藻類培養手法を介して列挙
English
Teilen
Übersicht
ソース: ドクターペッパー イアン博士チャールズ Gerba – アリゾナ大学所
示す著者: ブラッドリー ・ シュミッツ
藻類は、1 つの共通の特徴、すなわち光合成色素の保有微生物の非常に異種のグループです。環境、水で成長して、藻類によってプールの所有者に問題が発生します。藻類も海面、湖や貯水池、アオコの毒素を解放するためなどで問題が発生します。最近では、藻が藻類バイオ燃料によるエネルギーの新しい源として評価されています。青緑色藻類、シアノ バクテリアに分類される細菌実際に。シアノ バクテリアは光合成、だけでなく、また大気中の窒素ガスを修正する能力を持っています。他の藻類は真核生物、海草のような複雑な多細胞生物の単細胞生物に至るです。緑の藻、euglenoids、渦鞭毛藻、黄金の褐藻、珪藻、褐藻、紅藻が含まれます。土壌、藻類の人口が頻繁に 10 グラムあたり6 。これらの数字は、光合成に必要な日光が地表面下まで突き通すことができないので、主に細菌、放線菌、糸状菌の対応する番号より低いです。
藻は光合成であるため光合成・炭酸ガス、バイオマスの炭素からエネルギーを得る彼ら育てることができる完全無機栄養素と有機炭素基板なしで構成される成長媒体。有機基板の欠如には、従属栄養細菌の増殖ができなくなります。無機成長媒体を使用して、藻類はもともと土壌に存在または水は最確数 (MPN) 法で測定することができます。藻類自体が絶滅の危機に希釈されるよう、MPN 法は連続して、サンプルを希釈することに依存します。希釈する藻類の存在は、藻類の光合成に起因する緑のスライムは、通常、媒体の成長の肯定的な記号によって決まります。各希釈でレプリケート チューブの使用と指定された希釈する成長の肯定的なチューブの数の統計的評価できます藻オリジナル サンプルで現在の数を計算します。MPN テーブルを開発し、各希釈で使用される複製の数を含む特定の MPN 設計に固有を公開します。
Verfahren
Ergebnisse
Figure 2 is an example of representative results.
p1 is chosen to be the number of replicate tubes of the highest dilution (least concentrated in soil) that has the highest number of positive tubes. Here, the replicates from Tube B do not count, because those of Tube C are from a higher dilution. In contrast, the number of tubes from Tube D that show a positive sign of growth is less than those from Tube C. So, p1 = 5.
p2 and p3 are chosen to be the number of tubes in the next two higher dilutions that show a positive sign of growth. Thus, p2 = 3 and p3 = 1.
The value for p1 can be found by looking down the first column in Table 2. The same is done in the p2 column. Then, the value of p3 (across the top) intersects the two defined by the values of p1 and p2. In this example, the value is 1.1 organisms per mL.
Divide this value by the concentration of soil in the dilution to which you assigned p2. In this example, this is Tube D.
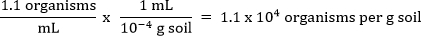
Thus, in this example, there were 1.1 x 104 algae cells per g of soil. This value is fairly typical of the number of algae found in soil.
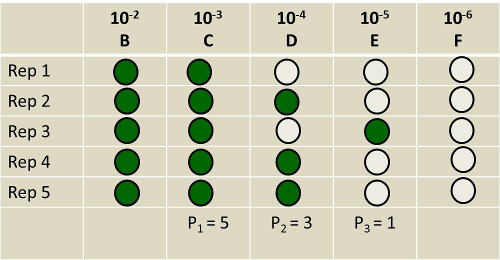
Figure 2. Hypothetical outcome of an algae enumeration experiment. Shaded tubes indicate the presence of algae. Un-shaded tubes represent the absence of algae.
| Most probable number for indicated values of p3 | |||||||
| p1 | p2 | 0 | 1 | 2 | 3 | 4 | 5 |
| 0 0 0 0 0 0 |
0 1 2 3 4 5 |
— 0.018 0.037 0.056 0.075 0.094 |
0.018 0.036 0.055 0.074 0.094 0.11 |
0.036 0.055 0.074 0.093 0.11 0.13 |
0.054 0.073 0.092 0.11 0.13 0.15 |
0.072 0.091 0.11 0.13 0.15 0.17 |
0.090 0.11 0.13 0.15 0.17 0.19 |
| 1 1 1 1 1 1 |
0 1 2 3 4 5 |
0.020 0.040 0.061 0.083 0.11 0.13 |
0.040 0.061 0.082 0.1 0.13 0.16 |
0.060 0.081 0.10 0.13 0.15 0.17 |
0.080 0.10 0.12 0.15 0.17 0.19 |
0.10 0.12 0.15 0.17 0.19 0.22 |
0.12 0.14 0.17 0.19 0.22 0.24 |
| 2 2 2 2 2 2 |
0 1 2 3 4 5 |
0.045 0.068 0.093 0.12 0.15 0.17 |
0.068 0.092 0.12 0.14 0.17 0.20 |
0.091 0.12 0.14 0.17 0.20 0.23 |
0.12 0.14 0.17 0.20 0.23 0.26 |
0.14 0.17 0.19 0.22 0.25 0.29 |
0.16 0.19 0.22 0.25 0.28 0.32 |
| 3 3 3 3 3 3 |
0 1 2 3 4 5 |
0.078 0.11 0.14 0.17 0.21 0.25 |
0.11 0.14 0.17 0.21 0.24 0.29 |
0.13 0.17 0.20 0.24 0.28 0.32 |
0.16 0.20 0.24 0.28 0.32 0.37 |
0.20 0.23 0.27 0.31 0.36 0.41 |
0.23 0.27 0.31 0.35 0.40 0.45 |
| 4 4 4 4 4 4 |
0 1 2 3 4 5 |
0.13 0.17 0.22 0.34 0.41 |
0.17 0.21 0.26 0.33 0.40 0.48 |
0.21 0.26 0.32 0.39 0.47 0.56 |
0.25 0.31 0.38 0.45 0.54 0.64 |
0.30 0.36 0.44 0.52 0.62 0.72 |
0.36 0.42 0.5 0.59 0.69 0.81 |
| 5 5 5 5 5 5 |
0 1 2 3 4 5 |
0.23 0.33 0.49 0.79 1.3 2.4 |
0.31 0.46 0.7 1.1 1.7 3.5 |
0.43 0.64 0.95 1.4 2.2 5.4 |
0.58 0.84 1.2 1.8 2.8 9.2 |
0.76 1.1 1.5 2.1 3.5 16 |
0.95 1.3 1.8 2.5 4.3 — |
Table 2. Most probable numbers for use with the experimental design in this exercise.
Applications and Summary
The MPN methodology is useful, because it allows estimation of a functional population based on a process-related attribution. In the example, the functional process was photosynthesis undertaken by algae, which allowed for growth in the absence of organic carbon. This allowed for total algal populations in soil to be enumerated.
MPN is also used to estimate the number of a particular type of microbial pathogens in water, such as Salmonella, utilizing the resistance of Salmonella to malachite green.
A further application is the estimation of mycorrhizal fungi by inoculating soil dilutions onto a plant host and looking for root colonization by the fungi.
Transkript
Algae are photosynthetic organisms that live in a variety of environments. Soil dwelling algae can be cultured in the laboratory, and their concentration enumerated using simple calculations.
Algae are a highly heterogeneous group of organisms that have one common trait, namely the possession of photosynthetic pigments, commonly chlorophyll. The vast majority of algae are microscopic, however, the exact definition of the group is controversial, and also includes seaweeds, which are typically macroscopic.
In the environment, algae can cause problems in surface waters such as lakes or reservoirs, forming algal blooms that deplete the water nutrients, blocking light passing beyond the water surface, and releasing toxins. The ability to enumerate algae in samples allows scientists to evaluate the health of an ecosystem, and the potential risk of algal overgrowth.
Algal populations in soils frequently occur at around ten thousand cells per gram. These numbers are typically lower than corresponding concentrations of bacteria, fungi, or actinomycetes, as algae require sunlight for photosynthesis, which cannot penetrate far below the soil surface.
This video will illustrate how to culture algae from soil in the laboratory, and how to enumerate the concentration of algae in the starting soil sample.
Algae have beneficial effects on ecosystems. Blue-green algae, or cyanobacteria, have the ability to fix nitrogen gas from the atmosphere, making them useful in increasing soil nitrogen in semi-arid environments and also as a potential tool for biofuel production.
Other algae are eukaryotic, and range from single-celled to complex multicellular organisms, like seaweeds. These include green algae, euglenoids, dinoflagellates and diatoms, brown algae, and red algae.
Algae are phototrophic, obtaining energy from photosynthesis and carbon for biomass from carbon dioxide. As a result, they can be grown in media consisting entirely of inorganic nutrients, without an added organic carbon substrate. This lack of organic substrate prevents the growth of heterotrophic bacteria, which are dependent on external organic carbon for growth.
To culture algae for enumeration, soil samples are serially diluted tenfold to 10-6 g soil per mL, and cultured in growth media. Several replicates are made for each dilution. They are then incubated in a well-lit area for up to 4 weeks to allow algal growth.
The presence of algae in any dilution is determined by a positive sign of growth in the medium, which will typically appear as a green slime. Finally, empirically developed MPN tables designed for algal growth are consulted, enabling the user to determine the original algal concentration based on growth in dilution replicates. The MPN method relies on the serial dilution of samples such that the algae are diluted to extinction, meaning that at some dilution, no algal growth ensues.
Now that we are familiar with the concepts behind growing and enumerating algae from samples, let’s take a look at how this is carried out in the laboratory.
To begin the experiment, first weight out 10 grams of moist soil that has either been collected moist from the field, or been rehydrated and remained moist for 2 to 3 days. The soil should but not saturated.
Next, prepare a ten-fold dilution series by adding the 10 grams of soil first to 95 mL of Modified Bristol’s solution, or MBS. Label this as suspension A.
After shaking vigorously, continue the dilution series by adding 1 mL of suspension A to 9 mL of MBS in a test tube. Continue this ten-fold dilution series another 4 times to give dilutions up to 10-6 g per mL.
Next, inoculate 5 replicate tubes, each containing 9 mL of MBS with 1 mL of each of the dilutions 10-1 to 10-5. This results in 5 replicates tubes for each dilution from 10-2 to 10-6. Cap the tubes loosely.
Finally, incubate the tubes for a full 4 weeks in an area exposed to sunlight. Observe the tubes for algal growth once every 7 days. Tubes exhibiting algal growth will appear green.
Most Probable Number, or MPN, analysis is a commonly used mathematical method to enumerate microorganisms grown from dilution of a concentrated initial substrate. By taking into account the dilution factors of the solutions, and the number of tubes which show positive signs of growth at each dilution, the most probable number of organisms per gram of original soil sample can be calculated using an MPN table and simple formula.
To calculate MPN, the highest dilution with the highest number of positive replicate tubes is assigned the label of p1, in this case, the replicates of tube C. In contrast, some of the tubes from D & E are negative with no signs of algal growth.
The number of tubes in the next two higher dilutions that show positive growth are labeled as p2 and p3. Here, p2 = D and p3 = E.
The value for p1 can be found by looking down the first column in the MPN table. The same should be done with the p2 column. Finally, the value of p3, across the top, is used to intersect the two defined by p1 and p2, to give a value of the most probable number of organisms per mL.
Next, to calculate the concentration of organisms per gram in the original soil sample, this value is divided by the concentration of soil in the dilution to which p2 was assigned. The following equation is used to define the actual number of organisms per gram of soil.
Algal enumeration and MPN analysis have a wide range of applications, some of which are explored here.
This culturing method of algal enumeration can be used in a variety of settings. It can be applied to rivers or lakes to determine algal levels, and assess the risks of harmful algal blooms. Alternatively, it can be used to assess the cleanliness and safety of waters more directly used by humans, including swimming pools, water fountains, or other drinking water sources. Ideally, in potable water samples and swimming pools, there are no algae present.
The MPN analysis for enumeration can also be applied to other non-algal microorganisms. For example, water quality can be assessed using indicator organisms such as coliforms or E. coli. Here, samples can be cultured with media containing chemicals that are altered to produce color or fluorescence in the presence of the indicator organisms. By performing multiple small replicates of this experiment in individual cells, with samples diluted to a known concentration, the ratio of positive cells can be referenced to an MPN table for the specific indicator organism, and the starting concentration in the samples determined.
Algae may also be cultured for commercial applications. For example, some types of biofertilizer utilize blue-green algae, which can act as symbionts with plants, aiding their fixture and take-up of nitrogen, which is particularly useful in aiding crop growth in areas with poor soil. Similarly, algae can be grown for biofuels, or as a source of nutrient rich food for livestock.
You’ve just watched JoVE’s introduction to algal culture and enumeration. You should now understand how to dilute soil samples for algal growth, how to culture algae in the laboratory, and how to enumerate the algal concentration of your starting samples. Thanks for watching!



