Automating Citrus Budwood Processing for Downstream Pathogen Detection Through Instrument Engineering
Summary
We engineered, fabricated, and validated an instrument that rapidly processes phloem-rich bark citrus budwood tissues. Compared to current methods, the budwood tissue extractor (BTE) has increased sample throughput and decreased the required labor and equipment costs.
Abstract
Graft-transmissible, phloem-limited pathogens of citrus such as viruses, viroids, and bacteria are responsible for devastating epidemics and serious economic losses worldwide. For example, the citrus tristeza virus killed over 100 million citrus trees globally, while "Candidatus Liberibacter asiaticus" has cost Florida $9 billion. The use of pathogen-tested citrus budwood for tree propagation is key for the management of such pathogens. The Citrus Clonal Protection Program (CCPP) at the University of California, Riverside, uses polymerase chain reaction (PCR) assays to test thousands of samples from citrus budwood source trees every year to protect California's citrus and to provide clean propagation units to the National Clean Plant Network. A severe bottleneck in the high-throughput molecular detection of citrus viruses and viroids is the plant tissue processing step.
Proper tissue preparation is critical for the extraction of quality nucleic acids and downstream use in PCR assays. Plant tissue chopping, weighing, freeze-drying, grinding, and centrifugation at low temperatures to avoid nucleic acid degradation is time-intensive and labor-intensive and requires expensive and specialized laboratory equipment. This paper presents the validation of a specialized instrument engineered to rapidly process phloem-rich bark tissues from citrus budwood, named the budwood tissue extractor (BTE). The BTE increases sample throughput by 100% compared to current methods. In addition, it decreases labor and the cost of equipment. In this work, the BTE samples had a DNA yield (80.25 ng/µL) that was comparable with the CCPP's hand-chopping protocol (77.84 ng/µL). This instrument and the rapid plant tissue processing protocol can benefit several citrus diagnostic laboratories and programs in California and become a model system for tissue processing for other woody perennial crops worldwide.
Introduction
Graft-transmissible phloem-limited pathogens of citrus, such as viroids, viruses, and bacteria, have caused devastating epidemics and serious economic losses in every citrus-producing area of the world. Citrus viroids are limiting production factors because of the exocortis and cachexia diseases they cause in economically important citrus types, such as trifoliate, trifoliate hybrids, mandarins, clementines, and tangerines1,2,3. In California, these viroid-sensitive citrus types are the basis of the growing and profitable market of "easy-peelers", following the shifting trend in consumers' preference for fruits that are easy to peel, segmented, and seedless4,5,6. Thus, citrus viroids are regulated under the California Department of Food and Agriculture (CDFA) "Citrus Nursery Stock Pest Cleanliness Program-Senate Bill 140", and the laboratories of CDFA's Plant Pest Diagnostics Branch perform thousands of citrus viroid tests annually7,8,9,10. Citrus tristeza virus (CTV) has been responsible for the death of over 100 million citrus trees since the beginning of the global epidemic in the 1930s3,9,10,11. In California, stem pitting and trifoliate breaking resistance isolates of the virus pose a serious threat to the $3.6 billion California citrus industry12,13,14. Consequently, CDFA classifies CTV as a regulated class-A plant pest, and the laboratory of the Central California Tristeza Eradication Agency (CCTEA) performs extensive field surveys and thousands of virus tests every year15,16. The bacterium "Candidatus Liberibacter asiaticus" (CLas) and the huanglongbing (HLB) disease are estimated to have caused close to $9 billion of economic damage to Florida as a result of a 40% reduction of citrus acreage, a 57% decrease in citrus operations, and a loss of almost 8,000 jobs17,18. In California, a hypothetical 20% reduction in citrus acreage due to HLB was predicted to result in more than 8,200 job losses and a reduction of over half a billion dollars in the state's gross domestic product. Therefore, the Citrus Pest and Disease Prevention Program spends over $40 million annually on surveys to test, detect, and eradicate CLas from California14,17,19,20.
A key element of the management of citrus viroids, viruses, and bacteria is the use of pathogen-tested propagative materials (i.e., budwood) for tree production. Pathogen-tested citrus budwood is produced and maintained within comprehensive quarantine programs that employ advanced pathogen elimination and detection techniques10,21. The Citrus Clonal Protection Program (CCPP) at the University of California, Riverside, tests thousands of budwood samples every year from citrus varieties newly imported into the state and the USA, as well as citrus budwood source trees, to protect California's citrus and support the functions of the National Clean Plant Network for Citrus10,17,22. To handle the large volume of citrus testing, high-throughput, reliable, and cost-effective pathogen detection assays are a fundamental component for the success of programs such as the CCPP7,10,22.
While molecular-based pathogen detection assays such as polymerase chain reaction (PCR) have allowed for significant increases in throughput in plant diagnostic laboratories, in our experience, one of the most critical bottlenecks in the implementation of high-throughput protocols is the plant tissue sample processing step. This is particularly true for citrus because the currently available protocols for the processing of phloem-rich tissues such as leaf petioles and budwood bark are labor-intensive, time-consuming, and require expensive and specialized laboratory equipment. These protocols require hand-chopping, weighing, freeze-drying, grinding, and centrifugation at low temperatures to avoid nucleic acid degradation8,23,24. For example, at the CCPP diagnostic laboratory, sample processing includes (i) hand-chopping (6-9 samples/h/operator), (ii) freeze-drying (16-24 h), (iii) pulverization (30-60 s), and (iv) centrifugation (1-2 h). The process also requires specialized supplies (e.g., heavy-duty safe-lock tubes, stainless steel grinding balls, adapters, blades, gloves) and multiple pieces of costly lab equipment (e.g., ultra-low freezer, freeze-dryer, tissue pulverizer, liquid nitrogen cryostation, refrigerated centrifuge).
As in any industry, equipment engineering and the automation of processes are key to lowering costs, increasing throughput, and providing high-quality, uniform product and services. The citrus industry needs low-cost tissue-processing instruments that require minimum skill to operate and, as such, are easy to transfer to diagnostic laboratories and field operations to allow high sample-processing capacity for rapid downstream pathogen detection. Technology Evolving Solutions (TES) and the CCPP developed (i.e., design and fabricate) and validated (i.e., tested with citrus samples and compared to standard laboratory procedures) a low-cost (i.e., eliminated the need for specialized laboratory equipment) instrument for the rapid processing of phloem-rich citrus tissues (i.e., budwood), named the budwood tissue extractor (BTE). As seen in Figure 1, the BTE includes a base component for power and controls, plus a removable chamber for the processing of citrus budwood. The BTE chamber is composed of a grinding wheel specifically designed to strip the phloem-rich bark tissues from the citrus budwood. The shredded bark tissue is ejected rapidly through a slide port into a syringe containing extraction buffer, filtered, and made ready for nucleic acid extraction and purification without any additional handling or preparation (Figure 1). The BTE system also includes a paperless sample tracking application and an integrated weighing application, which record the sample processing information in an online database in real time.
The BTE system has increased the CCPP's lab diagnostic capacity by over 100% and has consistently produced citrus tissue extracts suitable for the purification of high-quality nucleic acids and the downstream detection of graft-transmissible pathogens of citrus using PCR assays. More specifically, BTE has reduced the time for tissue processing from over 24 h to ~3 min per sample, replaced laboratory instruments costing over $60,000 (Figure 2, steps 2-4), and allowed for the processing of larger sample sizes.
This paper presents the BTE high-throughput citrus bark tissue processing, nucleic acid extraction, and pathogen detection validation data with citrus budwood samples from source trees, including all the appropriate positive and negative controls from the CCPP Rubidoux Quarantine Facility and Lindcove Foundation Facility, respectively. We also present the throughput and processing time changes compared to the current laboratory procedure (Figure 2). In addition, this work provides a detailed, step-by-step protocol for citrus pathogen testing laboratories and demonstrates how the BTE can support the functions of pathogen-clean nursery stock, survey, and eradication programs.
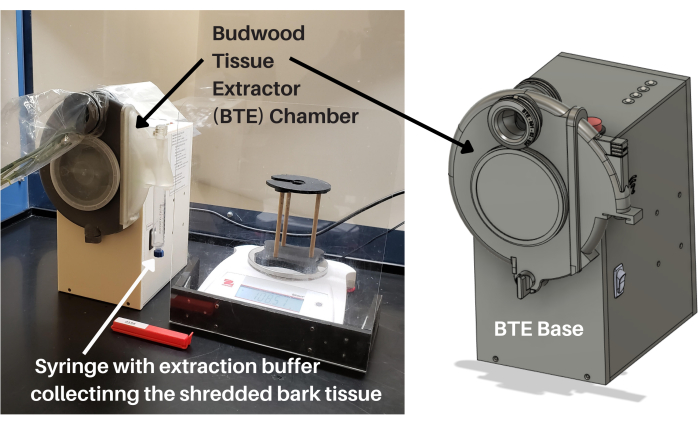
Figure 1: Budwood tissue extractor. The BTE includes a base component for power and controls, plus a removable chamber for the processing of citrus budwood. The BTE chamber is composed of a grinding wheel specifically designed to strip the phloem-rich bark tissues from citrus budwood. The shredded bark tissue is ejected rapidly through a slide port into a syringe, filtered, and made ready for nucleic acid extraction and purification without any additional handling or preparation. Abbreviation: BTE = budwood tissue extractor. Please click here to view a larger version of this figure.
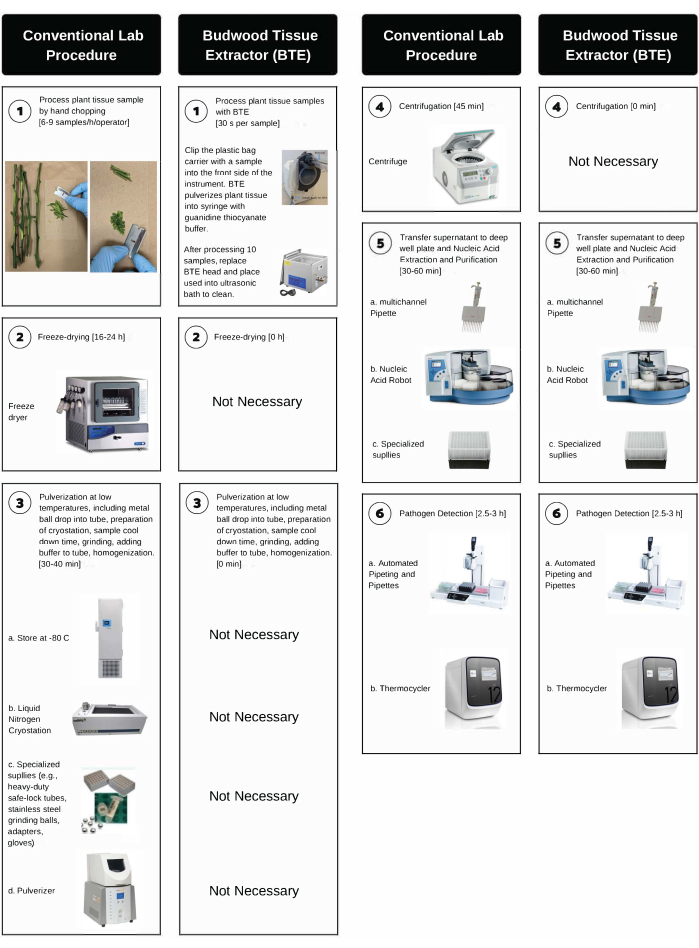
Figure 2: Step-by-step comparison between the conventional hand-chopping lab procedure and BTE processing. BTE processing involves high-throughput citrus bark tissue processing, nucleic acid extraction, and pathogen detection. The time for each step is indicated in parentheses. Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
With the advent of HLB citrus disease, to reduce losses, the citrus industry, regulatory agencies, and diagnostic laboratories have been urged to rely on high-throughput nucleic acid extraction methods combined with low-throughput manual sample processing and pathogen detection assays such as qPCR34 for the testing of individual trees, in combination with disease management practices35. California’s HLB positivity rate has gone from 0.01% in 2012 to 1.2% in 2020. Even thoug…
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge the Cahuilla people as the Traditional Custodians of the Land on which the experimental work was completed. We are grateful to Professor Norman Ellstrand at the University of California, Riverside, for providing lab space to carry out research activities for this project under the UCR California Agriculture and Food Enterprise (CAFÉ) Initiative. This research was supported by the CDFA – Specialty Crop Block Grant Program (grant no. 18-0001-055-SC). Additional support was also provided by the CRB project 6100; USDA National Institute of Food and Agriculture, Hatch project 1020106; and the National Clean Plant Network-USDA Animal and Plant Health Inspection Service (AP17PPQS&T00C118, AP18PPQS&T00C107, AP19PPQS&T00C148, & AP20PPQS&T00C049) awarded to Georgios Vidalakis.
Materials
| 0.08" Hex Trimmer line | PowerCare | FPRO07065 | Needed to replace blades. |
| 1 Hp, 8 gal air compressor | California Air Tools | 8010 | Quickly dry chambers after rinsed |
| 1.5 mL microcentrifuge tube | Globe Scientific | 111558B | Store sample in after swishing with syinges |
| 10 mL Syringe Set | Technology Evolving Solutions | TE006-F1-10A-G1000-E1 | Syringe material is cut into. 1 L bottle with guanidine thiocyanate buffer. WARNING – contains guanidine thiocyanate, hazardous waste service required – do not mix with bleach |
| 12" Ruler | Westcott | 16012 | To measure trimmer line before cutting |
| 12% Sodium Hypochlorite | Hasa | 1041 | Disinfects chambers after processing |
| -20 C Freezer | Insignia | NS-CZ70WH0 | Store sample after processing |
| 4" x 12" plastic bags | Plymor | FP20-4×12-10 | Bags to hold branches during shipping. O-rings attach bag to BTE chamber to seal |
| 6" Cotton Swab | Puritan | 806-PCL | Swab to remove clogs |
| 7 Gallon Storage Tote | HDX | 206152 | Holds sodium hypochlorite solution to disinfect chambers and water to rinse chambers |
| Air blow gun | JASTIND | JTABG103A | Directs air into the chambers at high pressure |
| Black Sharpie | Sharpie | S-19421 | Mark 1.5 mL tubes so you can identify sample later |
| Bottle Top Dispensor | Brand | Z627569 | Adjustable bottle top dispensor to dispense guandine into syringe |
| BTE Chamber | Technology Evolving Solutions | TE002BB-A05-E1 | Used to process budwood. Includes O-rings, BTE Slide, slide plunger, drain valve, lid, blade set, and blade set removal tool |
| Dish Soap | Dawn | 57445CT | Surfectant to improve sodium hypochlorite penetration into chamber |
| Fume hood with hepa filter | Air Science | P5-36XT-A | Fume hood with hepa filter (ASTS-030) to limit possible contamination and protect against chemical spills |
| Insulated foam shipping container | PolarTech | 261/J50C | Insulated shipping container to ship samples on ice after they are collected |
| Lab coat | Red Kap | KP14WH LN 46 | Lab coat to limit possible contamination and protect against chemical spills |
| Laptop | Microsoft | Surface | Wifi capable laptop to run TES GUI. Needed for initial setup and provides more indepth information about the tissue processing base |
| NFC Capable Phone | Samsung | Galaxy S9 | Phone to download and use TES phone app |
| NFC clip tag | Technology Evolving Solutions | TE005-Clip-E1 | Sample tag that can be linked with trees. Made to function with TES phone app |
| NFC Collar Tag | Technology Evolving Solutions | TE005-Collar-E1 | Tag that is attached to a tree. Made to function with TES phone app |
| Nitrile Gloves | Usa Scientific | 3915-4400 | Gloves to limit possible contamination and protect against chemical spills |
| Noise-Reducing Earmuff | 3M | 90565-4DC-PS | Protect ears while operating air compressor and tissue processing base |
| Polyurethane Recoil Air Hose | FYPower | 510019 | Attaches air gun to compressor |
| Saftey glasses | Solidwork | SW8329-US | Protect eyes for chemical and physical hazards |
| Spray bottle | JohnBee | B08QM81BJV | Spray bleach to deconatinate surfaces |
| Tissue Extractor Base | Technology Evolving Solutions | TE001-A-E1 | System to process plant tissue. Needs BTE or LTE chambers to function. Includes power cable, blade adapter, and 8/32" allen wrench |
| Tissue Processing Base Weight Scale | Technology Evolving Solutions | TE003-A05-200g-01-E1 | 200 g, 0.01 resolution weight scale that connects to tissue processing base to enforce weight ranges and/or link weights with sample. Includes scale, power cable, connection cable, 5ml syringe holder, tower air shield |
| Vermiculite | EasyGoProducts | B07WQDZGRP | Needed to transport hazardous waste (guanidine thiocyanate) using a hazardous waste disposal service |
| Wire Cutter | Boenfu | BOWC-06002-US | Wire cutters to cut trimmer line |
References
- Vernière, C., et al. Interactions between citrus viroids affect symptom expression and field performance of clementine trees grafted on trifoliate orange. Phytopathology. 96 (4), 356-368 (2006).
- Vernière, C., et al. Citrus viroids: Symptom expression and effect on vegetative growth and yield of clementine trees grafted on trifoliate orange. Plant Disease. 88 (11), 1189-1197 (2004).
- Zhou, C., Talon, M., Caruso, M., Gmitter, F. G., et al. Chapter 19 – Citrus viruses and viroids. The Genus Citrus. , 391-410 (2020).
- Trends and issues facing the U.S. citrus industry. Choices Magazine Online Available from: https://www.choicesmagazine.org/choices-magazine/theme-articles/trends-and-challenges-in-fruit-and-tree-nut-sectors/trends-and-issues-facing-the-us-citrus-industry (2021)
- Fruit and Tree Nuts Outlook. United States Department of Agriculture-Economic Research Service Available from: https://www.ers.usda.gov/webdocs/outlooks/98171/fts-370.pdf?v=5697 (2020)
- Forsyth, J., Fruits Damiani, J. C. i. t. r. u. s. Citrus Fruits. Types on the market. Encyclopedia of Food Sciences and Nutrition. , 1329-1335 (2003).
- Bostock, R. M., Thomas, C. S., Hoenisch, R. W., Golino, D. A., Vidalakis, G. Plant health: How diagnostic networks and interagency partnerships protect plant systems from pests and pathogens. California Agriculture. 68 (4), 117-124 (2014).
- Osman, F., Dang, T., Bodaghi, S., Vidalakis, G. One-step multiplex RT-qPCR detects three citrus viroids from different genera in a wide range of hosts. Journal of Virological Methods. 245, 40-52 (2017).
- Wang, J., et al. Past and future of a century old Citrus tristeza virus collection: A California citrus germplasm tale. Frontiers in Microbiology. 4, 366 (2013).
- Gergerich, R. C., et al. Safeguarding fruit crops in the age of agricultural globalization. Plant Disease. 99 (2), 176-187 (2015).
- Moreno, P., Ambrós, S., Albiach-Martí, M. R., Guerri, J., Peña, L. Citrus tristeza virus: A pathogen that changed the course of the citrus industry. Molecular Plant Pathology. 9 (2), 251-268 (2008).
- Yokomi, R. K., et al. Identification and characterization of Citrus tristeza virus isolates breaking resistance in trifoliate orange in California. Phytopathology. 107 (7), 901-908 (2017).
- Selvaraj, V., Maheshwari, Y., Hajeri, S., Yokomi, R. A rapid detection tool for VT isolates of Citrus tristeza virus by immunocapture-reverse transcriptase loop-mediated isothermal amplification assay. PLoS One. 14 (9), 0222170 (2019).
- Babcock, B. A. Economic impact of California’s citrus industry in 2020. Journal of Citrus Pathology. 9, (2022).
- Gottwald, T. R., Polek, M., Riley, K. History, present incidence, and spatial distribution of Citrus tristeza virus in the California central valley. International Organization of Citrus Virologists Conference Proceedings (1957-2010). 15, (2002).
- Yokomi, R., et al. Molecular and biological characterization of a novel mild strain of citrus tristeza virus in California. Archives of Virology. 163 (7), 1795-1804 (2018).
- Fuchs, M., et al. Economic studies reinforce efforts to safeguard specialty crops in the United States. Plant Disease. 105 (1), 14-26 (2021).
- The real cost of HLB in Florida. Citrus Industry Magazine Available from: https://citrusindustry.net/2019/07/30/the-real-cost-of-hib-in-florida/ (2019)
- McRoberts, N., et al. Using models to provide rapid programme support for California’s efforts to suppress Huanglongbing disease of citrus. Philosophical Transactions of the Royal Society B: Biological Sciences. 374 (1776), 20180281 (2019).
- Albrecht, C., et al. Action plan for Asian citrus psyllid and huanglongbing (citrus greening) in California. Journal of Citrus Pathology. 7 (1), (2020).
- Navarro, L., et al. The Citrus Variety Improvement Program in Spain in the period 1975-2001. International Organization of Citrus Virologists Conference Proceedings (1957-2010). 15 (15), (2002).
- Vidalakis, G., Gumpf, D. J., Polek, M. L., Bash, J. A., Ferguson, L., Grafton-Cardwell, E. E. The California Citrus Clonal Protection Program. Citrus Production Manual. , 117-130 (2014).
- Dang, T., Rao, A. L. N., Lavagi-Craddock, I., Vidalakis, G., et al. High-throughput RNA extraction from citrus tissues for the detection of viroids. In Viroids: Methods and Protocols. 2316, (2022).
- Osman, F., Vidalakis, G., Rao, A. L. N., Lavagi-Craddock, I., Vidalakis, G. Real-time detection of viroids using singleplex and multiplex quantitative polymerase chain reaction. Viroids: Methods and Protocols. 2316, (2022).
- Li, R., et al. A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. Journal of Virological Methods. 154 (1-2), 48-55 (2008).
- Saponari, M., Manjunath, K., Yokomi, R. K. Quantitative detection of Citrus tristeza virus in citrus and aphids by real-time reverse transcription-PCR (TaqMan). Journal of Virological Methods. 147 (1), 43-53 (2008).
- Damaj, M. B., et al. Reproducible RNA preparation from sugarcane and citrus for functional genomic applications. International Journal of Plant Genomics. 2009, 765367 (2009).
- Dang, T., et al. First report of citrus leaf blotch virus infecting Bearss lime tree in California. Plant Disease. 104 (11), 3088 (2020).
- Manchester, K. L. Use of UV methods for measurement of protein and nucleic acid concentrations. BioTechniques. 20 (6), 968-970 (1996).
- Teare, J. M., et al. Measurement of nucleic acid concentrations using the DyNA QuantTM and the GeneQuantTM. BioTechniques. 22 (6), 1170-1174 (1997).
- Imbeaud, S. Towards standardization of RNA quality assessment using user-independent classifiers of microcapillary electrophoresis traces. Nucleic Acids Research. 33 (6), 56-56 (2005).
- Menzel, W., Jelkmann, W., Maiss, E. Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. Journal of Virological Methods. 99 (1-2), 81-92 (2002).
- Vidalakis, G., Rao, A. L. N., Lavagi-Craddock, I., Vidalakis, G., et al. SYBR Green RT-qPCR for the universal detection of citrus viroids. Viroids: Methods and Protocols. , 211-217 (2022).
- Arredondo Valdés, R., et al. A review of techniques for detecting Huanglongbing (greening) in citrus. Canadian Journal of Microbiology. 62 (10), 803-811 (2016).
- Li, S., Wu, F., Duan, Y., Singerman, A., Guan, Z. Citrus greening: Management strategies and their economic impact. HortScience. 55 (5), 604-612 (2020).
- . CDFA California Citrus Pest and Disease Prevention Program Operations Subcomittee Meeting. Meeting Minutes Available from: https://www.cdfa.ca.gov/citrus/docs/minutes/2019/OpsSubcoMinutes-11062019.pdf (2019)

