Using Terminal Transferase-mediated dUTP Nick End-labelling (TUNEL) and Caspase 3/7 Assays to Measure Epidermal Cell Death in Frogs with Chytridiomycosis
Summary
We quantify epidermal cell death in frogs with chytridiomycosis using two methods. First, we use terminal transferase-mediated dUTP nick end-labelling (TUNEL) in situ histology to determine differences between clinically infected and uninfected animals. Second, we conduct a time series analysis of apoptosis over infection using a caspase 3/7 protein analysis.
Abstract
Amphibians are experiencing a great loss in biodiversity globally and one of the major causes is the infectious disease chytridiomycosis. This disease is caused by the fungal pathogen Batrachochytrium dendrobatidis (Bd), which infects and disrupts frog epidermis; however, pathological changes have not been explicitly characterized. Apoptosis (programmed cell death) can be used by pathogens to damage host tissue, but can also be a host mechanism of disease resistance for pathogen removal. In this study, we quantify epidermal cell death of infected and uninfected animals using two different assays: terminal transferase-mediated dUTP nick end-labelling (TUNEL), and caspase 3/7. Using ventral, dorsal, and thigh skin tissue in the TUNEL assay, we observe cell death in the epidermal cells in situ of clinically infected animals and compare cell death with uninfected animals using fluorescent microscopy. In order to determine how apoptosis levels in the epidermis change over the course of infection we remove toe-tip samples fortnightly over an 8-week period, and use a caspase 3/7 assay with extracted proteins to quantify activity within the samples. We then correlate caspase 3/7 activity with infection load. The TUNEL assay is useful for localization of cell death in situ, but is expensive and time intensive per sample. The caspase 3/7 assay is efficient for large sample sizes and time course experiments. However, because frog toe tip biopsies are small there is limited extract available for sample standardization via protein quantification methods, such as the Bradford assay. Therefore, we suggest estimating skin surface area through photographic analysis of toe biopsies to avoid consuming extracts during sample standardization.
Introduction
Amphibians are currently experiencing one the greatest losses of global biodiversity of any vertebrate taxa1. A major cause of these declines is the fatal skin disease chytridiomycosis, caused by the fungal pathogen Batrachochytrium dendrobatidis, Bd2. The pathogen superficially infects the epidermis, which can lead to the disruption of skin function resulting in severe electrolyte loss, cardiac arrest, and death3. Various potential host immune mechanisms against Bd are currently being studied, such as antimicrobial peptides4,5, cutaneous bacterial flora6, immune cell receptors7,8, and lymphocyte activity9,10. However, few studies explore whether epidermal apoptosis and cell death is an immune mechanism against this deadly pathogen.
Cell death, either through apoptosis (programmed cell death) or necrosis (unprogrammed death), in the epidermis may be a pathology of Bd infection. Previous research suggests that Bd infection may induce apoptosis because disruption of intracellular junctions is observed when skin explants are exposed to zoospore supernatants in vitro11. Additionally, degenerative epidermal changes in Bd-infected frogs are observed using electron microscopy12,13. Transcriptomic analyses indicate that apoptosis pathways are upregulated in infected skin14, and amphibian splenocytes undergo apoptosis when they are exposed to Bd supernatants in vitro15. Despite the growing volume of evidence suggesting that Bd can induce apoptosis and host cell death in vitro, in vivo studies that explore or quantify apoptosis mechanisms through the progression of infection are lacking. Further, it is unknown if the host uses apoptosis as a defensive immune strategy to combat Bd infection, or if apoptosis is a pathology of disease.
In this study, we aimed to detect epidermal cell death and apoptosis in infected animals in vivo using two methods: caspase 3/7 protein assay, and terminal transferase-mediated dUTP nick end-labelling (TUNEL) in situ assay. As each assay detects different aspects of cell death16, together these methods provide a full understanding of the mechanisms involved in cell death, and ensure an accurate measure of the effect. The caspase 3/7 assay quantifies the activity of effector caspases 3 and 7, which enables quantification of both the intrinsic and extrinsic apoptosis pathways. In contrast, the TUNEL assay detects DNA fragmentation, which is caused by cell death mechanisms including apoptosis, necrosis and pyroptosis17. We use the TUNEL assay to investigate the location of cell death within the epidermis of both clinically infected and uninfected animals using three different skin sections: the dorsum, the venter and the thigh of Pseudophryne corroboree. This method identifies the anatomical site of cell death, as well as distinguishing its location within specific epidermal layers. We then use the caspase 3/7 assay to conduct a time series quantification of apoptosis throughout an 8-week infection in Litoria verreauxii alpina. We take toe tip samples fortnightly from the same animals and are able to correlate pathogen infection load with caspase 3/7 activity.
Protocol
James Cook University approved animal ethics in applications A1875 for P. corroboree and A1897 and A2171 for L. v. alpina.
1. Animal Husbandry and Monitoring
- House animals individually, in an environment appropriate for the species, with an appropriate water, feeding and cleaning schedule. Check animals daily.
- Use adult individuals of the critically endangered Pseudophryne corroboree (for the TUNEL Assay, Section 4) and the threatened Litoria verreauxii alpina (for the Caspase 3/7 assay, Section 5), donated from captive breeding facilities. Maintain the animals at 15 – 18 °C, on a moss and gravel substrate, mist them daily with reverse osmosis water, and feed 3 times weekly with gut loaded crickets.
- Collect data on each individual once weekly. Swab the individuals for Bd as described in Step 2.1. Weigh each animal to the neared 0.1 g using a scale, and measure their snout to vent (SVL) length to the nearest 0.01 mm using dial callipers.
- After inoculation (as described in Section 3), check animals daily for clinical signs of infection (irregular skin slough, inappetence, leg redness, lethargy, or delayed righting reflex) in compliance with animal ethics guidelines. Euthanize animals if clinical signs and morbidity are present.
- Euthanize with an overdose of tricaine methanesulfonate (MS222) (0.1% w/v MS222 to pH 6 - 7 with sodium bicarbonate in aged tap water). Keep animals in MS222 for 10 min after all motion ceases and they display no response to stimuli.
2. Testing for Bd Infection
- Swabbing for Bd
- Swab each animal with a new sterile rayon swab (one swab per animal). Use the following swabbing method: five times on the venter, five times on each thigh, side, and limb. This is a total of 45 strokes.
- Gently rotate the swab during and between strokes to ensure effective capture of DNA. Break the swab tip off into 1.5 mL microcentrifuge tubes and store at -20 °C until extraction.
- DNA Extraction and qPCR assay
- Extract genomic DNA from the swabs by adding 50 µL of a commercially available DNA extraction reagent with 30 – 40 mg of 0.5 mm silica beads. Bead beat the samples in a bead beater cell disrupter at maximum speed for 2 min. Following the bead beater step, centrifuge the samples at 2,000 x g for 1 min.
- Incubate the samples at 100 °C for 10 min, and allow to cool. Centrifuge the samples at 5,000 x g for 3 min, and then transfer the supernatant to individual 1.5 mL micro centrifuge tubes and store at -20 °C until qPCR.
- Use quantitative PCR (qPCR) following Boyle et al. 200418 to analyze the infection load. Use the following modifications:
- Dilute DNA extraction samples 6:100 with double deionized water. Add 0.7 µL of bovine serum albumin (BSA) to each well to help prevent PCR inhibition. Run each sample in singlicate. On each plate use a negative (no template) control and a positive control with a series of dilution standards to estimate zoospore (infection) load.
3. Inoculation
- Culture Bd
- Prepare culture broth by adding 16 g of tryptone, 2 g of gelatin hydrolysate, and 4 g of lactose (TGHL) to 1 L of deionized water. Autoclave (121 °C for 40 min) and allow broth to cool.
- Inoculate Bd isolate (in this case, isolated from New South Wales isolate, AbercrombieR-L.booroologensis-2009-LB1, Passage number 11) in a TGHL broth and grow for 7 d at 23 °C, then transfer the broth culture to TGHL agar plates.
- To make plates, add 16 g of tryptone, 2 g of gelatin hydrolysate, 4 g of lactose (TGHL), and 10 g of bacteriological agar to 1 L of deionized water and autoclave (121 °C for 40 min). Once the mixture is cool enough to touch, but before it solidifies, pour the TGHL agar into 92 mm diameter culture plates so they are 1/4 to 1/3 full, in a Class II biological safety cabinet.
- When the agar has solidified and cooled completely, inoculate each plate with 0.5 mL of Bd from the liquid broth, and spread evenly. Allow Bd broth mixture to dry on plate for approximately 1 h and then seal plates with plastic paraffin film. Incubate plates agar side down at 23 °C for 5 – 7 d.
- Flood plates for zoospore inoculation solution
- Check zoospore motility under an inverted light microscope to ensure viability daily before inoculation. When zoospore release is high, with many zoospores swimming outside the sporangia, the culture is ready for inoculation.
- Flood each plate with 3 mL of aged tap water or artificial pond water, by pouring the water into the plate and incubating at room temperature for 10 min to allow the zoospores to release into the water. After the incubation period, decant the zoospore suspension into a new sterile container.
- Estimate zoospore concentration following manufacturer's instructions using a haemocytometer. Once the concentration of the zoospore suspension is known, dilute the suspension to a concentration of 1 x 106 zoospores per 3 mL with aged tap water.
- Inoculate animals
- Inoculate each animal by pouring 3 mL of inoculum mixture over its venter19 in individual 50 mL containers. Allow excess inoculum to collect into the base of the inoculation container. Leave each animal in individual inoculation container for 24 h to ensure infection, and after the 24 h inoculation, return each individual to a disinfected terrarium.
- Disinfect terraria using a 13% volume/volume (v/v) commercial bleach solution20, rinse at least twice with water, then allow to dry for no less than 24 h.
- For Bd-negative controls, mock inoculate the animals using the same methods as in 3.2 – 3.3, but flood Bd-free agar plates with 5 mL of aged tap water instead of Bd inoculated agar plates.
- Inoculate each animal by pouring 3 mL of inoculum mixture over its venter19 in individual 50 mL containers. Allow excess inoculum to collect into the base of the inoculation container. Leave each animal in individual inoculation container for 24 h to ensure infection, and after the 24 h inoculation, return each individual to a disinfected terrarium.
4. TUNEL Assay
- Euthanize animals that are showing clinical signs of chytridiomycosis, as described in step 1.3.1, and an equal number of Bd-negative control animals.
- Dissect skin (dorsal, ventral, and thigh) samples from each animal. Fix the skin samples for 2 h in 4% v/v phosphate buffered formaldehyde. The short and consistent fixing time allows the tissues to be fully fixed, but also allows for effective and accurate immunohistochemistry staining. Then transfer to 80% ethanol until embedding for sectioning.
- Embed skin in paraffin wax for histological preparation following standard methods21. Briefly, the protocol is as follows:
- Dehydrate tissues in a graded series of ethanol, and clear the ethanol with xylene. Embed the tissue in paraffin wax, and place all three skin samples for each individual in one paraffin block.
- Section skin using a microtome following standard histological preparation21. Section the tissue serially at 5 µm and then affix to hydrophilic glass slides. Place four serial histosections per skin type per slide. Make three slides per individual with serial skin sections, remember that each slide has four sections of each tissue affixed.
- Stain the slides in the following order:
- Stain the first slide with hematoxylin followed by eosin counterstaining (H&E). This slide is to visualize the location of Bd sporangia within the skin.
- Stain the second slide following a commercially available TUNEL assay for histological preparation and follow manufacturer's instructions, which are described below.
- First, deparaffinize the tissue sections in a coplin jar by washing the slides with three changes of xylene for 5 min per wash, and follow with two changes of 100% ethanol for 5 min each wash. Follow with one wash of 95% ethanol for 3 min and then 70% ethanol for 3 min. Finish with one wash of PBS for 5 min.
- Pretreat the tissue with freshly diluted protein digesting enzyme (proteinase K at a concentration of 20 μg/mL diluted in PBS), and add directly to the slide. Allow to incubate for 15 min. Wash with two changes of PBS in a coplin jar for 2 min each.
- Tap off the excess liquid and quench in 3.0% hydrogen peroxide in PBS in a coplin jar for 2 min at room temp. Rinse twice with PBS, for five minutes each wash.
- Tap off the excess liquid, then apply 75 µL/5 cm2 of the equilibrium buffer directly onto the tissue on the slide. Incubate for 10 s. Tap off the excess liquid around the section and apply 55 µL/5 cm2 of working terminal deoxynucleotidyl tranferase (TdT) enzyme. Incubate in a humidified chamber for 1 h at 37 °C.
- After the TdT incubation, put the slide in a coplin jar of working strength stop/wash buffer, agitate for 15 s and incubate for 10 min at room temperature. Wash the slide with three changes of PBS for 1 min per wash. Tap off the excess liquid.
- Apply anti-digoxigenin conjugate (rhodamine) that has been warmed to room temperature to the tissue, 65 µL/5 cm2. Incubate in a humidified chamber for 30 min at room temp and avoid exposure to light. Wash with four changes of PBS for 2 min per wash. Tap off the excess liquid.
- Finish by adding 15 µL of 0.5 – 1 µg/mL DAPI (4',6-diamidino-2-phenylindole) in slide mounting medium to the slide, which acts as a counter stain. Then cover with a cover slip and seal with nail polish or rubber cement. Allow slides to dry in the dark as the assay is light sensitive.
- Stain the third slide is stained using the TUNEL assay and DAPI counterstain, but use as a positive and negative control for each sample.
NOTE: Of the 4 histosections on the control slides, the first 2 are positive control replicates and the last 2 are negative control replicates.- For the positive controls, instead of step 4.5.2.2, pre-treat the tissue with DN buffer (30mM trizma base, pH 7.2, 4mM MgCl2, 0.1mM DDT) and let incubate at room temperature for 5 minutes. Then dissolve Dnase I in DN buffer for a final concentration of 0.1ug/mL, and apply it directly to the slide. Incubate for 15 minutes at room temperature. Then, wash the slide with 5 wash of dH2O for 3 minutes each wash. Blot off the excess liquid. Then resume TUNEL assay as directed in section 4.5.2.3.
- For the negative controls, do not add the terminal deoxynucleotidyl tranferase (TdT) enzyme to those samples (as described in section 4.5.2.4).
- Observe TUNEL slides immediately upon completion of the assay.
- Take photos at random intervals along each skin section at 200X using a fluorescent microscope, using filters for both the rhodamine stain, which reveals the apoptotic cells and appears red, and DAPI which reveals all nuclei and appears blue, so that an overlay of cells can be generated. Ensure at least 100 cells are photographed per skin section per sample. Store the slides in a dark microscope box at -20 °C.
- Count cells (blue DAPI stained) per image. Ensure at least 100 cells are counted per skin section. To reach 100 cells, count all cells within the image. If less then 100 cells are present in one image, count another image until at least 100 cells are reached per skin section per animal.
- Next, count the number of TUNEL positive cells in the same images (red rhodamine stained). TUNEL positive cells, which indicate apoptosis and not necrosis or background, are single cells with clear cell edges (membranes are intact with no lysis). Sometimes the cells shrink to form apoptotic bodies.
- Locate the areas counted in the TUNEL assay and analyze the corresponding regions on the H&E stained histosections. Confirm that the H&E stained sections correspond to sites of Bd infection, which ensures Bd-infected sites are used when counting apoptotic cells in the TUNEL assay.
5. Caspase 3/7 Assay
- Collect toe tips from each animal (both Bd– exposed and Bd– negative) once weekly through week 3 post inoculation, and fortnightly through the end of the experiment, up to 8 toes per individual.
- Cut the toe tip at the second phalange with flame-sterilized scissors, and place in a 1.5 mL microtube and freeze immediately at -80 °C.
- Extract proteins from frozen toe sample.
- Place samples in 100 µL of sample buffer (25 mM HEPES pH 7, 5 mM MgCl2) with two stainless steel beads (3.2 mm) in a 1.5 mL screw cap microtube. Lyse samples by 4 cycles of 1 min bead beating at maximum speed (in the same bead beater as used in step 1.2.1) followed by 3 min on ice. After lysis, centrifuge samples at 12,000 x g and 4 °C for 5 min. Collect supernatant to use in the assay.
- Quantify protein concentration per sample using the Bradford assay.
- In a 384 well plate, mix 10 µL of protein extract with 10 µL of Bradford reagent, and incubate for 2 min at room temperature. Perform each sample in duplicate, along with a series of 5 fold BSA dilution standards. Read the absorbance at 595 nm on an absorbance plate reader.
- Alternatively, if the toe tip samples are too small (the protein concentration is near the lowest standard as determined from the Bradford assay in step 4.3.1, or if the frogs that are sampled are under 3 g total weight), standardize samples by estimating skin surface area using photographs, instead of the Bradford assay.
- Before extracting the proteins from the sample for the caspase assay, photograph each toe at 40X magnification using an inverted light microscope.
- Analyze the toe sample using a computer imaging software, by estimating area of the toe. Do this by drawing a line around the edge of the toe clip, and have the imaging software estimate area within the shape. Then multiply that area by pi (3.14), which will approximate the surface area of the 3-dimentional skin sample (as length x cross-sectional area (pi x diameter) gives the outer surface area of a tube).
- Perform the caspase 3/7 assay.
- In a 384 well luminescence plate, add 10 µL of commercially available caspase 3/7 reagent and 10 µL of protein extract. Run each sample in triplicate.
- Mix the reagents by shaking the plate slowly for 15 s. Incubate the plate away from light for 30 min at room temperature. Measure luminescence using a luminescent plate reader.
Representative Results
TUNEL Assay
There were more TUNEL positive cells in the infected animals than in the uninfected control animals. The in situ location of TUNEL positive cells differed in infected and control animals. In control animals, there was an even distribution of TUNEL positive cells throughout the dermal and epidermal skin layers at low levels (See Figure 1A), but in the infected animals, the TUNEL positive cells were more frequent in the epidermis (Figure 1B). The sites of infection (as observed on the H&E stained slides) were observed as clumped Bd sporangia scattered through the thigh and ventral skin sections with none in the dorsum sections. While the TUNEL positive cells were more concentrated at and directly adjacent to sites of infected cells (Figure 1C), there was also more widespread TUNEL positive cells over the epidermis and in epidermal layers that were deeper than where Bd resided. In Bd infected animals there were more TUNEL positive cells in all three skin types analyzed, but a particularly high level in the thigh and venter skin (12.01 times higher (95% CI: 4.92 – 26.30) in the thigh skin and 22.31 times higher (95% CI 5.25 – 94.82) in the venter skin) (Figure 2).
Caspase 3/7 Assay
Over the course of infection there was a difference in caspase activity. In Bd infected animals, infection intensity was positively correlated with caspase 3/7 activity (Figure 3). There was also a difference between infected and uninfected animals through time, post inoculation. Caspase 3/7 activity decreased within the first few weeks after inoculation (with 48.36% less activity in infected animals), and then increased toward the end of week 7 (Figure 4) in Bd infected animals, but remained constant in uninfected individuals.
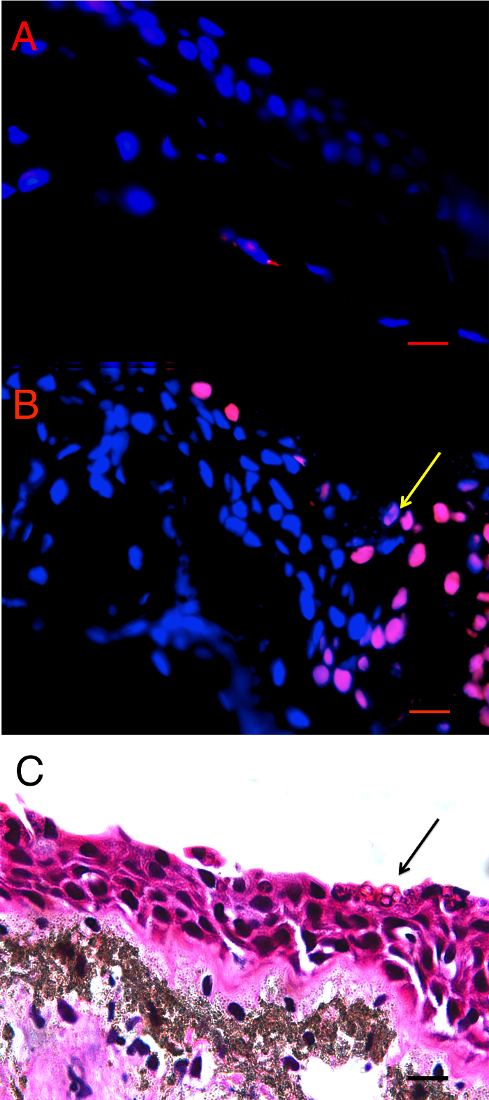
Figure 1: Terminal transferase-mediated dUTP nick end-labelling (TUNEL) in situ assay of infected and uninfected animals. A) Bd– control thigh skin section of Pseudophryne corroboree, and B) Bd+ thigh skin section of P. corroboree stained by in situ TUNEL assay. The blue is DAPI staining indicating nuclei of the cells, and the red is the rhodamine stain, which indicates DNA fragmentation characteristically caused by apoptosis. The yellow arrow indicates the position of the Bd cluster seen in panel C. C) P. corroboree section of thigh skin stained with H&E. The H&E section is serial to panel B. There is a cluster of empty Bd sporangia (arrow) and a few dark immature sporangia near the skin surface. For all three panels the epidermis is at the top of the photo. Comparing panels B and C shows that the rhodamine stained epidermal cells are concentrated around and below the cluster of Bd and where skin damage is visible, such as micro-vesicle formation between basal epidermal cells. 400X magnification and the scale bar indicates 0.03 mm. Adapted from Figure 2 in Brannelly et al. 2017 Peer J22. Please click here to view a larger version of this figure.
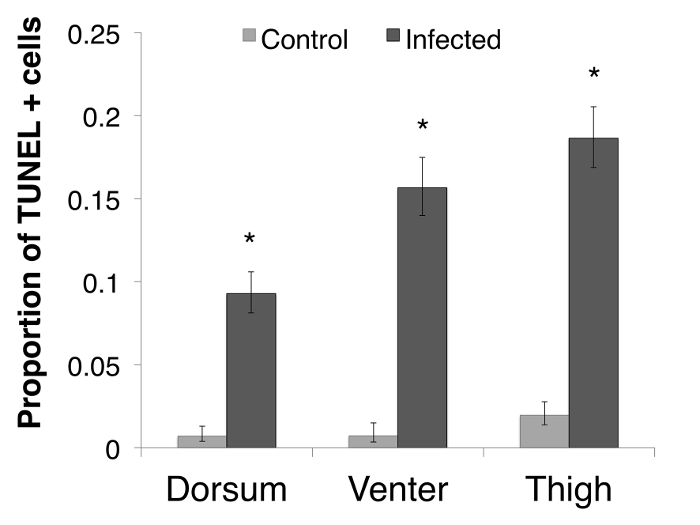
Figure 2: The proportion of TUNEL positive (TUNEL+) cells per skin type. The proportion of TUNEL positive apoptotic cells per skin type in P. corroboree, with infected animals indicating animals that succumbed to disease (n = 9) and uninfected control animals (n = 10). Error bars indicate 95% confidence intervals of a proportion and * indicates a significant increase in TUNEL+ cell proportions. In the dorsal skin, the infected animals had 14.38 (95% CI 3.32 – 62.24) times more TUNEL positive cells than control animals (Odds Ratio: Z = 3.57, p <0.01). In the thigh skin, infected animals had 12.01 (95% CI: 4.92 – 26.30; Odds Ratio: Z = 5.46, p <0.01) times more TUNEL positive cells than control animals (Pearson's Chi Squared: χ21 = 44.30, p <0.01). In the venter skin, infected animals had 22.31 (95% CI 5.25 – 94.82) times more TUNEL positive cells than control animals (Odds Ratio: Z = 4.21, p <0.01). Adapted from Figure 3 in Brannelly et al. 2017 Peer J22. Please click here to view a larger version of this figure.
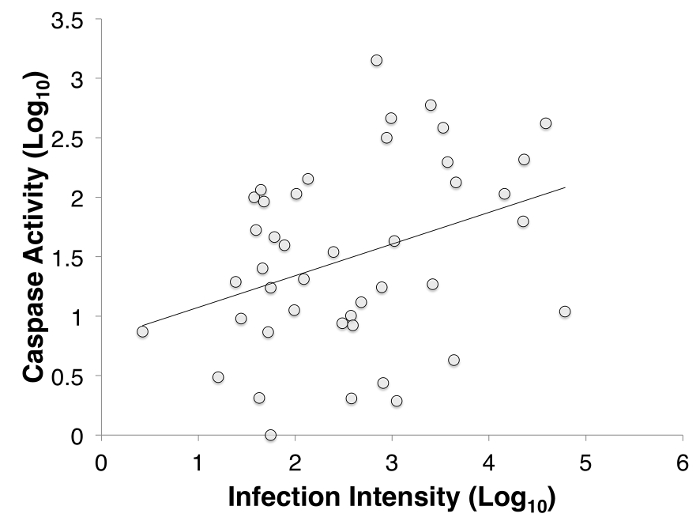
Figure 3: The correlation between infection intensity, Log10(zoospore equivalents), and caspase 3/7, Log10(Caspase) of inoculated Litoria verreauxii alpina over the course of the experiment. The correlation between infection intensity and caspase activity is 0.463, and the trend line has an equation of y = (0.229)x + 0.939. Adapted from Figure 4 in Brannelly et al. 2017 Peer J22. Please click here to view a larger version of this figure.
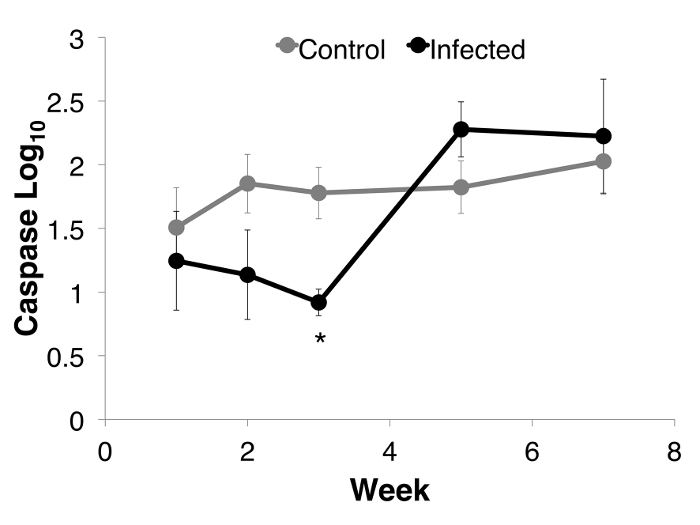
Figure 4: Caspase 3/7 activity through week 7 for each group of Litoria verreauxii alpina: Bd-infected animals that succumbed to disease (n = 4) and uninfected controls (n = 8). Caspase activity is defined as the luminescence reading controlled for by protein concentration per sample and then log base 10 transformed. The caspase activity (Log10 transformed) for each group per week. Error bars indicate standard error. * indicates that the infected animals differed from the uninfected controls at that week. (Linear mixed effects model: week, F4 = 11.974, p <0.01; week*status, F8 = 2.139, p = 0.037; Week 3 ANOVA, F2,18 = 5.512, p = 0.014), Adapted from Figure 5 in Brannelly et al. 2017 Peer J22. Please click here to view a larger version of this figure.
Discussion
We explored epidermal apoptosis and cell death as a potential mechanism of pathology of the deadly disease chytridiomycosis or a mechanism of disease resistance in Bd susceptible species. We used two methods of assessing cell death in the epidermis, TUNEL assay for in situ epidermal cell death analysis, and caspase 3/7 assay for monitoring epidermal cell death throughout the progress of infection. We found that cell death and apoptosis are correlated with infection load and cell death is significantly higher at the site of infection. In early infection, there is a decrease in apoptosis in the epidermis, but apoptosis increases as pathogen burden increases within the infected tissue.
Apoptosis in cells can be tested using numerous different approaches, each with benefits and limitations. It is important to study apoptosis and cell death using multiple assays in order to confirm findings. In this study, we explored two different assays to investigate epidermal cell death and apoptosis in frogs with chytridiomycosis. First, we trialled the TUNEL assay to assess cell death in situ. This test is time consuming, and expensive per sample, and slides must be read immediately as the fluorescent signal quickly fades. Despite these limitations, the results of this in situ experiment are important to further understanding the mechanisms of host-pathogen interactions. Information regarding localization can be determined using this method, and in our study apoptosis was present in high levels at the site of fungal infection and more diffusely at other sites. Additionally it must be noted that the TUNEL assay measures DNA damage, which can be caused by a number of different cell death mechanisms including apoptosis, necrosis and pyroptosis17. While there are signatures of apoptosis-associated cell death (such as single cells with membranes still intact, see protocol line 4.6.3, the classification relies on the interpretation of the researcher. Because the mechanism of cell death is not determined by the TUNEL assay, it is possible that another non-apoptosis cell death pathway may have caused the increase in TUNEL positive cells at morbidity of infected animals. The difference in what each assay measures might explain pattern differences in the two assays trialled here.
The caspase 3/7 assay can be used to quantify the effect of infection through time. However, as frog toe samples are small, there is limited sample for analysis and standardization. A common method for sample standardization is to determine the total protein concentration of each extract. As the Bradford protein quantification assay consumes sample, it is not an effective method for small extracts. In addition, we found that pigments within the frog skin precluded analysis by nano UV-Vis spectrometry. Small sample sizes also prevented standardization by dry weight. We suggest estimating skin surface area of the toe using photography, as the toe tip samples (these frogs were less than 3 g in body size) were too small to allow assays for both protein concentration and caspase concentration. We found that photographing the toes and using a skin surface area estimate is as effective as traditional protein concentration analyses.
In this study, we used two standard techniques for quantifying cell death and apoptosis, but adapted the methods for amphibians and small tissue samples. For the TUNEL assay, we euthanized the animal to ensure enough tissue was available for the assay, and allowed for the comparison of different skin locations. It is possible to adapt this method to smaller tissue samples, such as a toe webbing biopsy, which would not require euthanasia of the animal. Depending on the size of the animal, biopsies could allow for a time series test similar to our approach for the caspase 3/7 assay.
In future studies, we suggest the use of additional assays for exploring cell death and apoptosis over the course of chytridiomycosis infection because there is still much to learn of the causal mechanisms of chytridiomycosis pathogenesis. These results suggest that apoptosis and epidermal cell death may be important in the pathogenesis of Bd; however, more research is needed in order to determine the influence of apoptosis on disease outcomes, particularly in hosts that do not succumb to infection.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank the following people who assisted with husbandry and data collection: D. Tegtmeier, C. De Jong, J. Hawkes, K. Fossen, S. Percival, M. McWilliams, L. Bertola, M. Stewart, N. Harney, and T. Knavel; and M. Merces for assistance with dissections. We would also like to thank M. McFadden, P. Harlow and Taronga Zoo for raising the L. v. alpina, and G. Marantelli for raising the P. corroboree. We thank F. Pasmans, A. Martel for advice on apoptosis assays, C. Constantine, A. Kladnik and R. Webb for assistance with TUNEL assay, and T. Emeto and W. Weßels for help with protocol and kit for caspase 3/7 assay. This manuscript and protocol is adapted from Brannelly et al 2017 Peer J22.
Materials
| POLARstar Omega | BMG Labtech | Luminescent plate reader | |
| 384 well flat clear bottom plate | Corning | 3707 | |
| 384 well low flange white flat bottom plate | Corning | 3570 | |
| Agar Bacteriological (Oxoid) | Fisher | OXLP0011B | |
| Formal-Fixx 10% Neutral Buffered Formalin | Fisher | 6764254 | |
| Lactose Broth (Oxoid) | Fisher | OXCM0137B | |
| Sodium Bicarbonate | Fisher | BP328-500 | |
| Tricane-S (MS-222) | Fisher | NC0872873 | |
| Tryptone | Fisher | BP1421-500 | |
| Bovine Serum Albumin | Invitrogen | 15561020 | |
| Sterile rayon swab | Medical Wire & Equipment | MW-113 | |
| ApopTag Red In Situ Apoptosis Detection Kit | Merck Millipore | S7165 | |
| Coomassie Bradford reagent | Pierce | 23200 | |
| Caspase Glo 3/7 | Promega | G8090 | |
| HEPES buffer | Sigma Aldrich | H0887-20ML | |
| Magnesium chloride | Sigma Aldrich | 1374248-1G | |
| Gelatin hydrolysate Enzymatic | Sigma-Aldrich | G0262 | |
| PBS (Phosphate Buffered Saline), pH 7.2 (1X) | Thermo/Life | 20-012-043 | |
| Prepman | Thermo/Life | 4318930 | |
| TaqMan Fast Advanced Master Mix | ThermoFisher | 4444556 | |
| Parafilm | Bemis | PM996 | |
| Clorox bleach | Clorox | ||
| Ethanol, 200 Proof, Molecular Grade | Fisher | BP2818500 | |
| ZEISS Axio Scan florescent miscroscope | Carl Zeiss | Florescent microscope | |
| 3.2mm stainless steel beads | BioSpec | 11079132SS | |
| Primer ITSI-3 Chytr (5′-CCTTGATATAATACAGTGTGCCATATGTC-3′) | Taqman | Individual design for primers and probe | |
| Primer 5.8S Chytr (5′-TCGGTTCTCTAGGCAACAGTTT-3′) | Taqman | Individual design for primers and probe | |
| Minor groove binder probe Chytr MGB2(5′-CGAGTCGAAC-3′) | Taqman | Individual design for primers and probe | |
| Rotor-Gene qPCR Instruments | Qiagen | qPCR machine | |
| Microcentrifuge tubes 1.5ml | Fisher | 02-681-372 | |
| Cell culture petri plates | Nunc | 263991 | |
| Mini-beadBeater Zircornia-Silicate Beads, 0.5mm | BioSpec | 11079105Z |
References
- Stuart, S. N., et al. Status and trends of amphibian declines and extinctions worldwide. Science. 306 (5702), 1783-1786 (2004).
- Skerratt, L. F., et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 4, 125-134 (2007).
- Voyles, J., et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 326 (5952), 582-585 (2009).
- Woodhams, D. C., et al. Population trends associated with skin peptide defenses against chytridiomycosis in Australian frogs. Oecologia. 146 (4), 531-540 (2006).
- Woodhams, D. C., Voyles, J., Lips, K. R., Carey, C., Rollins-Smith, L. A. Predicted disease susceptibility in a Panamanian amphibian assemblage based on skin peptide defenses. Journal of Wildlife Diseases. 42 (2), 207-218 (2006).
- Woodhams, D. C., Rollins-Smith, L. A., Alford, R. A., Simon, M. A., Harris, R. N. Innate immune defenses of amphibian skin: antimicrobial peptides and more. Animal Conservation. 10, 425-428 (2007).
- Savage, A. E., Zamudio, K. R. MHC genotypes associate with resistance to a frog-killing fungus. PNAS. 108 (40), 16705-16710 (2011).
- Bataille, A., et al. Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proceeding of the Royal Society B. 282, 20143127 (2015).
- Fites, J. S., Reinert, L. K., Chappell, T. M., Rollins-Smith, L. A. Inhibition of local immune responses by the frog-killing fungus Batrachochytrium dendrobatidis. Infection and Immunity. 82 (11), 4698-4706 (2014).
- Brannelly, L. A., Webb, R. J., Skerratt, L. F., Berger, L. Effects of chytridiomycosis on hematopoietic tissue in the spleen, kidney and bone marrow in three diverse amphibian species. Pathogens and Disease. 74 (7), ftw069 (2016).
- Brutyn, M., et al. Batrachochytrium dendrobatidis zoospore secretions rapidly disturb intercellular junctions in frog skin. Fungal Genetics and Biology. 49 (10), 830-837 (2012).
- Berger, L., Hyatt, A. D., Speare, R., Longcore, J. E. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 68 (1), 51-63 (2005).
- Pasmans, F., et al. Chytridiomycosis related mortality in a midwife toad (Alytes obstetricans) in Belgium. Vlaams Diergeneeskundig Tijdschrift. 79 (6), 460-462 (2010).
- Ellison, A. R., et al. More than skin deep: Functional genomic basis for resistance to amphibian chytridiomycosis. Genome Biology and Evolution. 7 (1), 286-298 (2014).
- Fites, J. S., et al. The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science. 342 (6156), 366-369 (2013).
- Galluzzi, L., et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death and Differentiation. 16 (8), 1093-1107 (2009).
- Kelly, K. J., Sandoval, R. M., Dunn, K. W., Molitoris, B. A., Dagher, P. C. A novel method to determine specificity and sensitivity of the TUNEL reaction in the quantitation of apoptosis. American Journal of Cell Physiology. 284 (5), C1309-C1318 (2003).
- Boyle, D. G., Boyle, D. B., Olsen, V., Morgan, J. A. T., Hyatt, A. D. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organisms. 60 (2), 141-148 (2004).
- Brannelly, L. A., Webb, R., Skerratt, L. F., Berger, L. Amphibians with infectious disease increase their reproductive effort: evidence for the terminal investment hypothesis. Open Biology. 6 (6), 1-24 (2016).
- Webb, R., Mendez, D., Berger, L., Speare, R. Additional disinfectants effective against the amphibian chytrid fungus Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms. 74 (1), 13-16 (2007).
- Woods, A., Ellis, R. . Laboratory Histopathology: A Complete Reference. , (1994).
- Brannelly, L. A., Roberts, A. A., Skerratt, L. F., Berger, L. Epidermal cell death in frogs with chytridiomycosis. PeerJ. 5, e2925 (2017).

