Pluripotent Stem Cell Derived Cardiac Cells for Myocardial Repair
Summary
We present three novel and more efficient protocols for differentiating human induced pluripotent stem cells into cardiomyocytes, endothelial cells, and smooth muscle cells and a delivery method that improves the engraftment of transplanted cells by combining cell injection with patch-mediated cytokine delivery.
Abstract
Human induced pluripotent stem cells (hiPSCs) must be fully differentiated into specific cell types before administration, but conventional protocols for differentiating hiPSCs into cardiomyocytes (hiPSC-CMs), endothelial cells (hiPSC-ECs), and smooth muscle cells (SMCs) are often limited by low yield, purity, and/or poor phenotypic stability. Here, we present novel protocols for generating hiPSC-CMs, -ECs, and -SMCs that are substantially more efficient than conventional methods, as well as a method for combining cell injection with a cytokine-containing patch created over the site of administration. The patch improves both the retention of the injected cells, by sealing the needle track to prevent the cells from being squeezed out of the myocardium, and cell survival, by releasing insulin-like growth factor (IGF) over an extended period. In a swine model of myocardial ischemia-reperfusion injury, the rate of engraftment was more than two-fold greater when the cells were administered with the cytokine-containing patch comparing to the cells without patch, and treatment with both the cells and the patch, but not with the cells alone, was associated with significant improvements in cardiac function and infarct size.
Introduction
Human induced pluripotent stem cells (hiPSCs) are among the most promising agents for regenerative cell therapy because they can be differentiated into a potentially unlimited range and quantity of cells that are not rejected by the patient's immune system. However, their capacity for self-replication and differentiation can also lead to tumor formation and, consequently, hiPSCs need to be fully differentiated into specific cell types, such as cardiomyocytes (CMs), endothelial cells (ECs), and smooth muscle cells (SMCs), before administration. One of the simplest and most common methods of cell administration is direct intramyocardial injection, but the number of transplanted cells that are engrafted by the native myocardial tissue is exceptionally low. Much of this attrition can be attributed to the cytotoxic environment of the ischemic tissue; however, when murine embryonic stem cells (ESCs) were injected directly into the myocardium of uninjured hearts, only ~40% of the 5 million cells delivered were retained for 3-5 hr1, which suggests that a substantial proportion of the administered cells exited the administration site, perhaps because they were squeezed out through the needle track by the high pressures produced during myocardial contraction.
Here, we present novel and substantially more efficient methods for generating hiPSC-derived cardiomyocytes (hiPSC-CMs)2, endothelial cells (hiPSC-ECs)3, and smooth muscle cells (SMCs)4. Notably, this hiPSC-SMC protocol is the first to mimic the wide range of morphological and functional characteristics observed in somatic SMCs5 by directing the cells toward a predominantly synthetic or contractile SMC phenotype. We also provide a method of cell delivery that improves the engraftment rate of injected cells by creating a cytokine-containing fibrin patch over the injection site. The patch appears to improve both cell retention, by sealing the needle track to prevent the cells from exiting the myocardium, and cell survival, by releasing insulin-like growth factor (IGF) over a period of at least three days.
Protocol
All experimental procedures are performed in accordance with the Animal Guidelines of the University of Alabama at Birmingham.
1. Differentiating hiPSCs into hiPSC-CMs
- Coat the wells of a 6-well plate with pre-cooled growth-factor-reduced gelatinous protein mixture at 4 °C for overnight. Aspirate the gelatinous protein mixture before use. Seed the hiPSCs onto the pre-coated plates, and culture the cells (1 x 105 cell per well) at 5% CO2 and 37 °C in mTeSR1 medium supplement with 10 µM ROCK inhibitor.
- Refresh the medium daily until the cells reach 90% confluence; then, add growth-factor-reduced gelatinous protein mixture to the medium (0.5 mg gelatinous protein mixture per 6-well plate, 2 ml medium per well), and culture the cells for 5% CO2 and 37 °C two more days.
- To replace the medium, gently suck out the medium from the petri dish via vacuum without touching the cells, and add new medium using a transfer pipette.
- Initiate differentiation by replacing the medium with RPMI1640 medium supplemented with growth-factor-reduced gelatinous protein mixture, B27 without insulin, and 100 ng/ml Activin A; culture the cells at 5% CO2 and 37 °C for 24 hr.
- Replace the medium with RPMI1640 medium that has been supplemented with B27 without insulin, 10 ng/ml bone morphogenic protein 4 (BMP-4), and 10 ng/ml basic fibroblast growth factor (bFGF); culture the cells at 5% CO2 and 37 °C for 96 hr.
- Replace the medium with RPMI1640 medium supplemented with B27 and continue culture the cells at 5% CO2 and 37 °C; refresh the medium every 3 days.
- Observe clusters of contracting cells under the microscope ~3 days after initiating differentiation. Collect the clusters ~7 days after first observing contractions and wash them in Hanks Balanced Salt Solution.
- Dissociate the clustered-cells in the plate by incubating them in Hanks buffer containing 100 U/ml collagenase IV for 10 min at 37 ºC with gentle shaking; then, add 0.25% trypsin in ethylenediaminetetraacetic acid (EDTA) for 5 min.
- Neutralize the enzyme solution with 10% fetal bovine serum in RPMI/B27 medium and then resuspend the cells in RPMI/B27 medium.
- Count the number of cells by hemocytometer. Culture the cells on 10 cm dishes at 5% CO2 and 37 °C for at least 3 hr, which will allow the non-cardiomyocyte cells to adhere to the surface of the culture dish.
- Collect the cell suspension, which contains the hiPSC-CMs, and maintain the cells (around 1 x 106 cells per 10 cm dish) by culturing them on a gelatinous protein mixture -coated surface.
2. Differentiating hiPSCs into hiPSC-ECs
- Dissociate the hiPSC population into single cells by incubating them with chelating agent at 5% CO2 and 37 °C for 5 min. Transfer the cells to a 15 ml centrifuge tube containing fresh mTeSR1 medium.
- Spin down the cells at 200 x g for 5 min. Resuspend the cells in mTeSR1 medium supplemented with 10 µM ROCK inhibitor. Determine the cell density with a hemocytometer.
- Add 250 µl of 20 NIH units/ml thrombin solution to one well of a 24-well plate.
- Add 1 x 106 hiPSCs to 250 µl of a 12.5 mg/ml fibrinogen solution. And add the 250 µl of cell-containing fibrinogen solution to the thrombin-loaded well. Observe the mixture solidify to form a hiPSC-containing fibrin scaffold within min.
- Transfer the solidified, cell-containing scaffolds into the wells of a 6-well plate with the cover glass forceps, and initiate differentiation by culturing the scaffolds in 2 ml EBM2 medium supplemented with 2% B27 without insulin, activin-A (50 ng/ml), and BMP-4 (25 ng/ml) for 24 hr at 5% CO2 and 37 °C.
- Replace the medium by gently sucking out the old medium via vacuum without touching the cells. Add new EBM2 medium supplemented with B27 without insulin, vascular endothelial growth factor (VEGF, 50 ng/ml), erythropoietin (EPO, 100 ng/ml), and transforming growth factor β1 (TGFβ1, 10 ng/ml), and culture the scaffolds at 5% CO2 and 37 °C for 48 hr.
- Refresh the medium and culture the scaffolds at 5% CO2 and 37 °C for another 48 hr; then, release the cells from the scaffold by adding 200 U collagenase IV (100 U/ml) to the medium.
- Spin down the cells after they are released. Replace the medium with 2 ml EGM2-MV medium supplemented with 2% of B27, VEGF-A (50 ng/ml), and SB-431542 (10 µM); refresh the medium every two days.
- Approximately 10 days later (i.e., on ~Day 14 after differentiation was initiated), dissociate the cells with 100 U/ml collagenase IV, isolate hiPSC-ECs from the population of differentiated cells via flow-cytometry analyses for the expression of EC-specific marker proteins (e.g., CD31, CD144)3.
- Expand the isolated hiPSC-ECs via an established protocol3.
3. Differentiating hiPSCs into hiPSC-SMCs
- Seed the hiPSCs onto plates that have been coated with gelatinous protein mixture, and culture the cells in mTeSR1 medium at 5% CO2 and 37 °C, with daily medium changes, until confluent (~2 days).
- Initiate differentiation into mesodermal-lineage cells by culturing the cells with CHIR99021 (5 µM) and BMP-4 (10 ng/ml) in RPMI1640 medium and 2% B27 plus insulin for 3 days.
- To produce hiPSC-SMCs with a predominantly synthetic phenotype:
- Culture the cells with VEGF-A (25 ng/ml), fibroblast growth factor β (FGFβ, 5 ng/ml) in RPMI1640 medium, and 2% B27 minus insulin at 5% CO2 and 37 °C for 4 days.
- Culture the cells in VEGF-A (25 ng/ml), FGFβ (5 ng/ml) in RPMI1640 medium, and 2% B27 plus insulin at 5% CO2 and 37 °C for 2 days.
- Culture the cells in platelet-derived growth factor β (PDGFβ, 10 ng/ml), TGFβ (3 ng/ml) in RPMI1640, and 2% B27 plus insulin at 5% CO2 and 37 °C for 4 days.
- To produce hiPSC-SMCs with a predominantly contractile phenotype:
- Culture the cells for 4 days with VEGF-A (25 ng/ml) and FGFβ (5 ng/ml) in RPMI1640 and 2% B27 minus insulin.
- Culture the cells for 7 days with PDGFβ (5 ng/ml) and TGFβ (2.5 ng/ml) in RPMI1640 and 2% B27 plus insulin.
- Purify the final hiPSC-SMC populations by culturing the cells in lactate (4 mM) containing RPMI1640 metabolic medium for ~6 days.
4. Creating the IGF-containing Microspheres
- Heat olive oil to 45 °C in a water bath.
- Heat 5 ml of 10% gelatin solution to 50 °C.
- Add the gelatin to the olive oil, stir, and then rapidly cool to 5 °C by adding ice to the water bath.
- Twenty-five minutes later, add chilled (4 °C) acetone to the olive oil to induce microsphere formation.
- Maintain the temperature at 5 °C for 1 hr; then, collect the microspheres, wash them 5 times with pre-cooled acetone to remove the olive oil, and allow them to air-dry at 4 °C.
- Resuspend the microspheres in a solution of 70% ethanol and 0.25% glutaraldehyde overnight at 4 °C to induce cross-linking, and then neutralize the mixture with 100 mM glycine.
- Load IGF into the microspheres by mixing 5 mg microspheres with 15 µl distilled H2O containing 5 µg IGF and 0.1% bovine serum albumin for 30 min.
5. Creating the Patch over the Site of Injury and Injecting the Cells
- Suspend the hiPSC-derived CMs (2 million), SMCs (1 million), and ECs (1 million) together in 1 ml Minimum Essential Medium (MEM).
- Suspend 5 mg of microspheres in 1 ml fibrinogen solution (25 mg/ml).
- Fill one syringe with the cell-containing solution, another syringe with the fibrinogen/microsphere solution, and a third syringe with 1 ml of thrombin solution (80 NIH units/ml, supplemented with 2 µl 400 mM CaCl2 and 200 mM ε-aminocaproic acid).
- Surgically induce myocardial infarction in swine heart via ligation of left anterior descending coronary artery as described before2.
NOTE: In brief, female Yorkshire swine (~13 kg, 45 days of age) are sedated with intramuscular injection of Telazol/Xylazine (4.4 mg/kg), and intubated under general anesthesia with isoflurane (0.5-5%). A 4th intercostal thoracotomy is performed and left anterior descending coronary artery is exposed. The coronary artery is occluded by a ligature for 60 min and then the ligature is removed. Immediate electrical defibrillation is performed in the case of ventricular fibrillation. - Position a sterile plastic ring (~2.5 cm) on the epicardium of the infarcted region of swine hearts, and then simultaneously inject the microsphere/fibrinogen and thrombin solutions into the ring.
- Allow the mixture to solidify (~30 sec), and then insert the needle of the cell-containing syringe through the solidified mixture into the infarcted myocardium and inject the cells. Close the muscle layers, subcutaneous tissue, and the chest wall with 3-0 or 2-0 Monocryl suture depending on animal size. Buprenorphine 0.01-0.02 mg/kg intramuscular injection every 8 hours will be used to control pain for the first 3 days after surgery.
- Evaluate cardiac function using a cardiac magnetic resonance imaging (MRI) before surgical procedure, one week and four weeks after the surgical procedure2,3,4. After the final MRI studies, sacrifice the animal and process their hearts for histology and molecular analysis2,3,4.
Representative Results
Characterization of Differentiated hiPSC-CMs, -ECs, and -SMCs
The differential capacity of hiPSCs were evaluated2,3,4. Flow cytometry analyses of cardiac troponin T (cTnT) expression suggest that the purity of the final hiPSC-CM population can exceed 90% (Figure 1A, 1B, panel B1). Nearly all of the cells expressed slow myosin heavy chain (Figure 1B, panel A1), α-sarcomeric actin (Figure 1B, panel A2), while approximately 25% expressed the 2v isoform of myosin light chain (MLC2v) (Figure 1B, panel B2), which was found only in ventricular CMs4. The efficiency of the hiPSC-EC differentiation protocol (i.e., the percentage of CD31+ cells) was substantially higher when performed with hiPSCs from the GRiPS line (45.6%, Figure 1C, panel C2) than with PCBC16iPS cells (31.3%, Figure 1C, panel D2); populations of >95% purity were achieved via selection for CD31 expression, and >90% of the selected cells continued to express CD31 or CD144 for up to 4 weeks when cultured in EGM2-MV medium (without FBS) supplemented with B27, VEGF, and SB431542 (Figure 1D)3. More than 94% of the purified hiPSC-SMCs expressed smooth muscle actin (SMA), but synthetic hiPSC-SMCs were more likely than contractile hiPSC-SMCs to express collagen 1, connexin 43, or vimentin (Figure 1E). The migration and proliferation capability of hiPSC-SMCs were evaluated4. Synthetic hiPSC-SMCs were also more migratory and proliferative than contractile hiPSC-SMCs (Figure 1F, panels i and ii), while contractile SMCs contracted more strongly in response to carbachol treatment (Figure 1F, panels iii, iv and v).
Growth-factor Release from Gelatin Microspheres
ELISA measurements of IGF-1 levels in the medium from cultured, cell-free patches indicated that the growth factor was released from the microspheres over a period of at least 3 days (Figure 2A)2. The analyses were performed by loading 5 mg of IGF-containing microspheres with 5 µg IGF-1. A patch was generated by mixing the microspheres with 1 ml fibrinogen solution and 1 ml thrombin. The patch was cultured in 2 ml MEM. One ml of the medium was collected and replaced with 1 ml of fresh MEM each day (Figure 2B). Data were presented as Mean ± SEM.
Observations from an IR-injury Model
The effectiveness of our patch-mediated cell-transplantation protocol has been investigated in a swine model of ischemia-reperfusion injury2. A total of 6 million hiPSC-CMs, -ECs, and -SMCs (2 million of each cell type) were injected directly into the injured myocardial tissue. For animals in the CELL+Patch group, a patch composed of fibrin and IGF-1-containing microspheres was created over the site of injury before injection, while animals in the CELL group were treated with the same cell dose but without the patch; both treatments were withheld from animals in the MI group. Four weeks after injury and treatment, 9% of the cells delivered to animals in the CELL+Patch group were retained and continued to survive at the site of administration, compared to just 4% of the cells administered to animals in the CELL group (Figure 3A). Treatment with both the cells and the patch, but not with the cells alone, was also associated with significant improvements in measurements of cardiac function and infarct size (Figure 3B).
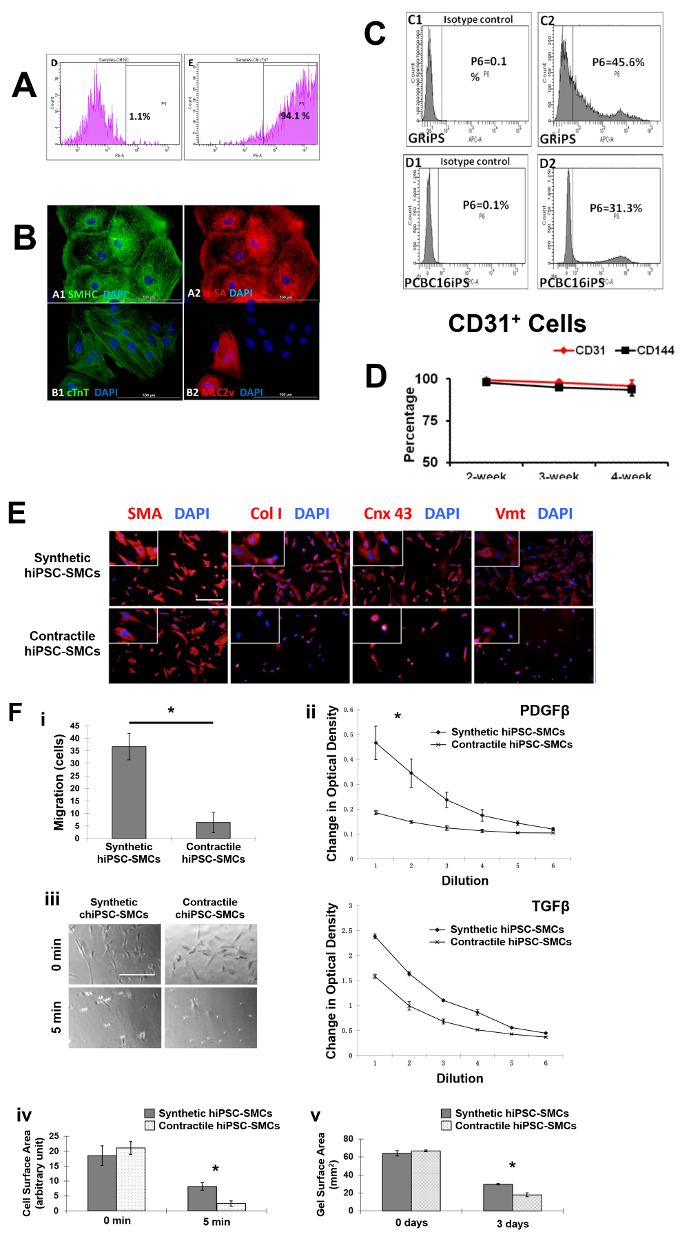
Figure 1: Differentiation of hiPSCs into cardiomyocytes, endothelial cells, and smooth muscle cells. Cardiomyocyte differentiation and the purity of hiPSC-CMs were evaluated via flow cytometry (A) and histology with different cardiac markers (B). The endothelial differentiation of hiPSC was determined with flow cytometry (C). The maintenance of the endothelial phenotype of hiPSC-ECs was assessed via analyzing the expression of CD31 and CD144 at 2, 3, and 4 weeks after the purification by flow cytometry (D). The smooth muscle cell differentiation of hiPSC was measured by various markers (E). And the migration and proliferation potential of synthetic smooth muscle cells vs. contractile smooth muscle cells derived from hiPSCs was assessed (F). Nuclei were counterstained with DAPI in the immunofluorescence staining. Scale bar = 100 µm in (B) and 200 µm (E and F). The migration and proliferation capability of synthetic hiPSC-SMCs were evaluated (F, panel i and ii). And contraction of contractile SMCs in response to carbachol treatment was assessed (F, panel iii-v). *p < 0.05, synthetic hiPSC-SMCs vs. contractile hiPSC-SMCs. A and B were modified from Ye L, et al.2. C and D were modified from Zhang S, et al.3. E and F were modified from Yang L, et al.4. Data were presented as mean ± standard error of the mean (SEM). Comparison between two groups was evaluated via the Student's T test, and comparison between multiple groups was assessed via one-way ANOVA. p <0.05 was considered significant. Please click here to view a larger version of this figure.
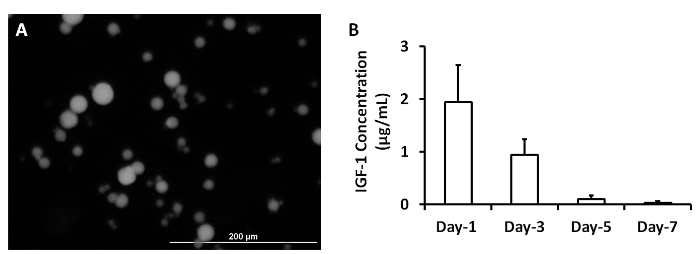
Figure 2: Release of growth factors from microspheres. IGF-containing microspheres was synthesized (A). IGF-1 release from microspheres was measured at 1, 3, 5 and 7 days after they were synthesized (B). Scale bar = 200 µm. Panel A was modified from Ye L., et al.2. Please click here to view a larger version of this figure.
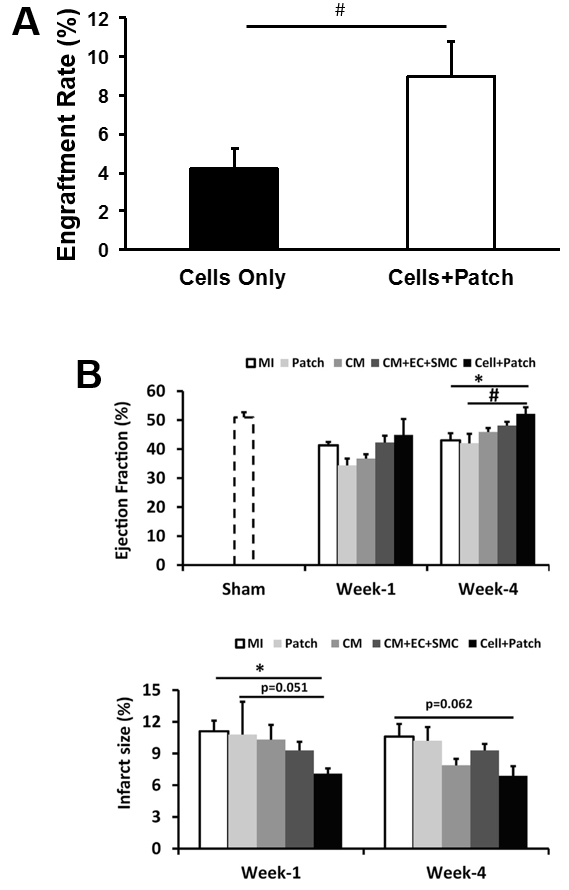
Figure 3: Assessment of efficiency of cell therapy with hiPSC-derived trilineage cells. The overall engraftment rate for the three cell population was assessed 4 weeks after cell transplantation (A). Cardiac function (as indicated by ejection fraction) and infarction size were evaluated via cardiac MRI at 1 and 4 weeks after cell transplantation (B). *p <0.05 versus MI; #p <0.05 versus Patch. A and B were modified from Ye L., et al.2. Data were presented as mean ± SEM. Comparisons among groups were analyzed for significance with one-way ANOVA. p <0.05 was considered significant. Results identified as significant via ANOVA were reanalyzed with the Tukey correction.
Discussion
Improved Yield/Purity of hiPSC-CMs
Conventional protocols for differentiating human stem cells into CMs are often limited by low yield and purity; for example, just 35-66% of hESC-CMs obtained via Percoll separation and cardiac body formation expressed slow myosin heavy chain or cTnT6. The purity of differentiated hiPSC-CM populations can be substantially increased by selecting for the expression of a reporter gene that has been linked to the promoter of a CM-specific protein7,8,9, but this technique necessarily requires the use of genetically modified cells, which are less desirable, particularly for clinical investigations. With the protocol presented here, 68% of the hiPSC-CMs expressed cTnT, and the purity was subsequently increased to >90% via micro-dissection and preplating without genetic manipulation or single-cell selection. The total yield was ~3-5 million hiPSC-CMs per well.
Improved Yield/Stability of hiPSC-ECs
The two most commonly used hiPSC-EC differentiation protocols are based on co-culturing with murine stromal cells10,11 and embryonic-body formation12,13. However, the co-culturing method could lead to xenogenic contamination with murine-lineage cells or proteins10, and only ~15% or less of the cells produced via the embryonic-body method assume an EC phenotype10,12,13. The hiPSC-EC differentiation protocol presented here eliminates the risk of xenogenic contamination and can be up to 3-fold more efficient than the methods that use embryonic bodies (depending on which hiPSC line is used). Furthermore, after purification via selection for CD31 expression, ~90% of the hiPSC-ECs maintained the EC phenotype for at least 4 weeks in vitro, compared to just two weeks when hiPSC-ECs are produced via conventional differentiation protocols12.
Phenotypic Specification of hiPSC-SMCs
The phenotype of an SMC (or a population of SMCs) is perhaps best described as a balance between predominantly contractile and synthetic characteristics, which can lead to substantial differences in morphology, marker expression, and activity. Thus, the utility of hiPSC-SMCs for a particular application may depend on which specific phenotype is generated. The hiPSC-SMC differentiation and purification protocols presented here are the first to produce predominantly contractile or synthetic SMC populations that are ~95% pure in just 2-3 weeks, and the risk of xenogenic contamination is minimal, because the procedures do not require the use of feeder cells.
Improved Engraftment Rate of Transplanted Cells
One of the primary obstacles to effective cell therapy is believed to be the extremely low number of administered cells that are engrafted by the myocardium14,15,16,17. Cytokines such as IGF-1 may improve engraftment by enabling the cells to better withstand the toxic microenvironment in ischemic tissues18,19,20, but the high pressures induced during contraction may squeeze the cells out through the needle track and into the peripheral circulation. Thus, the considerably higher rate of engraftment achieved by injecting the cells through an IGF-1-containing fibrin patch, which was more than 2-fold greater than the rate observed when the same cell dose was administered without the patch, can likely be attributed both to the cytoprotective effects of IGF-1 and to the patch itself, which formed a physical barrier that prevented the cells from being ejected into the epicardial space.
Critical Steps
To ensure the pluripotency of the hiPS cells, it is necessary to check the karyotype of the hiPSC line prior to differentiation, determine the expression of pluripotency markers (Nanog, SSEA1, and Oct4, et al.), and confirm their capability of teratoma formation. It is suggested to re-check their pluripotency when hiPSC lines have been passaged for more than 10 generations. The status of undifferentiated hiPSC is also crucial for the correct differentiation. To ensure the status of the hiPSC prior to the initiation of differentiation, it is recommended to: 1) use fresh medium (less than two weeks old), and refresh the medium daily before initiation of hiPSC differentiation; 2) reduce the enzymatic digestion time (less than 5 min), and gently pipette up and down the cells to prevent the unnecessary damage to the cells; 3) replate the cells at a density 1 x 105 cells per well of the 6-well plate; 4) always keep the cells in 37 °C and 5% CO2 incubator, and avoid keeping the cells out of the incubator for longer than 15 min.
Limitations of the Technique
The primary disadvantage of our method for combining injected hiPSC-derived cardiac cells and patch administration is the requirement for open-chest surgery; thus, this approach is unlikely to be directly translatable to clinical applications except as adjunctive therapy for patients who are undergoing concurrent coronary revascularization surgery. More widespread clinical use will require the development of a practical and minimally invasive delivery method, such as an endoscope- and catheter-based approach that could access the heart through the abdomen and diaphragm. Furthermore, one of the most critical safety concerns associated with cardiac cell therapy is the development of arrhythmogenic complications, and when monkeys were treated with intramyocardial injections of 1 billion hESC-derived CMs, all four of the animals developed spontaneous arrhythmias21. However, we observed no evidence of arrhythmogenic complications when 10 million hiPSC-derived CMs were injected into the hearts of swine with IR injury2, perhaps because the cell dose was 100-fold smaller.
Future Applications or Directions
Immune rejection likely plays an important role for the low engraftment rate. The CRISPR/Cas gene editing system may enable researchers to create a line of "universal donor" hiPSCs by knock out (KO) major histocompatibility complex (MHC) class I and class II. The hiPSC MHC KO-derived cells and tissues may have substantially higher rates of engraftment. As techniques improve, engraftment rates are likely to increase. At certain point of the increase the graft size, the large graft is expected to increase the arrhythmogenicity of the recipient heart. The reduction of grafts associated arrhythmogenicity could be achieved by using cells with increase of gap junction proteins expression by the gene editing technology.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported by US Public Health Service grants NIH RO1s HL67828, HL95077, HL114120, and UO1 HL100407-project 4 (to JZ), an American Heart Association Scientist Development Grant (16SDG30410018) and a Research Voucher Award from University of Alabama at Birmingham Center for Clinical and Translational Science (to WZ).
Materials
| Protocol 1 | |||
| mTeSR1 medium | Stem cell technologies | 5850 | |
| Growth-factor-reduced matrigel | Corning lifescience | 356231 | |
| Y-27632 | Stem cell technologies | 72304 | |
| B27 supplement, serum free | Fisher Scientific | 17504044 | |
| RPMI1640 | Fisher Scientific | 11875-119 | |
| Activin A | R&D | 338-AC-010 | |
| BMP-4 | R&D | 314-BP-010 | |
| bFGF | R&D | 232-FA-025 | |
| Collagenase IV | Fisher Scientific | NC0217889 | |
| Hanks Balanced Salt Solution (Dextrose, KCl, KH2PO4, NaHCO3, NaCl, Na2HPO4 anhydrous) | Fisher Scientific | 14175079 | |
| Fetal Bovine Serum | Fisher Scientific | 10438018 | |
| 6-well plate | Corning Lifescience | 356721 | |
| 10cm dish | Corning Lifescience | 354732 | |
| Cell incubator | Panasonic | MCO-18AC | |
| Materials | Company | Catalog Number | Comments |
| Protocol 2 | |||
| Versene | Fisher Scientific | 15040066 | |
| Fibrinogen | Sigma-Aldrich | F8630-5g | |
| Thrombin | Sigma-Aldrich | T7009-1KU | |
| EMB2 medium | Lonza | CC-3156 | |
| VEGF | ProSpec-Tany | CYT-241 | |
| EPO | Life Technologies | PHC9431 | |
| TGF-ß | Peprotech | 100-21C | |
| EGM2-MV medium | Lonza | CC-4147 | |
| SB-431542 | Selleckchem | S1067 | |
| CD31 | BD Bioscience | BDB555445 | |
| CD144 | BD Bioscience | 560411 | |
| 15 mL centrifuge tube | Fisher Scientific | 12565269 | |
| Eppendorff Centrifuge | Eppendorf | 5702R | |
| Materials | Company | Catalog Number | Comments |
| Protocol 3 | |||
| CHIR99021 | Stem cell technologies | 720542 | |
| PDGF-ß | Prospec | CYT-501-10ug | |
| Materials | Company | Catalog Number | Comments |
| Protocol 4 | |||
| Olive oil | Sigma-Aldrich | O1514 | |
| Gelatin | Sigma-Aldrich | G9391 | |
| Acetone | Sigma-Aldrich | 179124 | |
| Ethanol | Fisher Scientific | BP2818100 | |
| Glutaraldehyde | Sigma-Aldrich | G5882 | |
| Glycine | Sigma-Aldrich | G8898 | |
| IGF | R&D | 291-G1-01M | |
| Bovine serum albumin | Fisher Scientific | 15561020 | |
| Heating plate | Fisher Scientific | SP88850200 | |
| Water bath | Fisher Scientific | 15-462-10Q | |
| Materials | Company | Catalog Number | Comments |
| Protocol 5 | |||
| CaCl2 | Sigma-Aldrich | 223506 | |
 -aminocaproic acid -aminocaproic acid |
Sigma-Aldrich | A0420000 | |
| MEM medium | Fisher Scientific | 12561-056 | |
| Syringe | Fisher Scientific | 1482748 | |
| Anesthesia ventilator | Datex-Ohmeda | 47810 | |
| Anesthesia ventilator | Ohio Medical | V5A | |
| Defibrillator | Physiol Control | LIFEPAK 15 | |
| 1.5T MRI | General Electric | Signa Horizon LX | |
| 7T MRI | Siemens | 10018532 | |
| Gadolinium Contrast Medium (Magnevist) | Berlex | 50419-188-02 | |
| 2-0 silk suture | Ethilon | 685H | |
| 3-0 silk suture | Ethilon | 622H | |
| 3-0 monofilament suture | Ethilon | 627H |
Referenzen
- Qiao, H., et al. Death and proliferation time course of stem cells transplanted in the myocardium. Mol Imaging Biol. 11 (6), 408-414 (2009).
- Ye, L., et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 15 (6), 750-761 (2014).
- Zhang, S., Dutton, J. R., Su, L., Zhang, J., Ye, L. The influence of a spatiotemporal 3D environment on endothelial cell differentiation of human induced pluripotent stem cells. Biomaterials. 35 (12), 3786-3793 (2014).
- Yang, L., et al. Differentiation of Human Induced-Pluripotent Stem Cells into Smooth-Muscle Cells: Two Novel Protocols. PLoS One. 11 (1), e0147155 (2016).
- Rensen, S. S., Doevendans, P. A., van Eys, G. J. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 15 (3), 100-108 (2007).
- Xu, C., Police, S., Hassanipour, M., Gold, J. D. Cardiac bodies: a novel culture method for enrichment of cardiomyocytes derived from human embryonic stem cells. Stem Cells Dev. 15 (5), 631-639 (2006).
- Anderson, D., et al. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol Ther. 15 (11), 2027-2036 (2007).
- Huber, I., et al. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. FASEB J. 21 (10), 2551-2563 (2007).
- Kita-Matsuo, H., et al. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS One. 4 (4), e5046 (2009).
- Choi, K. D., et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 27 (3), 559-567 (2009).
- Woll, P. S., et al. Wnt signaling promotes hematoendothelial cell development from human embryonic stem cells. Blood. 111 (1), 122-131 (2008).
- Li, Z., Hu, S., Ghosh, Z., Han, Z., Wu, J. C. Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells. Stem Cells Dev. 20 (10), 1701-1710 (2011).
- Rufaihah, A. J., et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 31 (11), e72-e79 (2011).
- Beauchamp, J. R., Morgan, J. E., Pagel, C. N., Partridge, T. A. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 144 (6), 1113-1122 (1999).
- Qu, Z., et al. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 142 (5), 1257-1267 (1998).
- Tang, X. L., et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 121 (2), 293-305 (2010).
- Zeng, L., et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 115 (14), 1866-1875 (2007).
- Davis, M. E., et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 103 (21), 8155-8160 (2006).
- Li, Q., et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 100 (8), 1991-1999 (1997).
- Wang, L., Ma, W., Markovich, R., Chen, J. W., Wang, P. H. Regulation of cardiomyocyte apoptotic signaling by insulin-like growth factor I. Circ Res. 83 (5), 516-522 (1998).
- Chong, J. J., et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 510 (7504), 273-277 (2014).

