Evaluation of Changes in Hydration and Body Cell Mass with Bioelectrical Impedance Analysis after Exercise Program for Rheumatoid Arthritis Patients
Summary
This protocol assesses alterations in hydration and body cell mass status using bioelectrical impedance vectorial analysis following a dynamic exercise program designed for patients with rheumatoid arthritis. The dynamic exercise program itself is detailed, highlighting its components focused on cardiovascular capacity, strength, and coordination. The protocol details steps, instruments, and limitations.
Abstract
Rheumatoid arthritis (RA) is a debilitating disease that can result in complications such as rheumatoid cachexia. While physical exercise has shown benefits for RA patients, its impact on hydration and body cell mass remains uncertain. The presence of pain, inflammation, and joint changes often restrict activity and make traditional body composition assessments unreliable due to altered hydration levels. Bioelectrical impedance is a commonly used method for estimating body composition, but it has limitations since it was primarily developed for the general population and does not consider changes in body composition. On the other hand, bioelectrical impedance vectorial analysis (BIVA) offers a more comprehensive approach. BIVA involves graphically interpreting resistance (R) and reactance (Xc), adjusted for height, to provide valuable information about hydration status and the integrity of the cell mass.
Twelve women with RA were included in this study. At the beginning of the study, hydration and body cell mass measurements were obtained using the BIVA method. Subsequently, the patients participated in a six-month dynamic exercise program encompassing cardiovascular capacity, strength, and coordination training. To evaluate changes in hydration and body cell mass, the differences in the R and Xc parameters, adjusted for height, were compared using BIVA confidence software. The results showed notable changes: resistance decreased after the exercise program, while reactance increased. BIVA, as a classification method, can effectively categorize patients into dehydration, overhydration, normal, athlete, thin, cachectic, and obese categories. This makes it a valuable tool for assessing RA patients, as it provides information independent of body weight or prediction equations. Overall, the implementation of BIVA in this study shed light on the effects of the exercise program on hydration and body cell mass in RA patients. Its advantages lie in its ability to provide comprehensive information and overcome the limitations of traditional body composition assessment methods.
Introduction
Rheumatoid arthritis (RA) is a disabling disease that affects patients' functionality and independence due to acute joint pain, reduced muscle strength, and impaired physical function, all of which are associated with the inflammatory process inherent to the disease1,2. In advanced stages, persistent inflammation causes structural alterations leading to deformity, joint dysfunction, and rheumatoid cachexia, which is a poor prognostic factor for these patients3,4.
Rheumatoid cachexia is characterized by alterations in body composition, such as muscle loss with stable weight and increased fat mass, which can significantly impact the quality of life for these patients3,5,6. Various techniques are available to assess body composition, with the most widely used being bioelectrical impedance analysis (BIA). However, when conventional BIA analysis is used in subjects with altered body compositions, the estimations may be limited as they are based on prediction equations formulated for a healthy or normally hydrated population7,8.
A different approach, called bioelectrical impedance vector analysis (BIVA), utilizes the impedance vector based on graphical RXc. It utilizes impedance, resistance (R), and reactance (Xc) data corrected for height, resulting in a vector that provides information about the hydration state and integrity of the cell mass. BIVA is capable of classifying patients into categories such as dehydration, overhydration, normal, athlete, lean, cachectic, and obese, making it a valuable tool for RA patients8,9,10. Vectors located above or below the main axis (the left or right halves of the ellipses) have been associated with higher and lower cell mass in soft tissues, respectively. Forward and backward displacements of vectors parallel to the major axis are linked to dehydration and fluid overload. Athletes are defined as individuals with higher cell mass, potentially accompanied by dehydration. The lean classification refers to those with lower cell mass, potentially accompanied by dehydration, and the obese classification applies to individuals with higher cell mass, which may be accompanied by fluid overload. The classification of cachexia by BIVA is determined by high resistance and low reactance values, represented by the movement of the vector to the right of the graph, indicating a decrease in cell mass, potentially accompanied by an alteration in hydration status11 (Figure 1).
Conventional pharmacological treatments for RA primarily focus on reducing pain, inflammation, and joint damage progression, with limited attention given to alterations in body composition12. Among the non-pharmacological therapies commonly used in this population, exercise-based interventions have shown positive outcomes in improving functionality, fatigue, pain, joint mobility, aerobic capacity, muscle strength, endurance, flexibility, and psychological well-being. Importantly, these interventions have been shown to achieve these benefits without exacerbating symptoms or causing joint damage in patients without extensive pre-existing damage13,14,15,16,17. However, there is limited knowledge on implementing and evaluating changes in hydration and body cell mass status following exercise interventions in this population. These patients often experience pain, inflammation, and structural joint changes, limiting the types of activities they can engage in and further complicating body composition assessments using traditional approaches. This protocol aims to demonstrate how to evaluate changes in hydration and body cell mass status using bioelectrical impedance vectorial analysis after implementing a dynamic exercise program for patients with rheumatoid arthritis. Additionally, the protocol provides details of the dynamic exercise program, including cardiovascular capacity, strength, and coordination components, as well as the steps, instruments, limitations, and general considerations.
Protocol
The present protocol was approved by and followed the Human Research and Ethics Committee guidelines of the National Institute of Medical Sciences and Nutrition Salvador Zubirán (Ref.: 1347). Informed consent was obtained from the human participants before participation in this study. Only patients in functional class I to III without total or partial arthroplasties18,19 and who were not candidates for prostheses were included in this study. Exclusion criteria included patients with cardiovascular disease, cancer, chronic kidney disease, pregnancy, or other autoimmune diseases that overlap with RA.
1. Recruitment of participants
- Recruit patients.
NOTE: For the present study, twelve women with RA were recruited from the rheumatology outpatient clinic. - Ensure that the patients receive stable pharmacological treatment during the previous 6 months; which could include any of the following: antimalarial drugs (e.g., chloroquine, hydroxychloroquine), disease-modifying antirheumatic drugs (DMARD) (e.g., methotrexate, leflunomide), and glucocorticoids (e. g., prednisone)20.
NOTE: According to the assessment by the rheumatologist, changes in pharmacological treatment could be made during the intervention period, if necessary.
2. Participant pre-test
NOTE: Pre-tests were performed 1 week before beginning the dynamic exercise program. Multifrequency bioelectrical impedance analysis equipment (see Table of Materials) was used, and measurements were performed with patients fasting for 4 to 5 h.
- Steps before testing
- Ensure that these measurements are carried out by a standardized person with extensive experience.
- Clean the equipment using 0.05% chlorhexidine and ensure that the hands are washed.
- Explain the procedure to the patient and obtain the measurements for the weight (kg) and height (cm).
- Ask the patient to remove both shoes and their right sock as well as any metal objects that are in contact with their skin.
- Place the patient in a supine position for 5 min with both legs and arms extended and verify that they are not in contact with any part of their body.
- Measurement of BIA
- Clean the back of the hand and the right foot with 70% alcohol.
- Place two electrodes on the back of the hand: one on the third metacarpophalangeal joint and the other in the middle of the wrist at the level of the head of the ulna.
- Place two electrodes on the right foot: one at the third metatarsophalangeal joint and the other between the medial and lateral malleoli. There should be a gap of 5-10 cm between the electrodes.
- Connect the four cables of the equipment. Once connected, place the red clamps on the electrodes close to the fingernail and toenail; place the black clamps on the remaining electrodes. The cables must not cross each other.
- The impedance values (Z) of four different frequencies (5, 50, 100, and 200 kHz) will be shown on the equipment screen. Note the resistance and reactance values for the 50 kHz frequency. These values will be necessary to classify patients with cachexia.
NOTE: Bioelectrical impedance analysis using tetrapolar multifrequency equipment provides accurate resistance and reactance values at a single frequency of 50 kHz as well as the ratio between the 200 kHz and 5 kHz impedance values (200/5 kHz).
- Classification of cachexia by BIVA
- Download the BIVA tolerance R-Xc graph software (see Table of Materials) and open it.
NOTE: The software is a spreadsheet that can be seen at the bottom of seven worksheets. - Go to the second worksheet, Reference populations; choose a row that corresponds to the reference population; copy it; and paste it in row two, marked in yellow.
NOTE: The reference population is chosen according to the age range, race, sex, and BMI of the population to be evaluated. - Go to the fifth worksheet, Fächer, and insert the patient's data in the second row: in column A, enter the patient's ID; in column B, enter the number one; and for the next two columns, one can choose whether to enter the patient's name.
- In column E, enter the patient's sex, using M for men or F for women. In columns F and G, insert the previously noted resistance and reactance values at 50 kHz. Enter the height (cm) and weight (kg) in the next two columns.
- In column J, enter the number corresponding to the reference population chosen in the second worksheet.
- Insert a number between 1 and 10 in column K. It will be needed for the "Point graph" sheet; in the next column, enter the patient's age.
NOTE: One can choose values between 1 and 10 because there can be up to 10 patients to graph simultaneously in the BIVA tolerance software. - The options bar is at the top of the software. Find the complements option and click it. Then, select the calculate option that will be displayed and click it. Observe the resistance and reactance values adjusted by height and phase angle.
- Next, navigate to sheet 3, Point Graph, and observe a BIVA graph according to the chosen reference population. A dialogue box will be displayed. Select the group code entered in column K for step 2.3.6. Select OK, and the BIVA graph will be shown with the patient's vector drawn as a geometric figure.
- Observe the tolerance ellipses of 50%, 75%, and 95% as well as the quadrants I, II, III, and IV in the BIVA graph. To classify a patient with cachexia using BIVA, the vector must be in the lower right quadrant (quadrant IV) and outside the 75% tolerance ellipse (Figure 1).
NOTE: Patients whose vectors fall in any of the quadrants within the <75% tolerance ellipses will be considered with a normal body composition classification21.
- Download the BIVA tolerance R-Xc graph software (see Table of Materials) and open it.
3. Dynamic exercise program
NOTE: The program was applied and supervised by a physiotherapist. An intervention duration of 48 sessions per patient was estimated. The exercise sessions were carried out in a mechanotherapy gym within a physiotherapy area belonging to the Rheumatology and Immunology department of the "INCMNSZ" with a duration of 90 min, twice a week.
- Evaluation of the session
- Ask patients about the pain or discomfort they perceive in their joints.
NOTE: The visual analogue scale (VAS) was used to assess pain. If they reported pain on the VAS above 7/10 in any joint, a more specific evaluation was carried out by the physiotherapy department (e.g., electrotherapy was used if there was only pain, thermotherapy was used if there was stiffness present, and cryotherapy was used when there was both pain and inflammation). - Take vital signs before each exercise session.
- Ask patients about the pain or discomfort they perceive in their joints.
- Warm-up
NOTE: With a duration of 15 min, a general dynamic warm-up divided into phases was established. Activation phase: simple, gentle, and global movements were performed for all movement arcs while remaining in a static position, with 10 to 15 repetitions. Set-up phase: in this last part, gentle dynamic exercises were performed, which simulated the gestures of the movements that would be implemented in the work phase, with 10-15 repetitions.- Activation phase
- Choose the most suitable warm-up exercise, including upper- and lower-extremity joints and their range of motion.
- Upper extremity: Instruct the patient to reach a range of motion with no discomfort for each joint movement. The instructor must lead the patient through a normal-speed movement and instruct the patient to avoid a painful range of motion.
- Lower extremity: Instruct the patient to perform the warm-up in a standing position with both feet on the ground and on a stable surface. Instruct the patient to reach a non-painful movement speed through the range of motion for each joint while the patient sits in a chair.
NOTE: If some patients can stand for a long time, a sitting position must be reached, considering a stable chair with the back straight and the feet on the ground. The available ranges of motion of the hip, knee, ankle, and feet must be included.
- Choose the most suitable warm-up exercise, including upper- and lower-extremity joints and their range of motion.
- Set-up phase
- Instruct the patient to perform functional movement patterns that include more than two joints per segment (lower limb or upper limb).
- Give supervision during this stage to bring a sense of wellness during the movement and adjust the range of motion when the patient presents discomfort.
- Activation phase
- Work phase
NOTE: With a duration of 60 min, the work phase is divided into three stages of 20 min each.- Aerobic: perform the work on a treadmill.
NOTE: Select a treadmill with no default inclination.- Ensure the emergency stop device is working correctly and explain the safety measures to the patient. Advise the patient to wear sports shoes.
- Give the patients information about the adaptations that must be performed when the treadmill starts and must be performed properly to avoid unnatural gait movements.
- Establish a base speed for each patient, asking for a normal feeling during walking.
- Adjust the speed after 5 min on the treadmill. Using a pulse oximeter (see Table of Materials), measure the heart rate while the speed is increased until reaching a heart rate zone between 55% and 75%14,31of the HRmax.
NOTE: If the patient's heart rate goes over the 75% HRmax, the speed must be reduced to the ideal heart rate zone. Instruct the patient to look for a comfortable pace. - After 10 min, ask the patient for an assessment using a perceived effort rating scale.
NOTE: The modified Borg rating of the perceived exertion scale was used to assess perceived exertion. - Lower the treadmill speed to a comfortable pace for the patient's last 5 min. The speed must be lowered to a total stop when reaching 5 min.
- Ask the patient for any pain or discomfort after the use of the treadmill.
- Resistance exercises
NOTE: Directed joint mobility exercises were used in combination with muscle strength exercises. The workout consisted of a set of 8-10 repetitions per exercise. Soft (0.5-2.6 kg) and medium (0.7-3.2 kg) resistance bands were used, and the resistance was gradually increased every 2 weeks. The dosage of the exercise depended on the state of the patient at the time of the intervention.- Upper Extremity
- Instruct the patient to perform upper-extremity mobility while handling a wooden stick (<1 kg) with both hands.
- Teach the patient combined exercises that include the range of motion of more than two joints (e.g., shoulder and elbow flexion).
- Instruct the patient to hold a band above the ends. The patient must roll up their hand with the end of the band to ensure their grip.
NOTE: If the patient's hands have discomfort, the instructor must gently secure the band to their wrist. - Instruct the patient to put one end of the band on the floor and step it with their foot. Then, perform elbow flexion against the band's resistance. Elbow extension must work on eccentric contraction while returning to the neutral position.
NOTE: The patient must be standing with a stable foot base and good posture. If the patient indicates some discomfort, this exercise must be performed in a sitting position. - Instruct the patient to roll up a band on their hand, ensuring no excessive pressure is applied. The other end should be held by the free hand of the patient next to the body at hip level. Then, instruct the patient to flex the elbow at 90° with the elbow in a neutral position.
NOTE: The patient can rest for 20 s between movements.
- Lower extremity
- Instruct the patient to sit in a stable chair with 90° hip and knee flexion and tie the ends of the resistance band, making a loop band. The patient must surround their legs with the rubber band at the distal part of the femur (above the knee). In this position, instruct the patient to perform hip flexions for each leg up to 20 to 30 degrees above the starting position.
NOTE: For correct alignment, avoid hip rotation and knee flexion. If the patient indicates discomfort, reduce the range of motion. - Instruct the patient to sit in a stable chair with 90° hip and knee flexion and tie the ends of the resistance band, making a loop band. The patient must surround their legs with the rubber band at the distal part of the femur (above the knee). In this position, instruct the patient to perform a slight hip flexion (above 10° from the base position) and hip abduction.
NOTE: For correct alignment, avoid hip rotation and excessive knee flexion. If the patient indicates discomfort, reduce the range of motion. - Instruct the patient to sit in a stable chair with 90° hip and knee flexion and tie the ends of the resistance band, making a loop band. The patient must surround the nearest chair leg and their own leg with the rubber band at the ankle. Instruct the patient to return, at a slow tempo, to the base position.
NOTE: For correct alignment, the patient must maintain a comfortable sitting position and avoid hip flexion compensation. If needed, the patient can hold the base of the chair with their hands for more stability. The steps can be performed with one leg at a time or by changing sides. - Instruct the patient to hold a standing position. Then, ask the patient to tie the ends of the resistance band, make a loop band, and place the band around their ankles. Instruct the patient to perform reps of changing positions between sitting and standing.
NOTE: If the patient feels discomfort during the exercise, reassess and make the exercise easy by using a higher chair to reduce knee flexion or using a second chair where the patient can support himself and facilitate movement.
- Instruct the patient to sit in a stable chair with 90° hip and knee flexion and tie the ends of the resistance band, making a loop band. The patient must surround their legs with the rubber band at the distal part of the femur (above the knee). In this position, instruct the patient to perform hip flexions for each leg up to 20 to 30 degrees above the starting position.
- Upper Extremity
- Recreation games
NOTE: Consisting of the implementation of exercise series that involve gestures or movements adapted from a particular sport such as soccer, basketball, or volleyball, integrating flexibility and coordination components, 4 to 7 stations consisting of polyarticular movements and different exercises are created, and two series of 8 to 15 repetitions are worked (with difficulty increasing every 2 weeks).- Choose the most suitable exercise based on a sporting gesture for the patients each session and make an exercise station. Each station must be designed taking into account the limitations of the patient.
- Make a soccer goal with two chairs with a 1.3 m separation between them.
- Instruct the patients to hit a 30 cm plastic ball with their feet at a 3 m spot in front of the soccer goal.
- Control the difficulty by increasing the repetitions or sets per station and by adding new stations to the circuit.
NOTE: Example station designs: (1) Attach a "Ula Ula" ring to the tip of a 1.3 m wooden stick, place the patient at a 2 m throwing point in front of the ring, and instruct them to throw a 30 cm plastic ball with their arms to the "Ula Ula ring". Each patient must score at least 5 times and can score up to 10 times. (2) Attach a rope along the room walls to simulate a volleyball net. The rope must have a minimum height of 1. 7 m, and two patients must be in position on each side. Instruct the patients to pass a 40 cm air balloon over the rope at least 10 to 15 times each. (3) Place two patients with a 3 m separation between them and instruct the patients to throw a 30 cm plastic ball with their arms. Each patient must throw the plastic ball at least 10 times per arm. Patients must always supervise.
- Aerobic: perform the work on a treadmill.
- Cooling
NOTE: Cooling has a duration of 15 min and is composed of active static stretches.- Applied globally, stretching must be performed gently without putting stress on the joint. Stretching should not produce discomfort for the patient.
- Maintain each stretch for 15 to 20 s.
4. Post-test evaluation
NOTE: The post-test assessment must be scheduled during the week after the last exercise session.
- Repeat the measurement of body composition to obtain the BIVA classification, as described in the pre-test.
NOTE: To make a comparison between before and after the implementation of the dynamic exercise program, it is necessary to obtain the mean of the resistance difference divided by the height (dR/H), the mean of the reactance difference divided by the height (dXc/H), and the standard deviation and the Pearson correlation coefficient of the differences with the following equation8: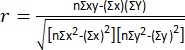
- To obtain the change in the resistance and reactance, download the BIVA confidence software (see Table of Materials) and open it.
NOTE: The software is a spreadsheet; at the bottom, you can see five worksheets. - In the fourth worksheet, "Paired data", check for ten columns where it will be necessary to insert the requested data.
- In column A, place the group ID. In column B, place the number of patients that were evaluated.
- In column C, insert the mean of d R/H obtained previously. In the next column, add the standard deviation.
- In column E, insert the mean of d Xc/H, and in the following column insert the standard deviation. In column G, insert the correlation coefficient obtained previously.
NOTE: In column H, choose to place 1, where one can display the confidence ellipse on the chart, or option 2 if you want to show the confidence ellipse and the difference mean vector. - In the following two columns, one can choose whether to place the names of the group and the equipment that was used to make the measurements.
- Once all the necessary data are complete, go to sheet 5, "Paired graph". There, a graph of the means of the difference is visible, and will be able to locate the vector of the resistance and reactance mean, in addition to the confidence ellipse.
- To evaluate whether the change is statistically significant, locate the complements option in the toolbar and click it. It will open a box with Hotelling's T2 test statistic8, allowing one to locate the value of p.
Representative Results
Results are presented for six female patients with RA who participated in a 48 session dynamic exercise program. The mean age of the patients was 52.7 ± 13.1 years, and their BMI was 26.8 ± 4.6. The average disease duration was 15.5 ± 6.1 years, and the disease activity, measured by Disease Activity Score 28, was classified as low activity with an average of 1.9 ± 1. Regarding disability, the Health Assessment Questionnaire Disability yielded an average score of 0.5 ± 0.3. For the six participants who did not undergo the exercise program, the mean age was 55.8 ± 7, and their BMI was 27.2 ± 4.8. The disease duration was 21.8 ± 10, and the disease activity was similar to the group that underwent the dynamic exercise program.
Table 1 displays the pharmacological treatment of the groups, as well as the concentrations of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). No changes in pharmacological treatment were required for any patient during the intervention period, according to the treating rheumatologist.
Figure 2A illustrates the initial status of the six patients before the implementation of the dynamic exercise program. Each patient was positioned outside the 75% tolerance ellipses in the RXc graph, indicating cachexia according to the BIVA classification. The average resistance before the exercise program was 630 ± 88, and the reactance was 46.5 ± 7.4. Figure 2B demonstrates the change in BIVA classification after the implementation of the dynamic exercise program for the six patients shown in Figure 2A. They were reclassified as normal, according to BIVA. The average resistance was 577 ± 54.9, and the reactance was 57.5 ± 11.4.
Figure 3A shows the six patients who did not participate in the exercise program. Two patients were classified as cachexia, one as normal, and two as lean. Figure 3B displays the change in BIVA classification after 6 months for the patients shown in Figure 3A. According to the BIVA classification, the patients initially classified as lean moved to cachexia, and the patient initially in the normal classification also moved to cachexia.
The mean change in resistance per height (dR/H) after the implementation of the dynamic exercise program was -55.9 ± 51, and the mean change in reactance per height (dXc/H) was 10.7 ± 10.3. These changes are associated with increased cell membrane surface and membrane integrity (Xc component) relative to fluid volume (R component), reflecting higher body cell mass and improved cell function and muscle functionality (Figure 4A). In the group that did not undergo the dynamic exercise program, no statistically significant changes were observed after 6 months (Figure 4B).
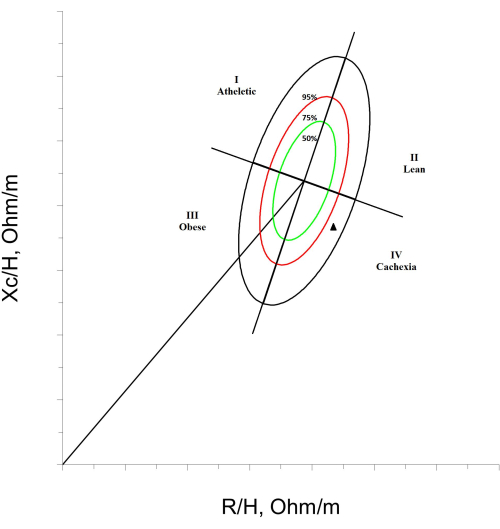
Figure 1: Cachexia classification by BIVA. An RXc graph is shown, divided into quadrants, with tolerance ellipses of 50%, 75%, and 95%. In the lower right corner, a patient with a cachexia BIVA classification, marked with a black triangle, is exemplified. Please click here to view a larger version of this figure.
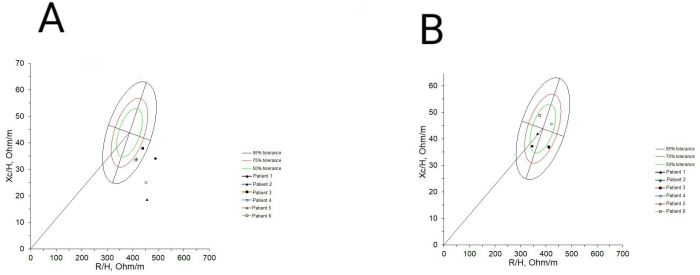
Figure 2: BIVA classification before and after implementing the dynamic exercise program. (A) The classification of the six patients before incorporating them into the exercise program is shown, and it can be observed that everyone had cachexia. (B) Changes in the BIVA classification after 48 sessions of the dynamic exercise program are shown, where it is observed that the six patients went from being classified with cachexia to being classified as normal. Please click here to view a larger version of this figure.
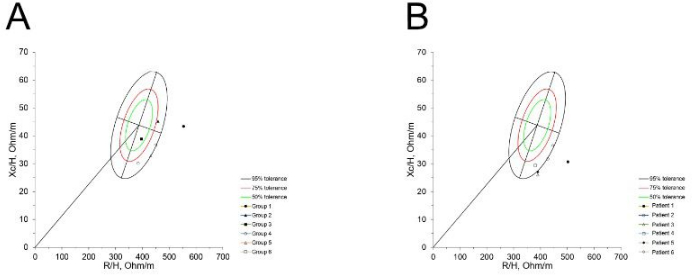
Figure 3: Basal BIVA classification and classification after six months in patients without an exercise program. (A) Classification of six patients at baseline measurement. (B) Changes after six months can be observed, where three patients shifted their classification to cachexia, while those who already had it remained unchanged. Please click here to view a larger version of this figure.
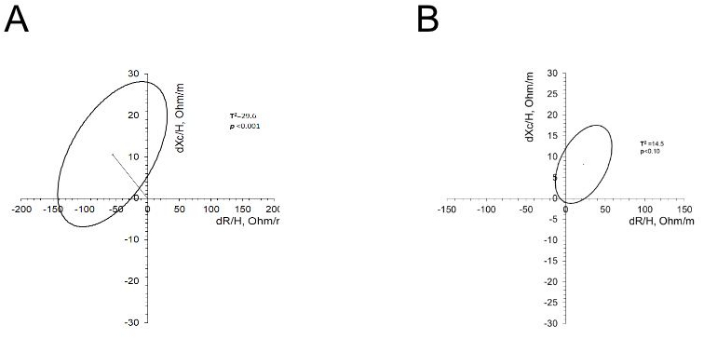
Figure 4: Changes in the R/H and Xc/H of patients who underwent an exercise program and those who did not. (A) The graph shows the vector of the mean of R/H and Xc/H and the confidence ellipse. The resistance decreased after the exercise program, while the reactance increased. (B) The graph shows the vector of the mean of R/H and Xc/H and the confidence ellipse. The resistance and reactance increased after six months. However, these changes were not statistically significant. Please click here to view a larger version of this figure.
| Variables | Dynamic exercise program | No dynamic exercise program |
| Age, years | 52.7 ± 13.1 | 55.8 ± 7 |
| Disease duration, years | 15.5 ± 6.1 | 21.8 ± 10 |
| Global Functional status, % | ||
| I | 33.3 | 33.3 |
| II | 66.6 | 33.3 |
| III | – | 33.3 |
| Disease activity score-28 | 1.9 ± 1 | 2.2 ± 0.8 |
| HAQ-Di, score | 0.5 ± 0.3 | 0.25 |
| BMI, kg/m2 | 26.8 ± 4.6 | 27.2 ± 4.8 |
| CRP, mg/dL | 1.2 ± 0.9 | 1.9 ± 1 |
| ESR, mm/h | 16.6 ± 8.5 | 12.5 ± 6.8 |
| Pharmacological treatment, % | ||
| Methotrexate | 100 | 83.3 |
| Sulfasalazine | 33.3 | 50 |
| Antimalarials | 66.6 | 16.6 |
| Leflunomide | – | 50 |
| Glucocorticoids | – | 33.3 |
| Glucocorticoid’s dose, mg | NA | 5 |
Table 1: Characteristics of the participants. The table displays the characteristics of six participants who underwent a dynamic exercise program for 48 sessions and six participants who did not undergo the exercise program. Data such as age, weight, disease duration, disease activity, disability, CRP and ESR concentrations, and prescribed pharmacological treatment are presented. Please click here to download this Table.
Discussion
In rheumatoid arthritis, the vicious circle of the disease has been described, which refers to the structural changes in joints caused by inflammation mechanisms; these changes, together with the chronic inflammatory state, lead to patients going through stages of great pain and inflammation, with structural changes in joints and as a consequence functional disability, that increase the risk of developing metabolic and cardiovascular diseases and alterations in body composition such as rheumatoid cachexia22. Exercise has been shown to reduce the symptoms of this disease, increase the quality of life, reduce the risk of other diseases23, and have a positive impact on the body composition of these patients. There are several methods to determine body composition; however, BIA is one of the most used because it is non-invasive, easily accessible, and simple to use. An analysis of body composition that is performed through BIA uses a low-frequency electric current. This current provides R values that are generated by the passage of the current through the fluids of the system, which allows an estimation of the intracellular and extracellular fluids24. Another measure provided by the BIA method is the Xc, which is the force that opposes the passage of the current through the cell membranes and allows an estimation of the cell mass of an individual; using the values of R, Xc, and body weight, it is possible to obtain through prediction equations the fat-free mass, total body water, and fat mass24. Several types of BIA equipment present different variabilities. The equipment described in this protocol is multifrequency equipment that measures impedance at different frequencies (5, 50, 50, 100, 200, and 500 kHz), which allows differentiation between intracellular and extracellular water because at lower frequencies, the impedance to current flow allows the determination of extracellular water, while at higher frequencies the impedance can be used to determine total body water and, by derivation, intracellular water25.
The use of BIA in clinical conditions such as AR presents some limitations because it is common in these patients to find total or partial arthroplasties. The surgical implants used are mainly made of metals such as steel, titanium, cobalt, and chromium, with the use of other materials such as ceramics, hydroxyapatites, and polyethylenes. These materials can increase or decrease electrical conductivity, and it is difficult to predict how they will affect the estimates of body composition26. On the other hand, deformities in the hands and feet are frequent and can influence the correct anatomical locations of the electrodes, which affects the results that are obtained. Another important limitation of the BIA method occurs when there are alterations in the distribution of body fluids or abnormal body geometries. Due to the pathophysiology of AR, using body composition estimates through the BIA method does not provide reliable data. To avoid this limitation and to be able to use the BIA method in populations with these alterations, it has been proposed to use the raw impedance data through BIVA, which presents the data through an RXc graph that represents specific sex and race and the tolerance ellipses of a comparative reference population27. The advantage of this method is that it provides information independent of body weight or prediction equations, so it is not influenced by hydration status or body alterations. This method can identify the hydration status through the R/H axis and the cell mass on the Xc/H axis28. It also allows us to make intra- and inter-subject comparisons; evaluate post-intervention changes in these variables; and categorize patients with cachexia, a condition that is reflected as an increase in R/H that has been associated with decreased muscle function and a decrease in Xc/h that has been associated with a loss of muscle strength, which is essential in patients with AR29. As for the limitations of BIVA,this is an indirect method to assess muscle function. We did not conduct an evaluation of muscle function or strength to support our findings. However, it is necessary to have the tolerance ellipses validated for the study population; using ellipses from different populations could lead to erroneous and invalid conclusions, and it is also essential to have the BIVA tolerance R-Xc graph software. Furthermore, it is worth noting that dual-energy X-ray absorptiometry (DXA) is widely considered the gold standard for measuring body composition. Although we did not directly compare the agreement between these two techniques, there are existing studies that have conducted such comparisons. These studies have found that the BIVA method demonstrates good concordance with DXA regarding total body water (TBW), extracellular water (ECW), and intracellular water (ICW). However, it should be noted that, to our knowledge, no specific comparison has been made regarding cell mass30.
One drawback of the BIVA method is the inability to assess fat mass or fat-free mass. Nevertheless, it offers the advantage of categorizing patients based on their cell mass and hydration status, which distinguishes it from DXA.
The determination of hydration status and cell mass using BIVA is a useful tool to assess changes in body composition in patients with AR, which may be derived from the pathophysiology of the disease, pharmacological treatments, and dietary or exercise interventions, so it is essential to apply in health services as an integral part of the evaluation of a patient with AR.
According to Hurkmans, dynamic exercise is characterized as an exercise therapy that involves sufficient intensity, duration, and frequency to enhance aerobic capacity and muscle strength and positively impact the functionality of patients with rheumatoid arthritis (RA)13. Based on the American College of Sports Medicine, dynamic exercise refers to the practice of aerobic exercise where the maximum heart rate (HRmax) is maintained between 55% and 80%31.
This type of exercise encompasses changes in body positions, enabling targeted work on joint mobility ranges. Moreover, it combines various components such as aerobic exercise, strength training, flexibility exercises, and coordination exercises. Our program is based on the Rheumatoid Arthritis Patients in Training (RAPIT) protocol, which has demonstrated its safety and effectiveness for patients with similar characteristics to ours15.
The exercise program presented here was designed to be applied to patients with RA with functional classes I to III. Patients with functional class IV are not suitable to carry out this program due to functional limitations and dependence when carrying out any activity17. The exercise program can be safely applied to overweight or obese patients with RA, as cardiovascular factors are taken into account to ensure safety. Patients with total or partial arthroplasties are also not suitable to carry out the program since joint dynamics are altered. It is not recommended to carry out this exercise protocol without prior supervision or instruction from an expert since it is necessary to understand the joint situation, disease activity, and disability level to avoid putting stress on the joints or causing pain or inflammation in the joints. The exercise program proposed in this study has an extended duration of 6 months. However, we did not assess changes in hydration or cellular mass before this period. Therefore, future investigations could explore interventions of shorter durations to determine if any changes occur in these aspects.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank professors Piccoli and Pastori of the Department of Medical and Surgical Sciences, University of Padova, Italy, for providing the BIVA software. Also, to Dr. Luis Llorente and Dra. Andrea Hinojosa-Azaola from the Department of Immunology and Rheumatology at the INCMNSZ for rheumatological assessment of patients. This work was supported by the CONACyT which sponsored the scholarship CVU 777701 for Mariel Lozada Mellado during his Ph.D. course study and through the Research Project Grant 000000000261652. The sponsor did not have any role in the study design or in the collection, analysis, or interpretation of data, nor in the writing of the report and in the decision to submit the paper for publication.
Materials
| Alcohol 70% swabs | NA | NA | Any brand can be used |
| bicycle ergometer | NA | NA | Any brand can be used |
| BIVA tolerance software 2002 | NA | NA | Is a sofware created for academic use, can be download in http://www.renalgate.it/formule_calcolatori/bioimpedenza.htm in "LE FORMULE DEL Prof. Piccoli" section |
| BIVA confidence software | NA | NA | Is a sofware created for academic use, can be download in http://www.renalgate.it/formule_calcolatori/bioimpedenza.htm in "LE FORMULE DEL Prof. Piccoli" section |
| Chair | NA | NA | Any brand can be used |
| Chlorhexidine | NA | NA | Any brand can be used, 0.05% |
| Examination table | NA | NA | Any brand can be used |
| Leadwires square socket | BodyStat | SQ-WIRES | |
| Long Bodystat 0525 electrodes | BodyStat | BS-EL4000 | |
| Plastic ball | NA | NA | Any brand can be used, 30 cm |
| Pulse oximeter | NA | NA | Any brand can be used |
| Quadscan 4000 equipment | BodyStat | BS-4000 | Impedance measuring range: 20 – 1300 Ω ohms Test Current: 620 μA Frequency: 5, 50, 100, 200 kHz Accuracy: Impedance 5 kHz: +/- 2 Ω Impedance 50 kHz: +/- 2 Ω Impedance 100 kHz: +/- 3 Ω Impedance 200 kHz: +/- 3 Ω Resistance 50 kHz: +/- 2 Ω Reactance 50 kHz: +/- 1 Ω Phase Angle 50 kHz: +/- 0.2° Calibration: A resistor is supplied for independent verification from time to time. The impedance value should read between 496 and 503 Ω. |
| Resistence bands | NA | NA | Any brand can be used, with resistence of 0.5 kg to 3.2 kg |
| Stationary bicycle | NA | NA | Any brand can be used |
| Treadmill | NA | NA | Any brand can be used |
| Wooden stick | NA | NA | Any brand can be used, 1.5m in large and <1kg |
Referenzen
- Aletaha, D., et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the Rheumatic Diseases. 62 (9), 1580-1588 (2010).
- Gamal, R. M., Mahran, S. A., Abo El Fetoh, N., Janbi, F. Quality of life assessment in Egyptian rheumatoid arthritis patients: Relation to clinical features and disease activity. Egyptian Rheumatologist. 38 (2), 65-70 (2016).
- Rall, L. C., Roubenoff, R. Rheumatoid cachexia: metabolic abnormalities, mechanisms, and interventions. Rheumatology. 43 (10), 1219-1223 (2004).
- Summers, G. D., Deighton, C. M., Rennie, M. J., Booth, A. H. Rheumatoid cachexia: A clinical perspective. Rheumatology. 47 (8), 1124-1131 (2008).
- Elkan, A. C., Engvall, I. L., Cederholm, T., Hafström, I. Rheumatoid cachexia, central obesity and malnutrition in patients with low-active rheumatoid arthritis: Feasibility of anthropometry, Mini Nutritional Assessment, and body composition techniques. European Journal of Nutrition. 48 (5), 315-322 (2009).
- Engvall, I. L., et al. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scandinavian Journal of Rheumatology. 37 (5), 321-328 (2008).
- Jacobs, D. O. Bioelectrical Impedance Analysis: Implications for Clinical Practice. Nutrition in Clinical Practice. 12 (5), 204-210 (1997).
- Santillán-Díaz, C., et al. Prevalence of rheumatoid cachexia assessed by bioelectrical impedance vector analysis and its relation with physical function. Clinical Rheumatology. 37 (3), 607-614 (2018).
- Piccoli, A., et al. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. The American Journal of Clinical Nutrition. 61 (2), 269-270 (1995).
- Espinosa-Cuevas, M. A., et al. Vectores de impedancia bioeléctrica para la composición corporal en población mexicana [Bioelectrical impedance vectors for body composition in Mexican population]. Revista de investigación clínica [Clinical research journal]. 59 (1), 15-24 (2007).
- Piccoli, A., Pillon, L., Dumler, F. Impedance vector distribution by sex, race, body mass index, and age in the United States: standard reference intervals as bivariate Z scores. Nutrition. 18 (2), 153-167 (2002).
- Maese, J., García De Yébenes, M. J., Carmona, L., Hernández-García, C. Estudio sobre el manejo de la artritis reumatoide en España (emAR II) [Study on the management of rheumatoid arthritis in Spain (emAR II)]. Características clínicas de los pacientes [Clinical characteristic of patients]. Reumatología Clinica. 8 (5), 236-242 (2012).
- Hurkmans, E., Van der Giesen, F. J., Vlieland, T. P. M. V., Schoones, J., Van den Ende, E. C. H. M. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database of Systematic Reviews. 4, CD006853 (2009).
- Baillet, A., et al. Efficacy of cardiorespiratory aerobic exercise in rheumatoid arthritis: Meta-analysis of randomized controlled trials. Arthritis Care & Research. 62 (7), 984-992 (2010).
- De Jong, Z., et al. Long-term follow-up of a high-intensity exercise program in patients with rheumatoid arthritis. Clinical Rheumatology. 28 (6), 663-671 (2009).
- García-Morales, J. M., et al. Effect of a dynamic exercise program in combination with Mediterranean diet on quality of life in women with rheumatoid arthritis. Journal of Clinical Rheumatology. 26 (2), S116-S122 (2019).
- Munneke, M., et al. Effect of a high-intensity weight-bearing exercise program on radiologic damage progression of the large joints in subgroups of patients with rheumatoid arthritis. Arthritis & Rheumatism. 53, 410-417 (2005).
- Hochberg, M., Chang, R., Dwosh, I., Lyndsey, S., Pincus, T., et al. The American College of Rheumatology 1991 Revised Criteria for the Classification of Global Functional Status in Rheumatoid Arthritis. Arthritis & Rheumatism. 35, 498-502 (1991).
- Nikiphorou, E., Konan, S., MacGregor, A. J., Haddad, F. S., Young, A. The surgical treatment of rheumatoid arthritis. Bone Joint Journal. 96 (10), 1287-1289 (2014).
- Jacqueline, B., et al. Rheumatoid Arthritis: A Brief Overview of the Treatment. Medical Principles and Practice. 27 (6), 501-507 (2019).
- Piccoli, A., Rossi, B., Pillon, L., Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney International. 46 (2), 534-539 (1994).
- Benatti, F. B., Pedersen, B. K. Exercise as an anti-inflammatory therapy for rheumatic diseases – Myokine regulation. Nature Reviews Rheumatology. 11 (2), 86-97 (2015).
- Cooney, J. K., et al. Benefits of Exercise in Rheumatoid Arthritis. Journal of Aging Research. 6, 297-310 (2011).
- Barbosa-Silva, M. C. G., Barros, J. D. Bioelectrical impedance analysis in clinical practice: a new perspective on its use beyond body composition equations. Current Opinion. Clinical Nutrition and Metabolic Care. 8 (3), 311-317 (2005).
- Mulasi, U., Kuchnia, A. J., Cole, A. J., Earthman, C. P. Bioimpedance at the bedside: current applications,limitations, and opportunities. Nutrition in Clinical Practice. 30 (2), 180-193 (2015).
- Steihaug, O. M., Bogen, B., Kristoffersen, M., Ranhoff, A. Bones, blood and steel: How bioelectrical impedance analysis is affected by a hip fracture and surgical implants. Journal of Electrical Bioimpedance. 8, 54-59 (2017).
- Nwosu, A. C., et al. Bioelectrical impedance vector analysis (BIVA) is a method to compare body composition differences according to cancer stage and type. Clinical nutrition ESPEN. 30, 59-66 (2019).
- Martins, P. C., Gobbo, L. A., Silva, D. A. S. Bioelectrical impedance vector analysis (BIVA) in university athletes. Journal of the International Society of Sports Nutrition. 18 (7), 1-8 (2021).
- Norman, K., Pirlich, M., Sorensen, J., Christensen, P., Kemps, M., Schütz, T., Lochs, H., Kondrup, J. Bioimpedance vector analysis as a measure of muscle function. Clinical Nutrition. 28 (1), 78-82 (2009).
- Stagi, S., et al. Usability of classic and specific bioelectrical impedance vector analysis in measuring body composition of children. Clinical nutrition. 41 (3), 673-679 (2022).
- Garber, C. E., et al. American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine & Science in Sports & Exercise. 43 (7), 1334-1359 (2011).

