Conducting Reactions Below Room Temperature
English
Teilen
Übersicht
Source: Laboratory of Dr. Dana Lashley – College of William and Mary
Demonstration by: Matt Smith
When new bonds are formed in the course of a chemical reaction, it requires that the involved species (atoms or molecules) come in very close proximity and collide into one another. The collisions between these species are more frequent and effective the higher the speed at which these molecules are moving. A widely used rule of thumb, which has its roots in the Arrhenius equation1, states that raising the temperature by 10 K will approximately double the rate of a reaction, and raising the temperature by 20 K will quadruple the rate:
(1) 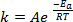
Equation (1) is often found in its logarithmic form:
(2) 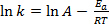
where k is the rate of the chemical reaction, A is the frequency factor (relating to frequency of molecular collisions), Ea is the activation energy required for the reaction, R is the ideal gas constant, and T is the temperature at which the reaction is taking place.
A higher temperature therefore means a reaction is completed much faster. Nonetheless, in some cases it is desirable to carry out reactions at low temperatures, in spite of the lowering effect on the rate of the reaction. A few scenarios in this regard are elaborated upon further below.
When it is useful to run a reaction below room temperature, chemists use cooling baths to maintain a certain temperature or temperature range. Reactions are cooled down to a desired temperature by placing the reaction flask inside an appropriate cooling bath. The reagents in the reaction never come in direct contact with the chemicals in the cooling bath. The cooling bath may consist of a single cryogenic (cooling) component (such as ice, dry ice, or liquid nitrogen) or may be a mixture of the cryogenic component with a certain solvent and/or an additive salt. The purpose of the solvent is to effectively transfer temperature of the cooling agent to the reaction flask, and the purpose of the additive is to lower (or depress) the freezing point of the mixture. (Note that it is possible for a substance to be both a solvent and an additive.)
Grundsätze
Verfahren
Applications and Summary
When is it useful to run a reaction at a low temperature?
In order to answer this question let us investigate four different applications:
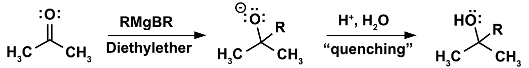
Application 1. Sometimes reactions are too vigorous and exothermic and the reaction mixture must be cooled in order to prevent spilling and pressure build-up due to gas development. A highly exothermic reaction can also become a safety hazard as the reaction mixture can rapidly boil over (many organic solvents usually have low boiling points) and spurt out. A very common application for this is the quenching or work-up step where a reaction initially carried out under anhydrous conditions is reacted with water and acid at the end in order to protonate the final product and to react off any remaining reactive intermediates and reactants. For example, in the Grignard reaction, a very common reaction in organic chemistry, the quenching step at the end will require cooling, even though an ice-water bath at 0 °C will suffice:
(4) 
Application 2. Cooling can also be required for addition steps at the beginning of a reaction, when an exothermic reaction would otherwise result in the boiling off of the organic solvent. This is undesirable, because reactions are best carried out in solvents. Having to add more solvent to compensate for the loss of solvent is not only wasteful and uneconomical but also tedious as solvents in many reactions require a prior drying step to make them anhydrous. Moreover, it is possible for certain reagents to thermally decompose at higher temperatures.
To avoid these occurrences in an exothermic reaction, a reagent is often added dropwise by syringe or dropping-funnel to a flask containing another reagent in solvent, while stirring and cooling. This way, the addition can be stopped anytime if the reaction becomes too vigorous. Often, the reaction must be cooled well below 0 °C and an ice-water bath does not suffice.
An example for a reaction where this is necessary is the addition of the strong base n-butyllithium (n-BuLi) to diisopropylamine to form lithium diisopropylamide (LDA).
(5) 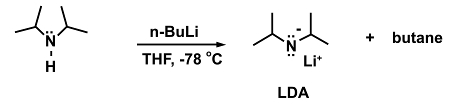
In the absence of a cooling bath the n-BuLi may decompose as higher temperatures are reached:
(6) 
Application 3. In some chemical reactions there is more than one possible product resulting from a competing chemical pathway. One product may be the result of the pathway with a more stable transition state, requiring less activation Energy (Ea1), while the other product may require more activation energy (Ea2) but is overall more stable. The former is called the kinetic product while the latter is called the thermodynamic (TD) product (see energy diagram in Figure 2).
By controlling the reaction temperature we can control which one of these products is formed. Because the kinetic product requires less activation energy it is the product that is formed at low temperatures. Conducting a reaction at low temperatures often ensures the formation of the kinetic product over the thermodynamic product.
A classical example in the realm of enolate chemistry is the reaction of 2-methylcyclohexanone with different bases under different reaction conditions. The reactant is an unsymmetrical ketone and therefore possesses two different types of α- hydrogens. Small bases, such as NaOH deprotonate the ketone at the more-highly substituted side, which results in the more stable, thermodynamic enolate (7). Bases, which are more sterically demanding, deprotonate the ketone on the less hindered side, resulting in the kinetic enolate (8). The formation of the kinetic enolate will have a much higher yield when the reaction is carried out at -78 °C as compared to room temperature. The two forms of the enolate can then be reacted with an appropriate electrophile, such as methyiodide, to form the α-alkylated products shown below.
(7)(8)
The sterically demanding base used to obtain the kinetic enolate is often LDA, the preparation of which was shown earlier in scheme (5). It is important to control the temperature to -78 °C to prevent the kinetic enolate to equilibrate back into the thermodynamic enolate. (Note: there is no significance to the temperature of -78 °C other than that it is easily obtained by a dry-ice-bath in acetone.)
Aside from temperature control, the addition order and manner of addition of reagents is crucial. For best results favoring the kinetic enolate, a solution of the ketone reactant is added dropwise to the LDA base in solvent. The anhydrous solvent used for the reaction with LDA is often THF. An example reaction is shown in scheme (9).
(9) 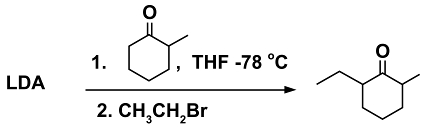

Figure 2. Energy diagram for a reaction that has a kinetic and a thermodynamic product.
Application 4. In some cases it is possible to regulate the reactivities of reagents with temperature. Consider for example the reduction of an ester. Reactions with the strong hydride reducing agent lithium aluminum hydride (LAH) result in the reduction of the ester all the way down to the respective primary alcohol (10). However, use of the bulky hydride reducing agent diisobutylaluminum hydride (DIBAL) allows for selective reduction of an ester to the respective aldehyde. Over-reduction to the primary alcohol can be avoided, so long as the reaction temperature is kept at below -78 °C (yet better down to -90 °C ) and only one stoichiometric equivalent of DIBAL is used (12). At temperatures above -70 °C, DIBAL becomes too reactive and will reduce the ester to the primary alcohol (11).
(10)-(12)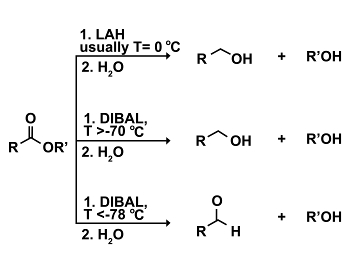
Referenzen
- Gordon, A. J., Ford, R. A. The Chemist's Companion – A Handbook of Practical Data, Techniques, and References. Chapter 11 (1973) ISBN: 978-0-471-31590-2.
- Rondeau, R. E. Slush baths. J. Chem. Eng. Data. 11, 124 (1966)
Transkript
Certain chemical reactions must be performed below room temperature for safety or to obtain the desired product.
A cooling bath allows for a system to be maintained at a certain temperature range for the duration of the reaction. This is achieved by placing the reaction flask into the bath, cooling the reaction without ever having direct contact with the reagents.
The bath is typically a well-insulated vessel such as a Dewar flask containing the cryogenic components necessary to reach the desired temperature. In simple setups like this, temperature is not stable, and the bath must be monitored and adjusted throughout the procedure.
This video will explore the different cooling baths regularly used to carry out reactions below room temperature.
During a chemical reaction the species involved must collide for new bonds to form. Raising the temperature increases the internal energy of the system and will cause these species to move more quickly, meaning they will collide more often. As a result, reactions proceed faster at higher temperatures.
However, in some cases, it is desirable to carry out reactions at low temperatures, despite the lowering of the rate of reaction. For example, some reactions are too vigorous, and must be cooled to prevent spilling and pressure build up. Highly exothermic reactions could also rapidly boil over and spurt out if not cooled, creating a safety hazard.
Cooling can be utilized to provide an economic benefit. For example, preventing the boiling off of a solvent or the decomposition of a reagent saves both time and resources.
Cooling is also frequently used to control which product is yielded by a reaction that has competing pathways. In these reactions the pathway with the lower activation energy is generated at lower temperatures, while the pathway with the higher activation energy is preferred at higher temperatures.
Now that you understand the importance of running reactions below room temperature, let’s take a look at how to prepare various types of cooling baths.
Ice-water baths are easy to set up, and are available in every teaching chemistry laboratory. While ice-water itself has a temperature of 0 °C, a melting-point depression can be achieved by the addition of certain salts.
This allows ice-water baths to reach a temperature of -40 °C. The final temperature can be adjusted by increasing or decreasing the concentration of salt additive.
To set up an ice-water bath, begin by weighing the appropriate amounts of ice and salt additive, as outlined in the ice-bath table found in the text protocol.
Next, add the salt to the ice. Pour a small amount of deionized water into the container. Using a stirring rod, mix the bath thoroughly.
Now that the bath has been set up, check with a thermometer to ensure that the desired temperature has been reached. If it has not, add more salt as necessary. When the correct temperature is reached, place the reaction vessel into the ice bath.
Ice-water baths do not retain their temperature long, and need to be adjusted every 20–30 min. To maintain the target temperature, it may be necessary to remove the liquid water and add more ice and salt.
For temperatures down to -78 °C, dry-ice baths are utilized. Dry-ice is solid carbon dioxide, so efficient heat-transfer from it to a reaction vessel requires a solvent. Because dry-ice sublimes at -78 °C, a solvent with a freezing point below that must be used if this temperature is to be reached. Solvents with higher freezing points can be utilized to create warmer dry-ice baths. To prepare a dry-ice bath, begin by putting on cryogenic protection gloves and safety goggles. Never let dry-ice touch bare skin.
For a 1 L bath, obtain about 1/3 of a block of dry-ice and break it into smaller pieces into the container.
Next, slowly add the chosen organic solvent to the dry-ice while stirring with a glass rod. There will be a vigorous fizzing as carbon dioxide gas develops.
Continue to slowly add solvent and stir until most of the dry-ice dissolves, forming a homogenous slurry. This ensures that heat transfer to the reaction flask is as uniform as possible.
Using a cold temperature thermometer or thermocouple, ensure that the bath has reached the desired temperature, then place the reaction vessel into the bath.
Monitor the bath in regular intervals, and add chunks of dry-ice when a rise in the bath temperature is noticed.
Finally, when the desired bath temperature is below what dry-ice can provide, liquid nitrogen is utilized. Liquid nitrogen has a melting point of -196 °C, and solvents are only needed when creating warmer baths.
Due to the extremely low temperatures of liquid nitrogen, a Dewar is the only acceptable vessel.
To prepare a liquid-nitrogen cooling-bath, begin by putting on safety goggles and cryogenic protection gloves. Use care when handling liquid nitrogen, as it can cause frostbite and permanent eye damage.
For a bath with additives, determine the appropriate organic solvent for the desired temperature, as shown in the liquid nitrogen table found in the text. Add the solvent to the Dewar, then slowly add the liquid nitrogen.
Insert a cold-temperature thermometer or thermocouple into the bath to ensure that the desired temperature has been reached. Then, place the reaction vessel into the bath.
For a bath without additives, simply add the appropriate amount of nitrogen to the Dewar to obtain a temperature as low as -196 °C.
Monitor the bath in regular intervals to see if additional nitrogen is needed.
Many different types of reactions across various scientific disciples utilize cooling baths to operate below room temperature.
Mechanical laboratory processes, much like very exothermic reactions, can also create undesirable heat.
In this example bulk barium copper tetrasilicate was prepared through both solid state and melt flux synthesis. Then, these layered materials were exfoliated using sonication techniques.
Sonication uses sound waves to agitate particles. However, because it is a high-energy process, it can create excess heat in a sample.
Therefore, an ice-water bath was used to cool the sample during the one-hour sonication process. Preventing this excess heating ensured the integrity and consistency of product yield.
In this example, a dry-ice bath was used to ensure that diiodomethyllithium was synthesized by deprotonation of diiodomethane.
Reagents were added to a round-bottomed flask containing a stir bar. Then, the round-bottomed flask was placed in a Dewar. Dry-ice and acetone were added to the Dewar, and the entire apparatus was covered to minimize exposure to light. Maintaining low system energy was essential for the stability of the product.
Dry-ice and liquid nitrogen baths are frequently used as cold traps to condense samples. In particular, these cold traps can aid the safe transport of air-sensitive compounds while preventing contamination of equipment. In this example, a liquid nitrogen cold trap was used to condense a volatile and oxidation sensitive sample, for later preparation for mass spectrometrical analysis.
The system was first cleaned and heated, to remove any potential contaminants. The lockable test tube was then submerged in liquid nitrogen, to allow for condensation of the sample through the Schlenk line. The sample was then removed for analysis through mass spectrometry.
You’ve just watched JoVE’s introduction to conducting reactions below room temperature. You should now understand ice-water, dry-ice, and liquid nitrogen cooling baths, and why they are chemically important.
Thanks for watching!
