Microelectrode Impalement Method to Record Membrane Potential from a Cannulated Middle Cerebral Artery
Summary
The primary goal of this article is to provide details of how to record membrane potential (Vm) from the middle cerebral artery using the microelectrode impalement method. The cannulated middle cerebral artery is equilibrated to gain myogenic tone, and the vessel wall is impaled using high resistance microelectrodes.
Abstract
Membrane potential (Vm) of vascular smooth muscle cells determines vessel tone and thus blood flow to an organ. Changes in the expression and function of ion channels and electrogenic pumps that regulate Vm in disease conditions could potentially alter Vm, vascular tone, and blood flow. Thus, a basic understanding of electrophysiology and the methods necessary to accurately record Vm in healthy and diseased states are essential. This method will allow modulating Vm using different pharmacological agents to restore Vm. Although there are several methods, each with its advantages and disadvantages, this article provides protocols to record Vm from cannulated resistance vessels such as the middle cerebral artery using the microelectrode impalement method. Middle cerebral arteries are allowed to gain myogenic tone in a myograph chamber, and the vessel wall is impaled using high resistance microelectrodes. The Vm signal is collected through an electrometer, digitized, and analyzed. This method provides an accurate reading of the Vm of a vessel wall without damaging the cells and without changing the membrane resistance.
Introduction
The membrane potential (Vm) of a cell refers to the relative difference of ionic charge across the plasma membrane and the relative permeability of the membrane to these ions. The Vm is generated by the differential distribution of ions and is maintained by ion channels and pumps. Ion channels such as K+, Na+, and Cl− contribute substantially to the resting Vm. Vascular smooth muscle cells (VSMCs) express more than four different types of K+ channels1, two types of voltage-gated Ca2+ channels (VGCC)2, more than two types of Cl− channels3,4,5, store-operated Ca2+ channels6, stretch-activated cation channels7,8, and electrogenic sodium-potassium ATPase pumps9 in their plasma membranes, all of which may be involved in the regulation of Vm.
The Vm of VSMCs depends on lumen pressure. In non-pressurized vessels, Vm varies from -50 to -65 mV, however, in pressurized arterial segments, Vm ranges from -37 to -47 mV10. Elevation of intravascular pressure causes VSMCs to depolarize11, decreases the threshold for VGCC opening, and increases calcium influx contributing to the development of myogenic tone12. On the contrary, in passive or non-pressurized vessels, membrane hyperpolarization, due to high K+ channel activity, will prevent VGCC from opening, resulting in limited calcium entry and a decrease in intracellular calcium, contributing to less vascular tone13. Thus, Vm due to changes in lumen pressure appears to play an essential role in vascular tone development, and both VGCC and K+ channels play a crucial role in the regulation of Vm.
Vm varies between vessel type and species. Vm is -54 ± 1.3 mV in guinea pig superior mesenteric arterial strips14, -45 ± 1 mV in the rat middle cerebral arteries at 60 mmHg lumen pressure12, and -35 ± 1 mV in rat parenchymal arteries at 40 mmHg lumen pressure15. The resting Vm recorded in unstretched rat lymphatic muscle is -48 ± 2 mV16. Vm of cerebral VSMCs is more negative than in peripheral arteries. In comparison, feline middle cerebral arteries were reported to have a Vm of approximately -70 mV, while mesenteric and coronary arteries were reported to have -49 and -58 mV, respectively17,18. Differences in the Vm across vascular beds may reflect the differences in the expression and function of ion channels and electrogenic sodium-potassium pumps.
Increases and decreases in Vm are referred to as membrane depolarization and hyperpolarization, respectively. These alterations in Vm play a central role in many physiological processes, including ion-channel gating, cell signaling, muscle contraction, and action potential propagation. At a fixed pressure, many endogenous and synthetic vasodilator compounds that activate K+ channels cause membrane hyperpolarization, resulting in vasodilation1,13. Conversely, sustained membrane depolarization is vital in agonist-induced or receptor-mediated vasoconstriction19. Vm is a critical variable that not only regulates Ca2+ influx through VGCC13 but also influences the release of Ca2+ from internal stores20,21 and Ca2+-sensitivity of the contractile apparatus22.
While there are several methods to record Vm from different cell types, data collected from the microelectrode impalement method of cannulated vessels appears to be more physiological than data obtained from isolated VSMCs. When recorded from isolated VSMC using current clamp methods, Vm is seen as spontaneous transient hyperpolarizations in VSMCs24. Isolated VSMCs are not in the syncytium, and the changes in the series resistance may contribute to the oscillatory behavior of Vm. On the other hand, oscillatory behavior is not observed when Vm is recorded from intact vessels, probably due to cell-cell contact between VSMCs that are in syncytium in the artery and are summated throughout the vessel leading to a stable Vm24. Thus, measurement of Vm from pressurized vessels using standard microelectrode impalement technique is relatively close to the physiological conditions.
Recording Vm from cannulated vessels could provide vital information, since Vm of VSMCs that are in syncytium is one of the major determinants of vascular tone and blood flow, and modulation of the Vm could provide a way to dilate or constrict blood vessels. Thus, it is essential to understand the methodology involved in recording Vm. This article describes intracellular recording of Vm from cannulated middle cerebral arteries (MCAs) using a microelectrode impalement method. This protocol will describe how to prepare MCAs, microelectrodes, set up the electrometer and perform the impalement method to record Vm. Also, representative data, common issues that were encountered when using this method and potential issues are discussed.
Protocol
The male rats were housed in the Animal Care Facility at UMMC, which is approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Animals had free access to food and water throughout the study. Animals were maintained in a controlled environment with temperature at 24 ± 2 °C, humidity levels of 60–80% and 12 h light/dark cycles. All protocols were approved by the Animal Care and Use Committee of UMMC.
1. Preparation of Equipment
- Place a dual channel differential electrometer amplifier (see the Table of Materials) close to the vessel chamber and at the desired location.
- Connect the output of the amplifier channel A or B to the channel input of the digitizer with a BNC-BNC cable.
- Mount the probe in the micromanipulator and place it near the microscope and the myograph. The recording setup must be installed on a vibration-free table.
- Place the knobs and switches on the front of the amplifier in positions that configure it for this experiment as described in the manual.
- Connect the bath ground to the circuit ground of the amplifier via with an appropriate electrode. Similarly, ensure that the cage is grounded to the chassis of the amplifier.
2. Preparation of Microelectrodes and Assembly
- Use borosilicate glass microelectrodes (see the Table of Materials) and pull the glass tip to have a 8–10 mm taper, diameter of <1 µm and resistance of 80–120 MΩ when filled with 3 M KCl.
- Use a standard puller to achieve a short gradual taper using the following settings: heat = 650; velocity = 20; pull = 25; time = 250 and loop twice for higher resistances and smaller tips. See the Table of Materials as the settings are instrument-specific.
NOTE: Tip diameters <1 µm will cause minimal damage to the cell when impaled.
- Use a standard puller to achieve a short gradual taper using the following settings: heat = 650; velocity = 20; pull = 25; time = 250 and loop twice for higher resistances and smaller tips. See the Table of Materials as the settings are instrument-specific.
- Fill the microelectrode with 3 M KCl using a microfiber syringe (see the Table of Materials).
- Slowly pull the plunger of the microfiber syringe up while injecting the 3 M KCl into the microelectrode to allow space for the fluid to fill and to prevent the formation of air bubbles inside the microelectrode.
- Fill the microelectrode until full and ensure that there are no air bubbles before placing it in the microelectrode holder. If bubbles are present, gently tap the microelectrode with a finger to remove the bubbles.
- Exercising care, firmly push the electrode shank into the holder through the bored hole. If excess fluid is present, remove it with a tissue.
- Connect the electrode holder assembly to the amplifier probe. Conduct an electrode test, adjust the input offset, verify zero setting and check the probe input leakage as per the amplifier manual.
- Measure electrode resistance using an electrode test as shown in Table 1.
- Note that a working electrode displays a positive DC voltage shift of 1 mV/MΩ at the channel output. On the other hand, if a large voltage appears at the channel output and on the meter, this indicates a blocked or broken electrode.
- Open the recording software, assign a name to the file and save it for future analysis in a storing software.
3. Isolation and Cannulation of the Middle Cerebral Artery
- Preparation the reagents.
- Prepare normal and low calcium physiological salt solution (PSS) as described in Table 2.
- Prepare the myograph.
- Rinse the myograph chamber (see the Table of Materials) with distilled water multiple times to keep it free of debris. Load the chamber with 5 mL of normal PSS.
- Fill both glass cannulas with filtered normal PSS using a 5–10 mL syringe. Carefully fill the entire cannula and the attached tubing without introducing any air bubbles.
- Prepare two monofilament nylon sutures (10-0, 0.02 mm) with a half-knot each using blunt forceps.
- Place the partially closed suture knots on both cannulas slightly away from the tip using dissection forceps under a dissection microscope. Later these knots will be slid off and tied carefully onto the cannulated arterial ends to secure the vessel.
- Isolate and cannulate the middle cerebral artery.
- Induce deep anesthesia in a Sprague Dawley rat by using 2–4% inhaled isoflurane.
- Decapitate the rat using guillotine under deep anesthesia.
- Carefully remove the skull using a bone cutter and a scissor.
- Remove the brain from the skull and place it in 5 mL of low calcium PSS on ice.
- Identify and dissect out an unbranched segment of rat middle cerebral artery (MCA) with an inner diameter of 100–200 μm from the brain using spring scissors and forceps.
- Mount the MCA onto the glass cannulas using fine forceps and secure by tightening the sutures in the myograph containing normal PSS.
- Close off the distal cannula so that there will be no flow within the MCAs.
- Connect the inflow pipette to a reservoir holding PSS to allow for control of intraluminal pressure which will be monitored with an in-line pressure transducer.
- Visualize the cannulated MCAs using a charge-coupled device camera (see the Table of Materials) mounted on an inverted microscope and an imaging software.
- Set the axial length of the MCA to an approximate length where it should appear neither rigid nor flaccid.
- Equilibrate the bath solution with O2 (95%) and CO2 (5%) at 37 °C to provide adequate oxygenation, temperature and to maintain pH at 7.4.
- Impale (penetrate the cell plasma membrane) the vascular smooth muscle cells.
- Connect the ground electrode and keep it immersed in the PSS of the myograph.
- Illuminate the vessel chamber and look through the microscope to visualize the tip of the microelectrode in the bath solution.
NOTE: Alternatively, one can visualize the MCA and microelectrode on a computer having an imaging software. - Use the controls of the micromanipulator to move the tip of the microelectrode close to the outer wall of the blood vessel. The micromanipulator and the tip of the microelectrode must be in a stable position in relation to the tissue.
NOTE: Before beginning experiments, confirm that the membrane voltage has stabilized. If the measured voltage is unstable, the connection between the electrode and the cell is not sealed, indicating a leak. - Begin the recording.
- Slowly move the tip of the microelectrode towards the vessel, aiming for the center of the vessel using course or fine control of the micromanipulator.
NOTE: Occasionally, a small deflection in the recording may be observed when the microelectrode tip contacts a muscle fiber membrane. - When the tip comes in close proximity to the vessel, advance the electrode forward in one rapid motion using the micromanipulator to impale the membrane of the muscle.
- At this point, one can begin observing the changes in Vm being recorded. Do not touch the micromanipulator when the microelectrode impales the membrane of the cell.
NOTE: The difference in voltage between the recording and reference electrode decreases from 0 mV to between -40 mV and -75 mV depending on the level of intravascular pressure or other excitatory or inhibitory stimuli. These readings characterize the transmembrane potential difference of the current cell. - Perform multiple impalements on a single vessel in different areas of the vessel without damaging VSMCs in order to get accurate measurements.
- After recording, use the manipulator to remove the microelectrode in one rapid movement.
- Stop the recording and save data files for further analysis.
Representative Results
The presented method can be reliably used to record Vm in cannulated vessels. A brief procedure describing how to isolate MCA from the brain is presented in Figure 1A. After separating the brain from the skull, the MCA was dissected out and placed in a Petri dish containing low calcium PSS. Part of the connective tissue that was attached was also dissected along with MCA using spring scissors and forceps to prevent damage to MCA during the isolation. Carefully, connective tissue was also removed, and the dissected MCA was ready to transfer to the myograph. MCA was mounted on the cannulas and tied using suture knots on both ends of the cannulas in the myograph. A schematic representation of a typical microelectrode impalement method setup is shown in Figure 1B. A microelectrode filled with 3 M KCl was connected to the electrometer via a holder in the probe. Channel output of the electrometer was connected to an analog input channel of a digitizer using a BNC-BNC cable. Digitizer output was further connected to an oscilloscope to visualize the signal in the recording software. The ground is established using an AgCl pellet wire that was extended from the chassis of electrometer to the bath solution in the myograph. Finally, digital traces were visualized in the recording software on the computer monitor.
The MCA was then incubated in freshly prepared warm PSS and was pressurized to 60 mmHg. Only vessels that gain tone were used for Vm recording. After the vessel gained significant tone, the microelectrode was advanced into the vessel wall. Arterial diameter and impalement of the artery were visualized using video microscopy. Impalement is considered successful when there is a rapid deflection to negative values, Vm is stable for ≥30 s, and the voltage returns abruptly to 0 mV upon removal of the electrode as shown in Figure 212,15. Our results suggest that, in an MCA that is pressurized to 60 mmHg, the Vm is ~ -43.2 ± 2.9 mV.
After a successful impalement and Vm stabilization, the drugs that change the Vm were perfused in the bath and changes in the Vm were recorded. We used 20 mM KCl to depolarize and 20 µM NS1619, a synthetic large conductance potassium channel opener, to hyperpolarize the membrane. Our results suggest that perfusion of the chamber with KCl depolarized the membrane by ~ 5.8 ± 0.18 mV. On the other hand, perfusion with NS1619 hyperpolarized the membrane by ~3.8 ± 0.4 mV.
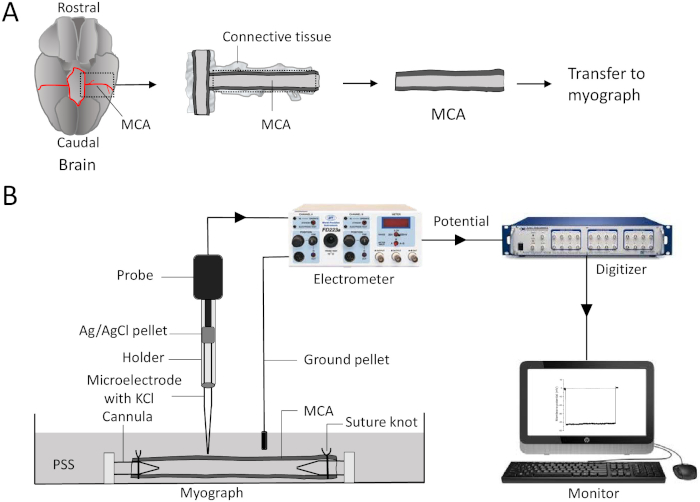
Figure 1: An illustration of isolation of middle cerebral arteries and recording of membrane potential using microelectrode impalement method. (A) Using spring scissors, cut the brain or connective tissue along the dotted lines. Transfer and mount the MCA between the two glass cannulas and secure it using sutures in the myograph chamber filled with PSS. (B) A microelectrode filled with 3 M KCl is inserted into the holder and is connected to the probe. Changes in transmembrane potential travel from the probe to an electrometer and to the digitizer via a BNC cable (BNC cable is connected from channel output of electrometer and to an analog input of a digitizer). The digitized potential is seen in the recording software on the monitor. The ground is established using an AgCl pellet wire connected from the electrometer to the bath solution in the myograph. MCA = middle cerebral artery; PSS = physiological salt solution; AgCl = silver chloride. Please click here to view a larger version of this figure.
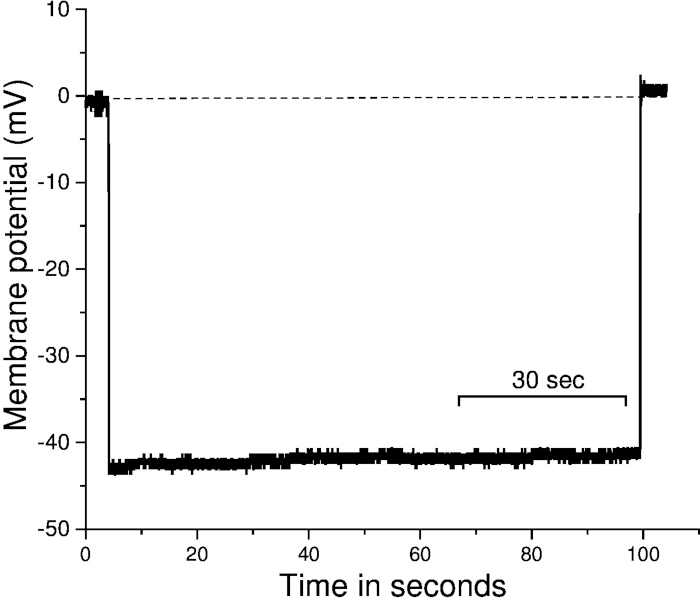
Figure 2: Recording of membrane potential using microelectrode impalement method from cannulated middle cerebral arteries. Representative trace of Vm. Impalement is considered successful if there is an abrupt deflection to negative values upon electrode entry, Vm is stable for ≥30 s, and the voltage returns abruptly to 0 mV upon removal of the electrode. The X-axis represent the time, and the Y-axis represents the membrane potential. The dotted line represents the adjusted baseline before the impalement of the vessel. Sec = seconds. Please click here to view a larger version of this figure.
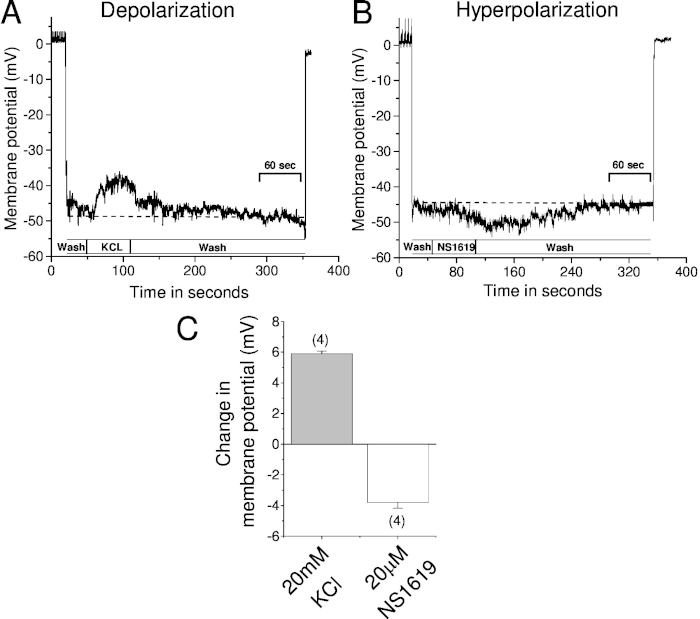
Figure 3: Recording of changes in the membrane potential using microelectrode impalement method when vessels are exposed to vasoactive agents. Vm was recorded using microelectrode impalement method from cannulated middle cerebral arteries before and after exposure to vasoactive agents. Sample trace represents (A) depolarization in response to 20 mM KCl and (B) hyperpolarization in response to 20 µM NS1619—a large conductance potassium channel agonist. (C) Summary bar graph of the changes in Vm before and after application of KCl and NS1619. The dotted line represents resting Vm. The number in the parenthesis represents the number of vessels used in the study. Note that Vm reached baseline as soon as the drug is washed out. Error bar represents the standard error of the mean. Sec = seconds. Please click here to view a larger version of this figure.
| Settings of electrometer to measure electrode resistance | |
| Meter Input | Channel A or B |
| Position toggle | In |
| Meter range | toggle to 200 mV |
| Electrode | In the bath |
| Mode | Operate to Electrode test |
| Meter indication | 1 mV/MΩ |
Table 1: Settings of electrometer to measure electrode resistance.
| Chemicals | low calcium PSS mM | Normal PSS mM | Company | Catalog | |
| 1 | NaCl | 119.00 | 119.00 | Sigma | S7653 |
| 2 | KCl | 4.70 | 4.70 | Sigma | P4504 |
| 3 | MgSO4 | 1.17 | 1.17 | Sigma | M7506 |
| 4 | CaCl2 | 0.05 | 1.60 | Sigma | C3881 |
| 5 | HEPES | 5.00 | 5.00 | Sigma | H7006 |
| 6 | Glucose | 10.00 | 10.00 | Sigma | G7021 |
| 7 | NaH2PO4 | 1.18 | 1.18 | Sigma | S0751 |
| 8 | NaHCO3 | 18.00 | 18.00 | Sigma | S5761 |
| pH: 7.4 | pH: 7.4 |
Table 2: Reagents used in the preparation of low calcium and normal physiological salt solution.
Discussion
This article provides the necessary steps on how to use a sharp microelectrode impalement method to record Vm from a cannulated vessel preparation. This method is widely used, and offers high-quality, consistent recordings of Vm that answer a wide range of experimental questions.
Some critical considerations and troubleshooting steps are described here to ensure success of the method. The quality of the microelectrode (its sharpness and resistance) and the cellular process it penetrates influence the stability and accuracy of Vm. If the signal continuously drifts or is above or below the amplifier’s recording range, it is most likely that the electrode is blocked, or the tip is broken. If the impalement is only partial, insufficient, or damages the cell, the recorded potential climbs back to 0 mV resulting in an unstable signal or no signal. In some cases, the contents of the cell can also block the very high resistance electrode. All these problems can be overcome by merely replacing the electrode with a new one.
For successful impalement, one must ensure that the total resistance of the cell membrane is unaltered, and the measured membrane potential is stable with no leaks between the electrode and the membrane. It is critical that the electrode is advanced towards the cell by a stable micromanipulator. When advanced to within a few micrometers of the cell, the tip potential will change resulting in a slight deflection in the detected voltage. To avoid damaging the tip of the electrode or stretching of the cell membrane, rapid advancement of the electrode is required. Successful impalement is characterized by a rapid drop in membrane potential followed by a stabilization around the resting potential of the membrane. If the potential reading fluctuates, it is possible that the tip of the electrode has disrupted the membrane causing sodium to leak into the cell and potassium to leak out of the cell, resulting in progressive depolarization. Another problem in this method is that junction potentials and electrode tip potentials can add an unnecessary artifact to the Vm recording. Junction potentials occur when differing conductors come into contact. There are two different types of junction potentials, liquid-metal, and liquid-liquid. Liquid-metal junctions are formed when the tip of the probe contacts the electrolyte in the micropipette. Liquid-liquid junctions, also called liquid junction potential, occur when two solutions of varying concentrations come into contact. The diffusion of the ions between the solutions contributes to the development of the potential. Besides, the properties of the glass electrode tip interacting with liquid can generate tip potentials. To minimize the junction as well as tip potentials, zeroing the measured potential in the bath could reduce the unwanted bias. During impalement, one cannot rule out the possibility that endothelial, rather than smooth muscle, Vm could inadvertently be sampled in some experiments. However, studies indicate that the endothelium and VSMC layers are electrically coupled in small arterioles and exhibit similar Vm responses23.
Ultimately, three steps in this process are critical to achieving successful recordings. First, vessels must be appropriately prepared, ensuring that they are not damaged in the process. Second, microelectrodes must be pulled to the right electrical properties. Finally, rapid impalement of the membrane without breaking the tip is crucial for accurate results. The investigator must understand electrophysiology, how to create viable microelectrodes, and how to set up and use an electrometer.
Despite its importance, this method has various limitations. First, the aggregate cost to procure all the equipment is high (~$30-40,000). Second, fresh vessels are needed for all these experiments; hence an animal is euthanized for each experiment, adding to the overall cost. Third, dissection of cerebral arteries, cannulation of the vessels is tedious, expensive and has a learning curve. Fourth, preparing microelectrodes, impalement, and recording Vm requires a thorough understanding of the electrophysiology. Finally, to establish this set up in the lab requires dedicated staff, time and effort.
Vm is an essential electrophysiological property that determines the vascular tone and thus blood flow to an organ. Vm could be altered by several vasoactive chemicals that are released from neurons, endothelium and blood components. While vasoconstrictors depolarize the membrane, dilators hyperpolarize the membrane. Several proteins including K+ channels, VGCC, sodium potassium ATPase, Ca2+ ATPase, Cl– channels, store-operated and stretch channels regulate the Vm. Alterations in any of these proteins in disease conditions could potentially alter Vm and thus vascular tone and blood flow. The microelectrode impalement method is useful to record resting as well as changes in Vm in response to vasoconstrictors and dilators. So, this method could be used reliably to understand normal and altered Vm associated with disease, and may be useful in the development of pharmacological agents designed to modulate Vm, vascular tone and blood flow.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by grants from the Intramural support research program (IRSP) from UMMC, AHA Scientist Development Grant (13SDG14000006) awarded to Mallikarjuna R. Pabbidi.
Materials
| Dissection instruments | |||
| Aneshetic Vaporiser | Parkland scientific | V3000PK | |
| Dissection microscope | Nikon Instruments Inc., NY | Eclipse Ti-S | |
| Kleine Guillotine Type 7575 | Harvard Apparatus, MA | 73-198 | |
| Littauer Bone Cutter | Fine science tools | 16152-15 | |
| Moria MC40 Ultra Fine Forceps | Fine science tools | 11370-40 | |
| Surgical scissors Sharp-Blunt | Fine science tools | 14008-14 | |
| Suture | Harvard Apparatus | 72-3287 | |
| Vannas Spring Scissors | Fine science tools | 15018-10 | |
| Electrophysiology Instruments | |||
| Charge-coupled device camera | Qimaging, , BC | Retiga 2000R | |
| Differential electrometer amplifier | WPI | FD223A | |
| In-line pressure transducer | Harvard Apparatus, MA | MA1 72-4496 | |
| Micromanipulator | Thor labs | PCS-5400 | |
| Microelectrodes | Warner Instruments LLC, CT | G200-6, | |
| Micro Fil (Microfiber syringe) | WPI | MF28G67-5 | |
| Microelectrode holder | WPI | MEH1SF | |
| Myograph | Living Systems Instrumentation, VT | CH-1-SH | |
| Puller | Sutter Instrument, San Rafael, CA | P-97 | |
| Vibration-free table | TMC | 3435-14 | |
| Softwares | |||
| Clampex 10 | Molecular devices | ||
| p Clamp 10 | Molecular devices | ||
| Imaging software | Nikon, NY | NIS-elements | |
| Chemicals | |||
| NaCl | Sigma | S7653 | |
| KCl | Sigma | P4504 | |
| MgSO4 | Sigma | M7506 | |
| CaCl2 | Sigma | C3881 | |
| HEPES | Sigma | H7006 | |
| Glucose | Sigma | G7021 | |
| NaH2PO4 | Sigma | S0751 | |
| NaHCO3 | Sigma | S5761 |
Referenzen
- Nelson, M. T., Quayle, J. M. Physiological roles and properties of potassium channels in arterial smooth muscle. American Journal of Physiology. 268, C799-C822 (1995).
- Hughes, A. D. Calcium channels in vascular smooth muscle cells. Journal of Vascular Research. 32 (6), 353-370 (1995).
- Large, W. A., Wang, Q. Characteristics and physiological role of the Ca(2+)-activated Cl- conductance in smooth muscle. American Journal of Physiology. 271 (2 Pt 1), C435-C454 (1996).
- Nelson, M. T., Conway, M. A., Knot, H. J., Brayden, J. E. Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. Journal of Physiology. 502 (Pt 2), 259-264 (1997).
- Yamazaki, J., et al. Functional and molecular expression of volume-regulated chloride channels in canine vascular smooth muscle cells. Journal of Physiology. 507 (Pt 3), 729-736 (1998).
- Gibson, A., McFadzean, I., Wallace, P., Wayman, C. P. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends in Pharmacol Sciences. 19 (7), 266-269 (1998).
- Davis, M. J., Donovitz, J. A., Hood, J. D. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. American Journal of Physiology. 262 (4 Pt 1), C1083-C1088 (1992).
- Setoguchi, M., Ohya, Y., Abe, I., Fujishima, M. Stretch-activated whole-cell currents in smooth muscle cells from mesenteric resistance artery of guinea-pig. Journal of Physiology. 501 (Pt 2), 343-353 (1997).
- Shelly, D. A., et al. Na(+) pump alpha 2-isoform specifically couples to contractility in vascular smooth muscle: evidence from gene-targeted neonatal mice. American Journal of Physiology-Cell Physiology. 286 (4), C813-C820 (2004).
- Coca, A., Garay, R. . Ionic Transport in Hypertension New Perspectives. , (1993).
- Harder, D. R. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circulation Research. 55 (2), 197-202 (1984).
- Knot, H. J., Nelson, M. T. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. Journal of Physiology. 508 (Pt 1), 199-209 (1998).
- Nelson, M. T., Patlak, J. B., Worley, J. F., Standen, N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. American Journal of Physiology. 259, C3-C18 (1990).
- Harder, D. R., Sperelakis, N. Action potentials induced in guinea pig arterial smooth muscle by tetraethylammonium. American Journal of Physiology. 237 (1), C75-C80 (1979).
- Nystoriak, M. A., et al. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. American Journal of Physiology-Heart and Circulatory Physiology. 300 (3), H803-H812 (2011).
- Yvonder Weid, P., Lee, S., Imtiaz, M. S., Zawieja, D. C., Davis, M. J. Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphatic Research and Biology. 12 (2), 66-75 (2014).
- Harder, D. R. Comparison of electrical properties of middle cerebral and mesenteric artery in cat. American Journal of Physiology. 239 (1), C23-C26 (1980).
- Harder, D. R. Heterogeneity of membrane properties in vascular muscle cells from various vascular beds. Federation Proceedings. 42 (2), 253-256 (1983).
- Cogolludo, A., et al. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circulation Research. 98 (7), 931-938 (2006).
- Ganitkevich, V., Isenberg, G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. Journal of Physiology. 470, 35-44 (1993).
- Yamagishi, T., Yanagisawa, T., Taira, N. K+ channel openers, cromakalim and Ki4032, inhibit agonist-induced Ca2+ release in canine coronary artery. Naunyn-Schmiedeberg’s Archives of Pharmacology. 346 (6), 691-700 (1992).
- Okada, Y., Yanagisawa, T., Taira, N. BRL 38227 (levcromakalim)-induced hyperpolarization reduces the sensitivity to Ca2+ of contractile elements in caninse coronary artery. Naunyn-Schmiedeberg’s Archives of Pharmacology. 347 (4), 438-444 (1993).
- Xia, J., Little, T. L., Duling, B. R. Cellular pathways of the conducted electrical response in arterioles of hamster cheek pouch in vitro. American Journal of Physiology. 269 (6 Pt 2), H2031-H2038 (1995).
- Jaggar, J. H., Porter, V. A., Lederer, W. J., Nelson, M. T. Calcium sparks in smooth muscle. American Journal of Physiology-Cell Physiology. 278 (2), C235-C256 (2000).

