记录水平眼跳。便携式saccadometers有不需要头枕( 见图1),这意味着会话的患者帕金森病更舒适,尤其是对于那些有严重的运动障碍患的优点。这里使用的saccadometer是重量轻,大约5厘米宽,10厘米高。该saccadometer measu通过使用直接红外线oculography的水库眼球运动:红外光源和传感器放置在内侧眼角利用光的前面从角膜反射的建立在毫秒间隔眼球的旋转位置。为了获取用于分析高质量的数据的saccadometer应该至少为1kHz与至少一个12位的分辨率的速率采样。在这里使用的saccadometer的视觉刺激三个红13 CD米-2由产生的光点建在低功率激光器,每个斑点对着某些0.1度,与在中线一个点,另两个在±10度( 即到右和左)。
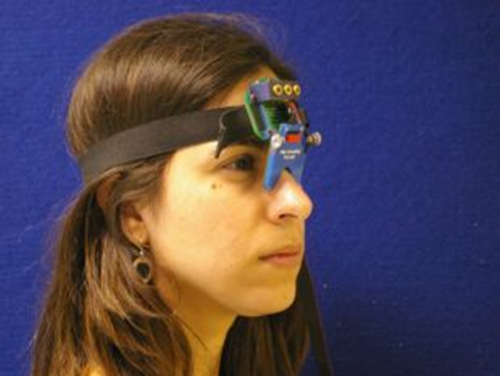
图1. Saccadometer。头戴式saccadometer附连到弹性带和搁在鼻梁。四微型激光投影视标来说明是粗糙面,和参加者的眼睛运动是用差红外反射换能器上的每个眼的鼻侧测量。由于激光目标与头部移动,头枕不是必需的。 请点击此处查看该图的放大版本。
Protocol
Representative Results
Discussion
在取得良好的质量扫视数据的最关键因素是确保提供给参与者指令是清楚和准确。例如,如果为antisaccadic任务指令是不完全清楚,参与者很可能代替执行prosaccades。录音也可能是被宠坏如果参与者不能清楚地看到刺激或saccadometer无法准确衡量眼位。因此,如果数据似乎是低质量的实验者应检查环境光线不要太亮,而且saccadometer正确地坐在鼻梁。
深部脑刺激使得神经活动的直接…
Disclosures
The authors have nothing to disclose.
Acknowledgements
Dr. Antoniades was supported by the National Institute of Health Research (NIHR) and by the Dementias and Neurodegenerative Diseases Research Network (DENDRON) and by the Wellcome Trust. Dr FitzGerald was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre.
Materials
| Saccadometer device ( Ober Consulting Poland) |
| Computer with Windows environment |
| Software, Latency Meter for downloading the raw data from the saccadometer. |
References
- Leigh, R. J., & Kennard, C. Using saccades as a research tool in the clinical neurosciences. Brain. 127, 460-477 (2004).
- Carpenter, R. H. The neural control of looking. Curr Biol. 10, R291-293 (2000).
- Leigh, R. J., & Zee, D. S. The Neurology of Eye Movements. New York: Oxford University Press, (2006).
- Antoniades, C. A., Xu, Z., Mason, S. L., Carpenter, R. H., & Barker, R. A. Huntington's disease: changes in saccades and hand-tapping over 3 years. Journal of Neurology. 257, 1890-1898 (2010).
- Blekher, T.M., Yee, RD., Kirkwood, SC., Hake, AM., Stout, JC., Weaver, MR., Foroud, TM. Oculomotor control in asymptomatic and recently diagnosed individuals with the genetic marker for Huntington's disease. in Vision Research. Vol. 44 2729-2736 (2004).
- Chan, F., Armstrong, I. T., Pari, G., Riopelle, R. J., & Munoz, D. P. Deficits in saccadic eye-movement control in Parkinson's disease. Neuropsychologia. 43, 784-796 (2005).
- Antoniades, C. A., Demeyere, N., Kennard, C., Humphreys, G. W., & Hu, M. T. Antisaccades and executive dysfunction in early drug-naive Parkinson's disease: The discovery study. Mov Disord. (2015).
- Antoniades, C. et al. An internationally standardised antisaccade protocol. Vision Res. 84, 1-5 (2013).
- Ober, J. K. et al. Hand-Held system for ambulatory measurement of saccadic durations of neurological patients. . In: Modelling and Measurement in Medicine. (2003).
- Temperli, P. et al. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 60, 78-81 (2003).
- Antoniades, C. A. et al. Deep brain stimulation: eye movements reveal anomalous effects of electrode placement and stimulation. PLoS ONE. 7, e32830 (2012).
- Yugeta, A. et al. Effects of STN stimulation on the initiation and inhibition of saccade in Parkinson disease. Neurology. 74, 743-748 (2010).
- Terao, Y., Fukuda, H., Ugawa, Y., & Hikosaka, O. New perspectives on the pathophysiology of Parkinson's disease as assessed by saccade performance: a clinical review. Clin Neurophysiol. 124, 1491-1506 (2013).
- Temel, Y., Visser-Vandewalle, V., & Carpenter, R. H. Saccadic latency during electrical stimulation of the human subthalamic nucleus. Curr Biol. 18, R412-414 (2008).
- Antoniades, C. A. et al. Deep brain stimulation abolishes slowing of reactions to unlikely stimuli. J Neurosci. 34, 10844-10852 1065-14.2014(2014).
- Rivaud-Pechoux, S. et al. Improvement of memory guided saccades in parkinsonian patients by high frequency subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry. 68, 381-384 (2000).
- Takikawa, Y., Kawagoe, R., Itoh, H., Nakahara, H., & Hikosaka, O. Modulation of saccadic eye movements by predicted reward outcome. Experimental brain research. Experimentelle Hirnforschung. 142, 284-291 (2002).
- Dorris, M. C., & Munoz, D. P. A neural correlate for the gap effect on saccadic reaction times in monkey. Journal of Neurophysiology. 73, 2558-2562, (1995).
- Hanes, D. P., & Schall, J. D. Countermanding saccades in macaque. Visual Neuroscience. 12, 929-937, (1995).
- Opris, I., Barborica, A., & Ferrera, V. P. On the gap effect for saccades evoked by electrical microstimulation of frontal eye fields in monkeys. Experimental brain research. Experimentelle Hirnforschung. 138, 1-7 (2001).
- Takagi, M., Frohman, E. M., & Zee, D. S. Gap-overlap effects on latencies of saccades, vergence and combined vergence-saccades in humans. Vision Res. 35, 3373-3388 (1995).
- Schall, J. D. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. Journal of Neurophysiology. 66, 559-579 (1991).
- Pare, M., & Hanes, D. P. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci. 23, 6480-6489 (2003).

