The Mouse Isolated Perfused Kidney Technique
Summary
The mouse isolated perfused kidney (MIPK) is a technique for keeping a mouse kidney under ex vivo conditions perfused and functional for 1 hr. The buffers and surgical technique are described in detail.
Abstract
The mouse isolated perfused kidney (MIPK) is a technique for keeping a mouse kidney under ex vivo conditions perfused and functional for 1 hr. This is a prerequisite for studying the physiology of the isolated organ and for many innovative applications that may be possible in the future, including perfusion decellularization for kidney bioengineering or the administration of anti-rejection or genome-editing drugs in high doses to prime the kidney for transplantation. During the time of the perfusion, the kidney can be manipulated, renal function can be assessed, and various pharmaceuticals administered. After the procedure, the kidney can be transplanted or processed for molecular biology, biochemical analysis, or microscopy.
This paper describes the perfusate and the surgical technique needed for the ex vivo perfusion of mouse kidneys. Details of the perfusion apparatus are given and data are presented showing the viability of the kidney's preparation: renal blood flow, vascular resistance, and urine data as functional, transmission electron micrographs of different nephron segments as morphological readouts, and western blots of transport proteins of different nephron segments as molecular readout.
Introduction
The isolated perfusion of organs has been the subject of an ongoing effort among physiologists for many decades1. The technique enables the function of the organ, without systemic influences such as blood pressure, hormones, or nerves, to be studied. Carl Eduard Loebell is considered to be the first to have described the successful perfusion of an isolated kidney, in 18492. Since then, the perfusion apparatus has undergone significant refinement. Frey and Gruber introduced an artificial lung for oxygenation and pulsatile pumps for continuous perfusion2. While early researchers mainly studied the kidneys of large mammals-namely, pigs2 and dogs3-the first report of the use of rat kidneys, by Weiss et al., was a milestone in the study of small-mammal-organ perfusion4. Schurek et al. reported the necessity of adding mammalian erythrocytes to the perfusate if sufficient renal tubular oxygenation was to be achieved5. Critical for long-term experiments was the introduction of continuous dialysis of the buffer by the same research group6. In 2003, Schweda et al. were the first to report a functional mouse isolated perfused kidney (MIPK)7, later refined by Rahgozar et al.18 and Lindell et al.14.
While technically more challenging than the rat isolated perfused kidney, the use of the MIPK bears the advantage of enabling the use of a wide array of genetically altered mice. This paper presents the details of the authors' method for perfusing isolated mouse kidneys for 1 hr. The method allows for the continuous assessment of renal flow rate, vascular resistance, hormone release, blood gas analysis, urine analysis, and the application of drugs. Following the procedure, kidneys could be processed for molecular and biochemical analysis, be fixed for microscopy, or transplanted into a recipient mouse (Figure 1).
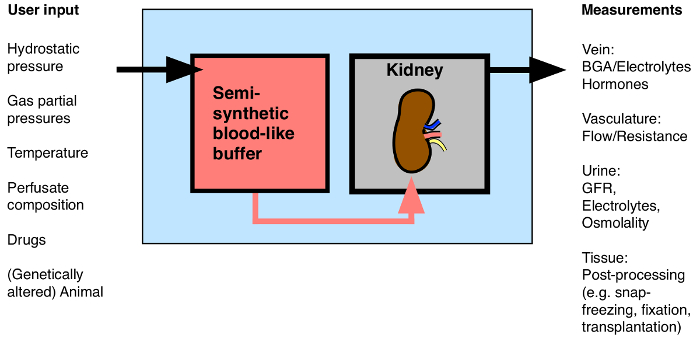
Figure 1: Overview of Possible Input/Output to the Isolated Perfused Kidney. BGA: Blood gas analysis. Please click here to view a larger version of this figure.
This technique likely will receive increasing attention over coming years, as many innovative applications are being discussed with the dawn of prolonged normothermic kidney perfusion prior to transplantation (with or without the application of anti-rejection or genome-editing drugs)8,9, 10, 11, the bioengineering of whole kidneys from decellularized scaffolds12, and the application of high doses of fluorescent dyes for multiphoton imaging13. It is also an ideal model with which to study the role of specific genes during acute kidney injury14.
A step-by-step protocol is given to allow other laboratories to perform isolated mouse kidney perfusion successfully. First, the composition and preparation of the buffer is specified. Then, the surgery is described in detail and the critical steps are shown. Third, data is presented that are representative of a successful preparation: renal blood flow, vascular resistance, glomerular filtration rate , and fractional electrolyte excretion-all as functional measurements of viability-and transmission electron micrographs of the morphology of different nephron segments of perfused kidneys fixed after 1 hr of perfusion.
Protocol
Representative Results
Discussion
The mouse isolated perfused kidney is a tool for studying kidney function in a controlled environment ex vivo for 1 hr, bridging the gap between in vivo experiments in intact animals, which may be flawed by the impact of numerous systemic factors, and in vitro experiments in isolated nephron segments or cultured cells, which necessarily neglect the impact of intact organ structure on function. There is, to the authors' knowledge, no alternative technique with which to perform this specific …
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Hans-Joachim Schurek for invaluable scientific advice. The authors would like to thank Monique Carrel and Michèle Heidemeyer for excellent technical assistance, David Penton Ribas and Nourdine Faresse for a critical reading of the manuscript and Carsten Wagner and Jürg Biber for the NaPi-2a antibody. This work was supported by the Swiss National Centre for Competence in Research “Kidney.CH” and by a project grant (310030_143929/1) from the Swiss National Science Foundation.
Materials
| Perfusion Circuit: | |||
| Moist chamber 834/8 | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-2901 | |
| Cannular with basket and side port | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-2947 | |
| Thermostat TC120-ST5 | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-4544 | |
| ISM 827/230V Roller Pump Reglo Analogue | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-0114 | |
| Reservoir jacketed for buffer solution 1L | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3438 | |
| Reservoir jacketed for buffer solution 0.5L | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3436 | |
| Pressure Transducer APT300 | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3862 | |
| TAM-D Plugsys Transducer | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-1793 | |
| SCP Plugsys servo controller | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-2806 | |
| Windkessel | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3717 | |
| HSE-USB data acquisition | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3330 | |
| Low-Flux Dialysator Diacap Polysulfone | B.Braun | 7203525 | |
| PE-Tubing for aorta cannulation 1.19mm I.D. x 1.70mm O.D. | Scientific Commodities Inc. | BB31695-PE/8 | |
| Name | Company | Catalog Number | Comments |
| Buffer reagents: | |||
| Aminoplasmal 10% | B.Braun | 134518064 | |
| Sodium pyruvate | Sigma-Aldrich | P2256-25G | |
| L-Glutamic acid monosodium salt hydrate | Sigma-Aldrich | G1626-100G | |
| L-(-)-Malic acid sodium salt | Sigma-Aldrich | M1125-25G | |
| Sodium-L-Lactate | Sigma-Aldrich | L7022-10G | |
| alpha-Ketoglutaric acid sodium salt | Sigma-Aldrich | K1875-25G | |
| NaCl | Sigma-Aldrich | 31434-1KG-R | |
| NaHCO3 | Sigma-Aldrich | S5761-5KG | |
| KCl | Sigma-Aldrich | 60130-1KG | |
| Urea | Sigma-Aldrich | U5378-500G | |
| Creatinine | Sigma-Aldrich | C4255-10G | |
| Ampicillin | Roche | 10835242001 | |
| MgCl2 * 6H2O | Sigma-Aldrich | M2393-500G | |
| D-Glucose | Sigma-Aldrich | G8270-1KG | |
| CaCl2 * 6H2O | Riedel-de-Haën | 12074 | |
| NaH2PO4 | Sigma-Aldrich | S9638-500G | |
| Na2HPO4 | Sigma-Aldrich | S0876-500G | |
| Antidiuretic Hormone dDAVP | Sigma-Aldrich | V2013-1MG | |
| FITC-Inulin | Sigma-Aldrich | ||
| Filter used for erythrocyte filtration | Macherey-Nagel | MN 615 | |
| BGA Analysis: | |||
| ABL 80 flex | Radiometer Medical ApS | ||
| Electron Microscope: | |||
| Philips CM100 TEM | FEI |
References
- Carrel, A., Lindbergh, C. A. The culture of whole organs. Science (New York, N.Y.). 81 (2112), 621-623 (1935).
- Skutul, K. . Über Durchströmungsapparate Pflüger’s Archiv. 123 (4-6), 249-273 (1908).
- Nizet, A. The isolated perfused kidney: possibilities, limitations and results. Kidney Int. 7 (1), 1-11 (1975).
- Weiss, C., Passow, H., Rothstein, A. Autoregulation of flow in isolated rat kidney in the absence of red cells. Am J Physiol. 196 (5), 1115-1118 (1959).
- Schurek, H. J., Kriz, W. Morphologic and functional evidence for oxygen deficiency in the isolated perfused rat kidney. Lab Invest. 53 (2), 145-155 (1985).
- Stolte, H., Schurek, H. J., Alt, J. M. Glomerular albumin filtration: a comparison of micropuncture studies in the isolated perfused rat kidney with in vivo experimental conditions. Kidney Int. 16 (3), 377-384 (1979).
- Schweda, F., Wagner, C., Krämer, B. K., Schnermann, J., Kurtz, A. Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. AJP Renal Physiol. 284 (4), F770-F777 (2003).
- Moers, C., Smits, J. M., et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 360 (1), 7-19 (2009).
- Nicholson, M. L., Hosgood, S. A. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. 13 (5), 1246-1252 (2013).
- Worner, M., Poore, S., Tilkorn, D., Lokmic, Z., Penington, A. J. A low-cost, small volume circuit for autologous blood normothermic perfusion of rabbit organs. Art Org. 38 (4), 352-361 (2014).
- Kaths, J. M., Spetzler, V. N., et al. Normothermic Ex Vivo Kidney Perfusion for the Preservation of Kidney Grafts prior to Transplantation. J Vis Exp. (101), e52909 (2015).
- Song, J. J., Guyette, J. P., Gilpin, S. E., Gonzalez, G., Vacanti, J. P., Ott, H. C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nature Med. 19 (5), 646-651 (2013).
- Hall, A. M., Crawford, C., Unwin, R. J., Duchen, M. R., Peppiatt-Wildman, C. M. Multiphoton imaging of the functioning kidney. JASN. 22 (7), 1297-1304 (2011).
- Lindell, S. L., Williams, N., Brusilovsky, I., Mangino, M. J. Mouse IPK: A Powerful Tool to Partially Characterize Renal Reperfusion and Preservation Injury. Open Transplant J. 5, 15-22 (2011).
- Wagner, C., De Wit, C., Kurtz, L., Grünberger, C., Kurtz, A., Schweda, F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 100 (4), 556-563 (2007).
- Czogalla, J., Vohra, T., Penton, D., Kirschmann, M., Craigie, E., Loffing, J. The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflüger’s Archiv. , (2016).
- Poosti, F., et al. Precision-cut kidney slices (PCKS) to study development of renal fibrosis and efficacy of drug targeting ex vivo. Dis Model Mech. 8 (10), 1227-1236 (2015).
- Rahgozar, M., Guan, Z., Matthias, A., Gobé, G. C., Endre, Z. H. Angiotensin II facilitates autoregulation in the perfused mouse kidney: An optimized in vitro model for assessment of renal vascular and tubular function. Nephrology. 9 (5), 288-296 (2004).

