Bulk and Thin Film Synthesis of Compositionally Variant Entropy-stabilized Oxides
Summary
The synthesis of high quality bulk and thin film (Mg0.25(1-x)CoxNi0.25(1-x)Cu0.25(1-x)Zn0.25(1-x))O and (Mg0.25(1-x)Co0.25(1-x)Ni0.25(1-x)CuxZn0.25(1-x))O entropy-stabilized oxides is presented.
Abstract
Here, we present a procedure for the synthesis of bulk and thin film multicomponent (Mg0.25(1-x)CoxNi0.25(1-x)Cu0.25(1-x)Zn0.25(1-x))O (Co variant) and (Mg0.25(1-x)Co0.25(1-x)Ni0.25(1-x)CuxZn0.25(1-x))O (Cu variant) entropy-stabilized oxides. Phase pure and chemically homogeneous (Mg0.25(1-x)CoxNi0.25(1-x)Cu0.25(1-x)Zn0.25(1-x))O (x = 0.20, 0.27, 0.33) and (Mg0.25(1-x)Co0.25(1-x)Ni0.25(1-x)CuxZn0.25(1-x))O (x = 0.11, 0.27) ceramic pellets are synthesized and used in the deposition of ultra-high quality, phase pure, single crystalline thin films of the target stoichiometry. A detailed methodology for the deposition of smooth, chemically homogeneous, entropy-stabilized oxide thin films by pulsed laser deposition on (001)-oriented MgO substrates is described. The phase and crystallinity of bulk and thin film materials are confirmed using X-ray diffraction. Composition and chemical homogeneity are confirmed by X-ray photoelectron spectroscopy and energy dispersive X-ray spectroscopy. The surface topography of thin films is measured with scanning probe microscopy. The synthesis of high quality, single crystalline, entropy-stabilized oxide thin films enables the study of interface, size, strain, and disorder effects on the properties in this new class of highly disordered oxide materials.
Introduction
Since the discovery of high-entropy metal alloys in 2004, high-entropy materials have attracted significant interest due to the properties such as increased hardness1,2,3, toughness4,5, and corrosion resistance3,6. Recently, high-entropy oxides7,8 and borides9 have been discovered, opening up a large playground for material enthusiasts. Oxides, in particular, can demonstrate useful and dynamic functional properties such as ferroelectricity10, magnetoelectricity11,12, thermoelectricity13, and superconductivity14. Entropy-stabilized oxides (ESOs) have recently been shown to possess interesting, compositionally-dependent functional properties15,16, despite the significant disorder, making this new class of materials particularly exciting.
Entropy-stabilized materials are chemically homogeneous, multicomponent (typically having five or more constituents), single-phase materials where the configurational entropic contribution (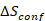 ) to the Gibbs free energy (
) to the Gibbs free energy (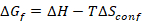 ) is significant enough to drive the formation of a single phase solid solution17. The synthesis of multicomponent ESOs, where cationic configurational disorder is observed across the cation sites, requires precise control over the composition, temperature, deposition rate, quench rate, and quench temperature7,16. This method seeks to enable the practitioner the ability to synthesize phase pure and chemically homogeneous entropy-stabilized oxide ceramic pellets and phase pure, single crystalline, flat thin films of the desired stoichiometry. Bulk materials can be synthesized with greater than 90% theoretical density enabling the study of the electronic, magnetic, and structural properties or use as sources for thin film physical vapor deposition (PVD) techniques. As the entropy-stabilized oxides considered here have five cations, thin film PVD techniques that employ five sources, such as molecular beam epitaxy (MBE) or co-sputtering, will be presented with the challenge of depositing chemically homogenous thin films due to flux drift. This protocol results in chemically homogenous, single crystalline, flat (root-mean-square (RMS) roughness of ~0.15 nm) entropy-stabilized oxide thin films from a single material source, which are shown to possess the nominal chemical composition. This thin film synthesis protocol may be enhanced by the inclusion of in situ electron or optical characterization techniques for real-time monitoring of the synthesis and refined quality control. Expected limitations of this method stem from laser energy drift which may limit the thickness of high quality films to be below 1 μm.
) is significant enough to drive the formation of a single phase solid solution17. The synthesis of multicomponent ESOs, where cationic configurational disorder is observed across the cation sites, requires precise control over the composition, temperature, deposition rate, quench rate, and quench temperature7,16. This method seeks to enable the practitioner the ability to synthesize phase pure and chemically homogeneous entropy-stabilized oxide ceramic pellets and phase pure, single crystalline, flat thin films of the desired stoichiometry. Bulk materials can be synthesized with greater than 90% theoretical density enabling the study of the electronic, magnetic, and structural properties or use as sources for thin film physical vapor deposition (PVD) techniques. As the entropy-stabilized oxides considered here have five cations, thin film PVD techniques that employ five sources, such as molecular beam epitaxy (MBE) or co-sputtering, will be presented with the challenge of depositing chemically homogenous thin films due to flux drift. This protocol results in chemically homogenous, single crystalline, flat (root-mean-square (RMS) roughness of ~0.15 nm) entropy-stabilized oxide thin films from a single material source, which are shown to possess the nominal chemical composition. This thin film synthesis protocol may be enhanced by the inclusion of in situ electron or optical characterization techniques for real-time monitoring of the synthesis and refined quality control. Expected limitations of this method stem from laser energy drift which may limit the thickness of high quality films to be below 1 μm.
Despite the significant advances in the growth and characterization of thin film oxide materials10,18,19,20,21, the correlation between stereochemistry and electronic structure in oxides can lead to significant differences in the final material stemming from seemingly insignificant methodological differences. Furthermore, the field of multicomponent entropy-stabilized oxides is rather nascent, with only two current reports of thin film synthesis in the literature7,16. ESOs lend themselves particularly well to this process, circumventing challenges that would be presented by chemical vapor deposition and molecular beam epitaxy. Here, we provide a detailed synthesis protocol of bulk and thin films ESOs (Figure 1), in order to minimize materials processing difficulties, unintended property variations, and improve the acceleration of discovery in the field.
Protocol
Representative Results
Discussion
We have described and shown a protocol for the synthesis of bulk and high-quality, single crystalline films of (Mg0.25(1-x)CoxNi0.25(1-x)Cu0.25(1-x)Zn0.25(1-x))O (x = 0.20, 0.27, 0.33) and (Mg0.25(1-x)Co0.25(1-x)Ni0.25(1-x)CuxZn0.25(1-x))O (x = 0.11, 0.27) entropy-stabilized oxides. We expect these synthesis techniques to be applicable to a wide range of entropy-stabilized oxide compositions as more are discovere…
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded in part by National Science Foundation grant No. DMR-0420785 (XPS). We thank the University of Michigan's Michigan Center for Materials Characterization, (MC)2, for its assistance with XPS, and the University of Michigan Van Vlack laboratory for XRD. We would also like to thank Thomas Kratofil for his assistance with bulk materials preparation.
Materials
| MAGNESIUM OXIDE 99.95% | Fisher | AA1468422 | |
| COBALT(II) OXIDE, 99.995% | Fisher | AA4435414 | |
| NICKEL(II) OXIDE 99.998% | Fisher | AA1081914 | |
| COPPER(II) OXIDE 99.995% | Fisher | AA1070014 | |
| ZINC OXIDE 99.99% | Fisher | AA8781230 | |
| TRICHLROETHLENE SEMICNDTR 9 | Fisher | AA39744K7 | |
| ACETONE SEMICNDTR GRD 99.5% | Fisher | AA19392K7 | |
| 2-PROPANOL ACS 99.5% | Fisher | A416S4 | |
| Mineral oil, pure | Acros Organics | AC415080010 | |
| alumina crucible | MTI Corporation | eq-ca-l50w40h20 | |
| ZIRCONIA (YSZ) GRINDING MEDIA | Inframat Advanced Materials | 4039GM-S010 | |
| SiC paper 320/600/800/1200 | South Bay Technology | SDA08032-25 | |
| MgO (100) substrate, 5x5x0.5 mm, 1SP | MTI Corporation | MGa050505S1 | |
| OXYGEN COMPRESSED ULTRA HIGH PURITY GRADE, 99.999% | Cryogenic Gases | OXYUHP | |
| NITROGEN COMPRESSED EXTRA DRY GRADE | Cryogenic Gases | NITEX |
References
- Tsai, M. H., Yeh, J. W. High-Entropy Alloys: A Critical Review. Mater Res Lett. 2 (3), 107-123 (2014).
- Yeh, J. W., et al. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv Eng Mater. 6 (5), 299-303 (2004).
- Gao, M. C., Carney, C. S., Dogan, N., Jablonksi, P. D., Hawk, J. A., Alman, D. E. Design of Refractory High-Entropy Alloys. Jom. 67 (11), 2653-2669 (2015).
- Gludovatz, B., Hohenwarter, A., Catoor, D., Chang, E. H., George, E. P., Ritchie, R. O. A fracture-resistant high-entropy alloy for cryogenic applications. Science. 345 (6201), 1153-1158 (2014).
- Zou, Y., Ma, H., Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat Commun. 6, 7748 (2015).
- Poulia, A., Georgatis, E., Lekatou, A., Karantzalis, A. E. Microstructure and wear behavior of a refractory high entropy alloy. Int J Refract Met Hard Mater. 57, 50-63 (2016).
- Rost, C. M., et al. Entropy-stabilized oxides. Nat Commun. 6, 8485 (2015).
- Jiang, S., et al. A new class of high-entropy perovskite oxides. Scripta Mater. 142, 116-120 (2018).
- Gild, J., et al. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci Rep. 6 (October), 37946 (2016).
- Schlom, D. G. others Strain Tuning of Ferroelectric Thin Films. Annu Rev Mater Res. 37, 589-626 (2007).
- Zhao, T., et al. Electrical control of antiferromagnetic domains in multiferroic BiFeO3 films at room temperature. Nat Mater. 5 (10), 823-829 (2006).
- Borisov, P., Hochstrat, A., Chen, X., Kleemann, W., Binek, C. Magnetoelectric Switching of Exchange Bias. Phys Rev Lett. 94 (11), 117203 (2005).
- Weidenkaff, A., Robert, R., Aguirre, M., Bocher, L., Lippert, T., Canulescu, S. Development of thermoelectric oxides for renewable energy conversion technologies. Renew Energy. 33 (2), 342-347 (2008).
- Pickett, W. E. Electronic structure of the high-temperature oxide superconductors. Rev Mod Phys. 61 (2), 433-512 (1989).
- Berardan, D., Franger, S., Dragoe, D., Meena, A. K., Dragoe, N. Colossal dielectric constant in high entropy oxides. Phys Status Solidi – Rapid Res Lett. 10 (4), 328-333 (2016).
- Meisenheimer, P. B., Kratofil, T. J., Heron, J. T. Giant Enhancement of Exchange Coupling in Entropy-Stabilized Oxide Heterostructures. Sci Rep. 7 (1), 13344 (2017).
- Miracle, D. B. High-Entropy Alloys: A Current Evaluation of Founding Ideas and Core Effects and Exploring "Nonlinear Alloys.". Jom. , 1-7 (2017).
- Mannhart, J., Schlom, D. G. Oxide Interfaces-An Opportunity for Electronics. Science. 327 (5973), 1607-1611 (2010).
- Mundy, J. A., et al. Atomically engineered ferroic layers yield a room-temperature magnetoelectric multiferroic. Nature. 537 (7621), 523-527 (2016).
- Martin, L. W., Chu, Y. H., Ramesh, R. Advances in the growth and characterization of magnetic, ferroelectric, and multiferroic oxide thin films. Mater Sci Eng R Rep. 68 (4), 89-133 (2010).
- Saremi, S., et al. Enhanced Electrical Resistivity and Properties via Ion Bombardment of Ferroelectric Thin Films. Adv Mater. 28 (48), 10750-10756 (2016).
- Cullity, B. D., Weymouth, J. W. Elements of X-ray Diffraction. Am J Phys. 25 (6), 394-395 (1957).
- Rijnders, G. J. H. M., Koster, G., Blank, D. H. A., Rogalla, H. In situ monitoring during pulsed laser deposition of complex oxides using reflection high energy electron diffraction under high oxygen pressure. Appl Phys Lett. 70 (14), 1888-1890 (1997).
- Sullivan, M. C., et al. Complex oxide growth using simultaneous in situ reflection high-energy electron diffraction and x-ray reflectivity: When is one layer complete?. Appl Phys Lett. 106 (3), 031604 (2015).
- Eres, G., et al. Time-resolved study of SrTiO3 homoepitaxial pulsed-laser deposition using surface x-ray diffraction. Appl Phys Lett. 80 (18), 3379-3381 (2002).
- Fleet, A., Dale, D., Suzuki, Y., Brock, J. D. Observed Effects of a Changing Step-Edge Density on Thin-Film Growth Dynamics. Phys Rev Lett. 94 (3), 036102 (2005).
- Luca, G. D., Strkalj, N., Manz, S., Bouillet, C., Fiebig, M., Trassin, M. Nanoscale design of polarization in ultrathin ferroelectric heterostructures. Nat Commun. 8 (1), 1419 (2017).
- De Luca, G., Rossell, M. D., Schaab, J., Viart, N., Fiebig, M., Trassin, M. Domain Wall Architecture in Tetragonal Ferroelectric Thin Films. Adv Mater. 29 (7), (2017).
- Gruenewald, J. H., Nichols, J., Seo, S. S. A. Pulsed laser deposition with simultaneous in situ real-time monitoring of optical spectroscopic ellipsometry and reflection high-energy electron diffraction. Rev Sci Instrum. 84 (4), 043902 (2013).
- . MDC Vacuum Products | Vacuum Components, Chambers, Valves, Flanges & Fittings Available from: https://mdcvacuum.com/DisplayContentPageFull.aspx?cc=b8ca254a-cdc0-4b71-8603-af10ce18bbcb (2018)
- Dijkkamp, D., et al. Preparation of Y-Ba-Cu oxide superconductor thin films using pulsed laser evaporation from high Tc bulk material. Appl Phys Lett. 51 (8), 619-621 (1987).
- Biegalski, M. D., et al. Relaxor ferroelectricity in strained epitaxial SrTiO3 thin films on DyScO3 substrates. Appl Phys Lett. 88 (19), 192907 (2006).
- Schlom, D. G., Chen, L. Q., Pan, X., Schmehl, A., Zurbuchen, M. A. A Thin Film Approach to Engineering Functionality into Oxides. J Am Ceram Soc. 91 (8), 2429-2454 (2008).
- Damodaran, A. R., Breckenfeld, E., Chen, Z., Lee, S., Martin, L. W. Enhancement of Ferroelectric Curie Temperature in BaTiO3 Films via Strain-Induced Defect Dipole Alignment. Adv Mater. 26 (36), 6341-6347 (2014).

