Studying Wnt Signaling During Patterning of Conducting Airways
Summary
The use of reporter mice coupled to whole mount and section staining, microscopy and in vivo assays facilitates the analysis of mechanisms underlying the normal patterning of the respiratory tract. Here we describe how these techniques contributed to the analysis of Wnt signaling during tracheal development.
Abstract
Wnt signaling pathways play critical roles during development of the respiratory tract. Defining precise mechanisms of differentiation and morphogenesis controlled by Wnt signaling is required to understand how tissues are patterned during normal development. This knowledge is also critical to determine the etiology of birth defects such as lung hypoplasia and tracheobronchomalacia. Analysis of earliest stages of development of respiratory tract imposes challenges, as the limited amount of tissue prevents the performance of standard protocols better suited for postnatal studies. In this paper, we discuss methodologies to study cell differentiation and proliferation in the respiratory tract. We describe techniques such as whole mount staining, processing of the tissue for confocal microscopy and immunofluorescence in paraffin sections applied to developing tracheal lung. We also discuss methodologies for the study of tracheal mesenchyme differentiation, in particular cartilage formation. Approaches and techniques discussed in the current paper circumvent the limitation of material while working with embryonic tissue, allowing for a better understanding of the patterning process of developing conducting airways.
Introduction
Respiratory tract development is initiated by embryonic day 9 (E9) with the appearance of Nkx2.1 positive cells in the ventral endodermal foregut1,2. Esophageal-tracheal tube separation will resolve by E11.5 when the tubes can be distinguished as distinct entities, each surrounded by mesenchymal tissue3. Wnt signaling plays a key role in the specification of the respiratory tract as deletion of Wnt2 and Wnt2b, expressed by the splanchnic mesenchyme and deletion of β-catenin from the endodermal respiratory epithelium will result in lung agenesis4,5. Our previous studies determined that deletion of Wls, a cargo receptor mediating secretion of all Wnt ligands, from the endodermal respiratory tract results in lung hypoplasia, defects in pulmonary vascular development and mis-patterning of the tracheal mesenchyme6,7. These data support the importance of the epithelial-mesenchymal cross talk in cell differentiation and specification, as it has also been shown in other studies8,9.
The study of the earliest stages of lung development relies upon genetic, in vitro and ex vivo techniques that have allowed us to better understand mechanisms driving respiratory identity10-16. Whole lung explant cultures at the air liquid interphase have been widely utilized to study the effects of growth factors in early stages of pulmonary branching morphogenesis10,17,18. While this method is used as readout of morphological changes, such as branching morphogenesis, and gene expression modulation, it is limited to the study of early stages of the developmental process, as the culture itself does not support the development of vasculature17. Development of tracheal cartilage requires longer incubation times that may be not compatible with this culture technique.
To analyze the role of Wnt signaling during respiratory tract formation, we have adapted standard techniques to meet the needs of our embryonic studies. We have modified volumes, staining times, processing cycling for paraffin embedding and timing for clearing of tracheal-lung tissue. The main goal of optimizing the techniques described in the present study was to analyze the earliest stages of tracheal development in mice that take place from E11 to E14.5. Using the reporter mice line Axin2LacZ we precisely determined sites of Wnt/β-catenin activity in the developing tracheal mesenchyme. We have also adapted lectin staining procedure for whole mount tracheal tissue. Thus, we were able to visualize mesenchymal condensations and predict sites where chondrogenesis will take place. Staining of whole mount and sections of embryonic tissue obtained from WlsShhCre mice, coupled with advanced microscopy techniques, allowed us to unveil the role of Wnt ligands produced by the tracheal epithelium in tracheal patterning.
Protocol
Animals were housed in pathogen-free conditions. Mice were handled according to protocols approved by CCHMC Institutional Animal Care and Use Committee (Cincinnati, OH USA). Mice utilized throughout these studies were maintained in a mixed background.
1. Whole Mount X-galactosidase Staining
- Euthanize pregnant female at E11.5 to E14.5, by CO2 inhalation. Place animals in CO2 chamber, charge the chamber with CO2. Maintain animals in chamber for minimum of 5 min. Perform secondary method of euthanasia by cervical dislocation.
- Clean abdominal area with ethanol (EtOH) 70% and perform laparotomy with a single abdominal midline incision. Utilize surgical scissors and standard pattern (straight serrate) forceps.
- Isolate uterine horns using forceps and fine scissors and immediately place tissue in petri dish containing chilled PBS solution. Maintain tissue on ice until proceeding to isolation of embryos.
- Isolate embryos from uterine horns. Dissect embryonic tracheal lung tissue using dissecting microscope. Utilize fine tip forceps and scissors to hold and puncture the uterine tissue and concepti releasing the embryos.
- Remove remaining embryonic membranes. With assistance of needle blades cut head of embryos and lower body part below the diaphragm. Locate liver as a landmark.
- After isolation of the thoracic region of the embryo, proceed to remove lateral sides of the body wall, spine and heart using needle blades. Take care while removing the heart to avoid injuring tracheal tissue. Finally, carefully, separate the esophagus from the trachea by pulling the esophagus with assistance of needle blades. Maintain tissue in PBS solution on ice.
- Place tissue in 4 ml screw cap vials using glass or transfer pipettes. Fix tracheal lung tissue in 4% paraformaldehyde (PFA) in PBS for 30 min. After fixation, rinse tissue with PBS. While rinsing, prepare X-gal staining solution (add 5 mM K3Fe(CN)6, (100 μl 0.5 M stock) 5 mM K4Fe(CN)6 (100 μl 0.5 M stock) 2 mM MgCl2, (20 μl 1 M stock) 0.01% NaDOC (50 μl 2% stock), 0.02% NP4O (100 μl 2% stock), 1 mg/ml X-gal (500 μl of 20 mg/ml stock) and 9.13 ml of distilled water to bring to a final volume of 10 ml).
- Remove any remaining PBS and add 2 ml of X-gal staining solution to the glass vial. Place vials in tray on shaker. Stain tissues 1 to 2 hr in X-gal staining solution. Incubate the tissue in X-gal solution for 4 hr for further processing and embedding.
- Stop the reaction by washing tissues in 3% dimethyl sulfoxide-PBS. Rinse in PBS, 3 times for 5 min each wash and store in 70% ethanol.
- Fill Petri dishes with a 1% agarose/PBS solution. Allow agarose to solidify. Using glass transfer pipettes, place the tissue on agarose coated plates filled with PBS solution. Photograph whole mounts using a dissecting microscope. Select appropriate filter for brightfield.
- To better distinguish sites of X-gal staining process samples for paraffin embedding and sectioning. Place samples in fine screen cassettes.
- To process samples for paraffin embedding use an automated processor and set up a short cycle as follows: six changes of alcohol: 5 min first change and 3 min per each remaining change. Three changes of Xylene: 5 min first change and 3 min next two changes. The previous steps are performed at 30 °C. Finally, perform three changes of paraffin at 62 °C: 25 min, 8 min and 5 min.
- Orient samples for embedding with tissue lying flat on the bottom of embedding boat. Carefully add paraffin to fill the embedding boat. Cool immediately on cold plate of embedding station until paraffin solidifies. Remove block from embedding boat. Using a microtome, generate 6 μm sections. Mount sections on pretreated-slides and dry slides on slide warmer set at 42 °C for 1 hr.
- Bake slides overnight in oven at 56 ºC. Place slides in racks. Deparaffinize slides with three changes of 100% Xylene, 10 min each. Use staining dishes filled with 200 ml of Xylene.
- Rehydrate slides using graded EtOH series (100%, 70%, 50% and 30%, 1 min per step) to PBS before counterstaining with Nuclear Fast Red for 10 to 30 sec. Rinse slides with tap water three times for 5 min each time to remove excess of Nuclear Fast Red.
- Dehydrate slides in a graded EtOH series (50%, 70% and 100% EtOH) before washing in three changes of Xylene (20 dips each change) and cover slipping with Xylene based mounting media.
2. Lectin Staining
- Dissect E13.5 and E14.5 tracheal lung tissue as described in steps 1.1 to 1.4. Using glass transfer pipettes place tissue in screw cap vials. Fix tracheal lung tissue with 2 ml of 2% PFA solution overnight at 4 °C. After fixation, rinse samples in PBS three times for 10 min each wash.
- Prepare blocking buffer, usually a final volume of 10 ml of solution. Add 0.1 g of BSA (1%) to 8 ml of PBS. Allow BSA to dissolve. Add 0.2 ml of goat serum (2%) 0.03ml of Triton X-100 (0.3%). Bring final volume to 10 ml with PBS. Block samples for 1 hr in 0.5 ml of blocking buffer at room temperature.
- Remove blocking buffer using a transfer pipette and replace with lectin solution comprised of 50 μg/μl of lectin, 10% goat serum and PBS. Use 250 μl of lectin solution per sample. Incubate overnight at 4 °C. Make sure to cover the glass vial containing tissue with aluminum foil. After incubation with lectin solution, rinse samples in PBS three times for 10 min.
- Place explants in agarose coated petri dishes containing enough PBS to cover samples. Photograph whole mounts using a fluorescence-dissecting microscope. Select the appropriate filter to detect fluorescence. Lectin PNA is usually coupled to GFP.
3. Whole Mount Immunofluorescence Staining and Confocal Microscopy
- Isolate tracheal lung tissue, transfer to 4 ml glass screw cap vials and fix overnight in 4% PFA. E11.5 tissue can be fixed in 2% PFA. Store in methanol 100%. Samples can be stored up to several months at -20 °C.
- Remove methanol using transfer pipette and permeabilize samples using Dent's Bleach (4 parts Methanol, 1 part DMSO and 1 part 30% H2O2) for 2 hr. Hydrate tissue in a graded series of methanol diluted in PBS as follows: 100% methanol, 75% methanol, 50% methanol, 25 % methanol, 100% PBS. Incubate 10 min at room temperature during each step of hydration.
- Block tissue in 0.5% blocking reagent diluted in PBS (see Materials for details) for two hours at room temperature with agitation.
- Dilute primary antibody in blocking solution (Sox9 1:100, Nkx2.1 1:200, αSMA 1:250) and incubate samples overnight at 4 °C. Wash samples with PBS five times at room temperature. Perform each wash for 1 hr.
- Apply secondary antibody at a 1:500 dilution in 0.5% blocking solution. Carefully select secondary antibodies to prevent binding among secondary antibodies. Incubate overnight at 4 °C in dark room. Wash samples three times for 20 min at room temperature. Dehydrate samples in a graded series of methanol diluted in PBS as follows: 25% methanol, 50% methanol 75% methanol, 100% Methanol, 10 min each step.
NOTE: Ensure that samples remain covered with aluminum foil and minimize exposure to light. Tissue can now be stored at 4 °C for several weeks until photographed. - Transfer samples to custom made stage plates, remove methanol and clear with approximately 200 μl of Murray's clear solution19,20 (2 parts Benzyl benzoate, 1 part Benzyl alcohol) immediately before imaging. Photograph using a confocal microscope. Process and analyze image using imaging software.
4. Cell Proliferation
- Inject E11.5 pregnant mice intra-peritoneally with a solution of BrdU at a concentration of 100 μg BrdU/g of body weight. Sacrifice female as described in section 1 of the protocol and isolate embryos at E12.5 or E13.5. Using knife blades, excise heads and lower part of body below thoracic cavity.
- Place thoracic tissue in 4 ml screw cap vials and fix in 1 ml of 4% PFA overnight. Wash samples with PBS twice for 5 min and dehydrate samples through an Ethanol series diluted in distilled water up to 70% ethanol as follows: 30%, 50% and 70% ethanol, for 5 min each step.
- Place samples in medium size screen cassettes. Process tissue for paraffin embedding using and automated processor. Set up a cycle as follows: six changes of alcohol for 8 min each, three changes of Xylene for 6 min each, three changes of paraffin, for 25 min, 9 min and 8 min.
- Embed in paraffin orienting the tissue in desired position to generate 6 μm transverse or longitudinal sections as described in section 1. Place sections on slides and allow tissue to adhere to slide on slide warmer set at 42 °C for 1 hr.
- Bake slides, on their sides, overnight in oven at 56 °C. Place slides in plastic racks. De-paraffinize slides by immersion in three changes of 100 % Xylene, 10 min per change. Use 200 ml of Xylene to completely submerge slides. Rehydrate slides using graded EtOH series (100%, 70%, 50% and 30%, 5 min per step with agitation) to PBS (5 min).
- Perform antigen retrieval using 10 mM citrate buffer, pH 6. To prepare 100 ml of citrate buffer mix 18 ml of 0.1 M Citric acid monohydrated and 82 ml of 0.1 M Sodium citrate.
- Place samples in plastic coplin jars filled with antigen retrieval solution and microwave for 6.5 min at high power. Add distilled water to coplin jar to compensate for evaporated-citrate buffer and reheat at 40% power for 6 min.
- Fill coplin jar to top with water, and reheat again at 40% power for 6 min. Microwave times may vary based on microwave used.
- Allow slides to cool for 20 min at room temperature before washing slides in distilled water for 1 min, followed by PBS for 5 min. After the PBS wash, block slides for 2 hr in blocking solution containing TBS (2.425 g of Tris base and 8.765 g of NaCl diluted in 1 L of distilled water) 10% Donkey serum and 1% BSA.
- Add primary antibodies diluted in blocking solution (TBS containing 10% Donkey serum and 1% BSA). BrdU (mitosis marker), Nkx2.1 (respiratory tract epithelium), Sox9 (cells that will give rise to cartilage) and αSMA (smooth muscle cells). Incubate slides overnight, at 4 °C. Use overlay method to conserve antibodies. Apply 180 μl of antibody to a gasket and then attach slide as if cover slipping.
- Detach gasket by dipping slides into distilled water. After removal of the gasket, wash unbound primary antibody by performing six 5 min washes with TBS containing 0.1% Tween-20.
- Incubate slides with secondary antibody diluted in blocking solution at a dilution of 1:200, for 1 hr at room temperature. Select secondary antibodies carefully to prevent undesired binding among them. Remove unbound secondary antibody by performing three five minute washes with TBS containing 0.1% Tween.
- Wash slides in 0.1 M Tris base twice for 5 min and then 0.05 M Tris base twice for 5 min. Slides may be left in 0.05 M Tris base while coversliping using mounting media with or without DAPI (1.5 μg/ml). Store slides in slide folder at 4 °C and protect from light by covering folder with aluminum foil.
- Visualize staining and photograph using an automated fluorescence microscope. Count labeled-cells and total cells per field photographed at 20X and 40X to determine ratios of proliferating cells to total cells.
Representative Results
Wnt/β-catenin activity
Whole mount Lac-Z staining was detected in tracheal-lung tissue of embryos isolated from reporter Axin2Lac-Z mice11. Sites of staining indicate Wnt/β-catenin activity. Analysis of sections of whole mount staining determined that Wnt/β-catenin activity was present in the mesenchyme of the trachea and in mesenchyme of peripheral regions of developing lungs. In WlsShhCre embryos (wherein secretion of Wnt ligands from the epithelium of respiratory tract was abrogated) Lac-Z staining was almost absent (Figure 1).
Mesenchymal condensations in tracheal mesenchyme
A critical step in chondrogenesis is the process by which mesenchymal cells fated to give rise to cartilage condense. These tight aggregations of cells are known as mesenchymal condensations. To test whether the lack of cartilage observed in WlsShhCre mice was due to an absence of mesenchymal condensations, tracheal lung tissues from gestational ages E12.5 to E14.5 were stained with PNA lectin. At E13.5 and E14.5, fluorescence was detected as periodic bands in the tracheal mesenchyme at sites where cartilage will be formed. Lack of detectable fluorescence in the tracheal tissue of WlsShhCre embryos indicates that endodermal Wnt signaling to the tracheal mesenchyme is necessary for mesenchymal condensations (Figure 2).
Respiratory tract cell specification
Whole mount immunofluorescence of tracheal lung tissues determined the expression pattern of Sox9, Nkx2.1 and αSMA at E11.5. In the tracheal tissue, Sox9 expression is limited to the mesenchyme of the trachea as a continuous stripe (arrow in Figure 3A and B) while in the developing lung Sox9 is observed at the periphery of the respiratory epithelium (arrow heads in Figure 3A, B and C). Nkx2.1 presents a distinct expression pattern restricted to the epithelium of the trachea of the lung. Preventing secretion of Wnt ligands from epithelium causes diminished expression of Sox9 in tracheal mesenchyme but does not affect Sox9 expression in peripheral lung epithelium. Expression of αSMA is not readily detected in tracheal mesenchyme, however staining was detected at the level of the larynx (yellow arrow, Figure 3) and stomach (Figure 3).
Cell proliferation in developing trachea
Proliferation in epithelium and mesenchyme of the trachea was detected after in vivo labeling of tissue with BrdU. Sections of tracheal tissue were stained with antibodies recognizing BrdU, Sox9 and αSMA. The proliferation profile in the tracheal mesenchyme indicates that the levels of Sox9 stained cells undergoing proliferation is higher than the level of αSMA stained cells undergoing proliferation. This proliferation pattern seems to be reverted in WlsShhCre tracheas (Figure 4).
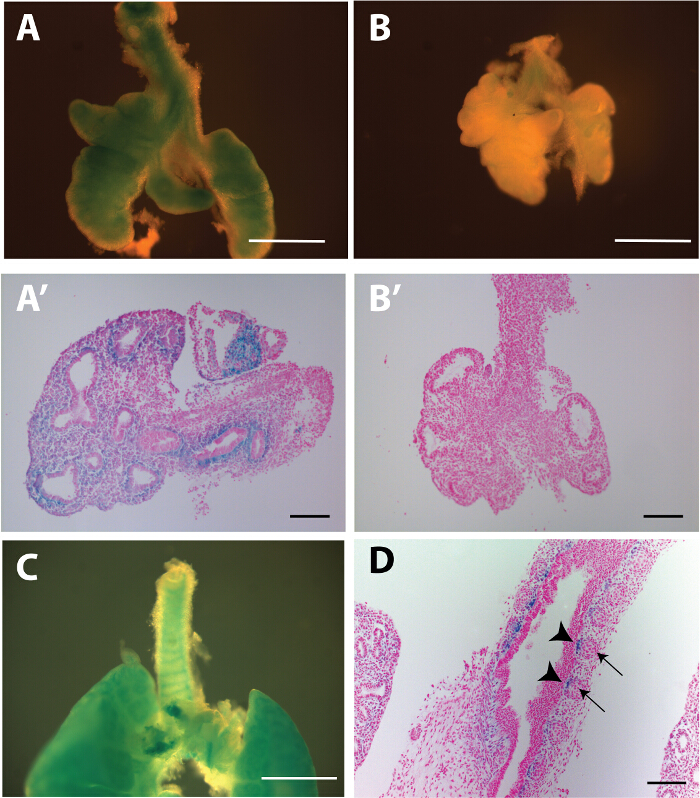
Figure 1: Wnt/β-catenin activity is operative in tracheal mesenchyme. E13.5 tracheal lung tissue, isolated from Axin2-LacZ and WlsShhCre:Axin2-LacZ mice, were stained with X-gal (A and B). Sections of tracheal lung tissue were counterstained with fast red (A', B'). Note the lack of X-gal staining in WlsShhCre;Axin2-Lac-Z mice demonstrating absence of Wnt/β-catenin activity (B'). E14.5 tissue from Axin2 LacZ mice stained with X-gal depicts sites of future cartilaginous rings (C); however, Axin2 activity was restricted to the periphery (arrow head) of the mesenchymal condensations (arrow) (D). Scale bar A, B and C = 500 μm; B', D' and D = 100 μm. Please click here to view a larger version of this figure.
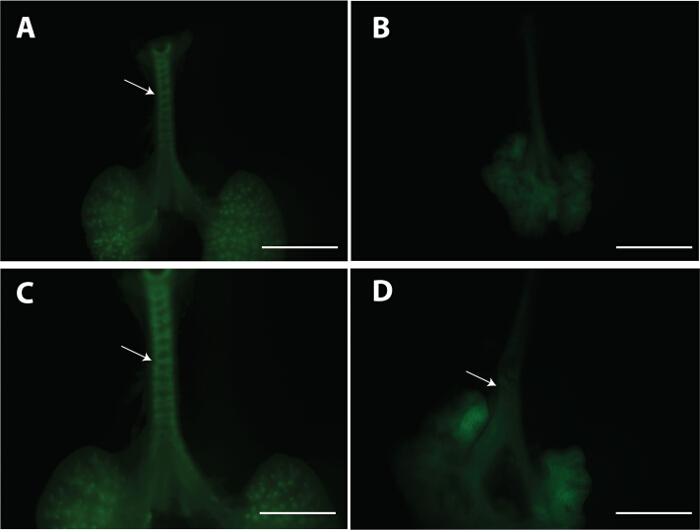
Figure 2: Mesenchymal condensation in developing tracheal tissue. E13.5 tracheal-lung explants were stained with PNA lectin. Control tracheal tissue depicting the regions were mesenchymal cells condensed is shown (arrows A, C). No mesenchymal condensations were detected in WlsShhCre mice (B and arrow in D). C and D depict lower magnification of tracheal-lung tissue shown in A and B. Scale bar A and B = 1 mm; C and D = 2 mm. Please click here to view a larger version of this figure.
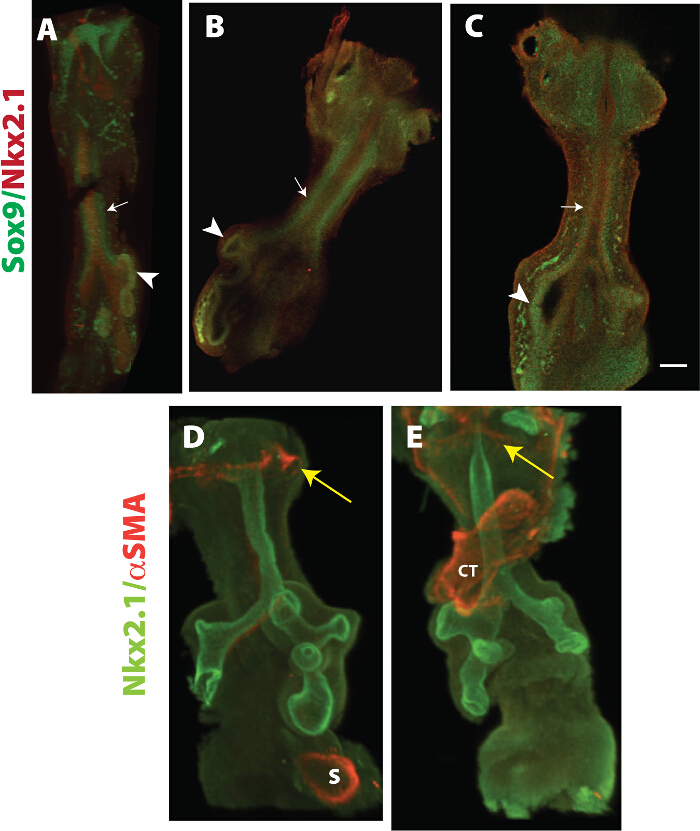
Figure 3: Expression pattern in prechondrogenic whole mount trachea. Nkx2.1 and Sox9 staining was detected in E11.5 explants. Panel A depicts an image of a control tissue, while panel B shows an optical section of a 20 μm control tissue. Note the lack of mesenchymal expression of Sox9 in WlsShhCre tissue (arrow in C), but maintenance of peripheral expression of Sox9 (arrowhead in C). αSMA staining was detected at low levels in trachealmesenchyme of E11.5 tissue but observed in the laryngeal region (yellow arrowhead in D and E), stomach (S) and remaining cardiac tissue (CT) (E). Nkx2.1 is expressed in epithelium of developing trachea and lungs (D, E). Scale bar = 10 µm. Please click here to view a larger version of this figure.
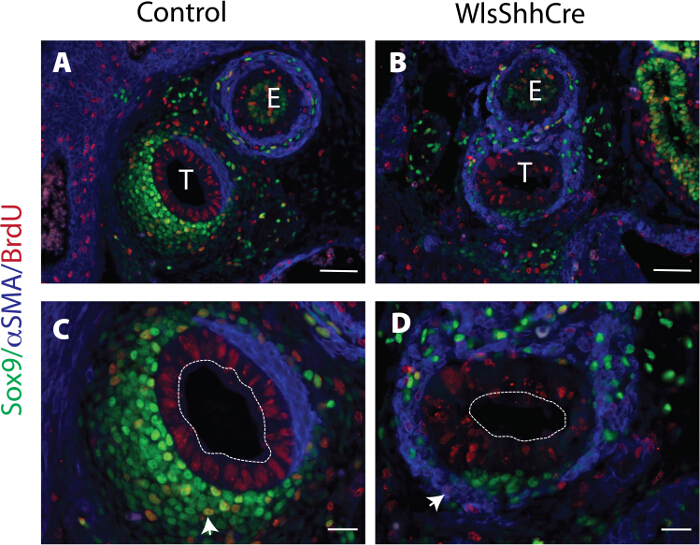
Figure 4: Cell proliferation pattern in prechondrogenic mesenchyme. Sections of E13.5 embryos were stained with anti BrdU, Sox9 and αSMA antibody. Panels C and D are higher magnifications of A and B respectively. Dotted lines represent limits of the tracheal epithelium. Note Sox9 stained proliferative cell (arrowhead C) and αSMA stained proliferative cell (arrowhead in D). T = trachea, E = esophagus. Scale bar A and B = 50 μm, C and D = 20 μm. Please click here to view a larger version of this figure.
Discussion
Events underlying the morphogenesis of the respiratory tract are not completely understood, particularly the processes required for the patterning of the conducting airways. Previous studies have utilized ex vivo techniques wherein developing explants are cultured at the air-liquid interphase or embedded in matrigel21,22. These studies have shown how growth factors influence the patterning of the developing trachea and the formation of tracheal cartilage. A limitation to these studies is that the architecture of the tissue is not properly maintained and therefore, they may not quite recapitulate the in vivo process of chondrogenesis.
The approach that we undertook to study the role of Wnt signaling in the process of conducting airways patterning included in vivo studies coupled with whole mount staining techniques, immunofluorescence and imaging. X-gal staining of whole mount tissue followed by sectioning and counter-staining, presents advantages over the staining of paraffin sections. Whole mount staining allows for direct detection of sites where Wnt/β-catenin activity is operative. Furthermore, sectioning of the stained explants determined precise sites in the mesenchyme wherein the activity was located as development progressed. The latter is a better and accurate readout than detection of expression of β-galactosidase enzyme in paraffin sections. While enzymatic activity could be detected in sections, this procedure will require cryosections that will not always be easy to generate and may not preserve the architecture of small samples. A limitation in the protocol described in this paper, is that processing of samples for paraffin embedding after whole mount staining will cause a loss of the signal. Therefore, samples need to remain longer in X-gal solution and a shorter cycle for paraffin embedding should be used to obtain good signal on sections.
Processing of small tissue for paraffin embedding will result in reduction of the size of the tissue. This is an undesired result while working with reduced amount of tissue. Therefore, we developed a short processing cycle that simultaneously allows for adequate preservation of the structure without causing reduction in the size of the tissue.
On the other hand, whole mount immunofluorescence procedure, modified after the technique described by Ahnfelt-Ronne23, allowed for tridimensional visualization of developing tracheal and pulmonary tissue, with a defined expression pattern of proteins encoded by key transcription factors such as Nkx2.1 and Sox9. When desired, the whole mount staining can be presented as confocal-aided z stacks of images, depicting different depths of the tracheal lung tissue. While imaging small explants, clearing of the tissue should be performed in a stage plate. We utilized thick metallic custom made stage plates with optical bottom where samples are cleared and imaged. This prevents the loss of material while transferring small tissue after clearing step. While whole mount immunofluorescence is a suitable technique for staining of small tissue, we acknowledge limitations to the technique. In particular, we observed dissimilar performances of antibodies that recognize antigen better in sections as opposed to whole mount. This issue may preclude the performance of the technique if no alternative antibodies are available. It is also possible that the use of methanol and clearing with Murray's clear may have a negative impact in the signal observed. In the future, we will test other non-organic clearing solutions and adjust the protocol properly for this purpose24. A final consideration is that whole mount will allow the study of a few proteins expressed in a tissue at one given time.
Previous reports have shown the usefulness of PNA lectin staining to detect mesenchymal condensations in sections of tracheal tissue21,25. In the present study, we report a methodology by which the staining was performed in whole mount, which facilitates a better visualization of the regions of the trachea wherein the cartilage will be formed. While performing lectin staining care should be placed in using the appropriate concentration of lectin to not over-stain, as commercially available PNA-lectin is coupled to GFP. Auto-fluorescence of the tissue may obscure the results making difficult to distinguish bands corresponding to mesenchymal condensations.
Finally, in vivo labeling of tissue with BrdU followed by sectioning and immunofluorescence staining is a feasible technique to determine cell proliferation that presents advantages over typical and static staining such as PPH3 or PCNA26,27. The latter technique presents limitations, as staining will vary depending on the cell cycle stage. This problem is eliminated by our described technique. Combining in vivo labeling followed by embedding, sectioning and immunofluorescence allowed us to determine that epithelial Wnt ligands balance the differential proliferation of Sox9 and α-SMA expressing cells that will give rise to tracheal cartilage and muscle7.
In summary, a multiple-technique approach to studying the patterning of the developing respiratory tract allows for a deep analysis of the role of Wnt signaling in conducting airway morphogenesis. These studies determined the precise sites of Wnt/β-catenin in the tracheal tissue, as well as inductive and instructive signaling provided by the tracheal epithelium that is required for specification, differential proliferation of tracheal mesenchyme cell lineages, as well as cartilage formation. Our future directions include the optimization of the whole mount staining for recently identified Wls target genes, as well as performing whole mount immunofluorescence in combination with lectin staining.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We acknowledge the assistance of Mike Muntifering and Matt Kofron with confocal imaging and Gail Macke with histological procedures. This work was partially supported by National Institutes of Health-NHLBI (K01HL115447 to D.S.).
Materials
| Anti Sox9 ab. | Millipore | AB5535 | 1:400 , rabbit |
| Anti Sox9 ab. | Santa Cruz | Sc-20095 | 1:50, rabbit |
| Anti Smooth Muscle Actin ab. | Sigma | A5228 | 1:2k, mouse |
| Anti NKX2.1 ab. | Seven Hills | n/a | 1:100, guinea pig |
| Anti NKX2.1 ab. | Seven Hills | n/a | 1:400, mouse |
| Anti Brdu ab. | Abcam | AB1893 | 1:200, sheep |
| Anti Brdu ab. | Santa Cruz | Sc-32323 | 1:4k, mouse |
| PNA Lectin | Sigma | L 7381 | |
| Secondary antibodies | Life technologies | Alexa fluor Molecular probes | |
| K3Fe(CN)6 | Sigma | P8131 | |
| K4Fe(CN)6 | Sigma-Aldrich | P3289 | |
| MgCl2 | Sigma-Aldrich | M9272 | |
| NaDOC | Life Technologies | 89905 | |
| NP4O | Life Technologies | 85124 | |
| Alcian Blue 8GX | Sigma | A-3157 | |
| Fisher brand super-frost plus | Fisher | 12-550-15 | |
| PFA (16%) | EMS | 15710 | |
| PBS | Gibco | 70011-044 | |
| Fetal Calf Serum | Sigma | 11K413 | |
| Blocking reagent | Invitrogen | Component of TSA kit #2 ( T20932) | |
| BrDu | Sigma | B5002-5g | |
| Vectashield mounting medium | Vector labs | H-1000 | |
| Permount | Fisher | SP15-500 | |
| Tissue-loc cassettes Histoscreen | Fisher | C-0250-GR | |
| Biopsy cassettes | Premiere | BC0109 | Available in different colors |
| Nuclear fast red Kernechtrot 0.1% | Sigma | N3020 | |
| Citric acid | Sigma | C1909-500G | |
| Sodium citrate tribasic dihydrate | Sigma | S4641-1Kg | |
| Trizma hydrochloride | Sigma | T5941-500G | |
| Xylene | Pharmco-AAPER | 399000000 | |
| Ethanol | Pharmco-AAPER | 111000200 | |
| Micro knives | FST | 10318-14 | |
| Dumont #5 ceramic coated | FST | 11252-50 | |
| Dumont #5CO | FST | 11295-20 | |
| Dumont # 5 | FST | 91150-20 | |
| Thermo/Shandon Excelsior ES | Thermo Fisher | ||
| Microtome | Leica | RM2135 | |
| Nikon i90 | Nikon | Wide field microscope | |
| NikonA1Rsi | Nikon | Confocal microscopy. Settings:NikonA1 plus camera, scanner: Galvano, detector:DU4. Optics Plan Apo lambda 10x. Modality: Widefield fluorescence laser confocal. | |
| Leica MS 16 FA | Leica | Fluorescence Dissecting microscope | |
| Zeiss | Zeiss | Automated fluorescence microscope | |
| Leica Application suite | Leica | Leica imaging software | |
| NIS | Nikon | Nikon imaging software | |
| IMARIS | Bitplane | Imaging processing software |
Referenzen
- Maeda, Y., Dave, V., Whitsett, J. A. Transcriptional control of lung morphogenesis. Physiol Rev. 87, 219-244 (2007).
- Morrisey, E. E., Hogan, B. L. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 18, 8-23 (2010).
- Fausett, S. R., Klingensmith, J. Compartmentalization of the foregut tube: developmental origins of the trachea and esophagus. Wiley Interdiscip Rev Dev Biol. 1, 184-202 (2012).
- Goss, A. M., et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 17, 290-298 (2009).
- Harris-Johnson, K. S., Domyan, E. T., Vezina, C. M., Sun, X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 106, 16287-16292 (2009).
- Cornett, B., et al. Wntless is required for peripheral lung differentiation and pulmonary vascular development. Dev Biol. 379, 38-52 (2013).
- Snowball, J., Ambalavanan, M., Whitsett, J., Sinner, D. 34;Endodermal Wnt signaling is required for tracheal cartilage formation". Dev Biol. , (2015).
- Shannon, J. M., Hyatt, B. A. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 66, 625-645 (2004).
- Shannon, J. M., Nielsen, L. D., Gebb, S. A., Randell, S. H. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 212, 482-494 (1998).
- Li, C., et al. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 287, 86-97 (2005).
- Loscertales, M., Mikels, A. J., Hu, J. K., Donahoe, P. K., Roberts, D. J. Chick pulmonary Wnt5a directs airway and vascular tubulogenesis. Development. 135, 1365-1376 (2008).
- Yin, Y., et al. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol. 319, 426-436 (2008).
- Shu, W., et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 283, 226-239 (2005).
- Bretholz, A., Morrisey, R., Hoffman, R. S. The use of OpdA in rat models of organic phosphorus (OP) poisoning. Toxicology. 257, (2009).
- Goss, A. M., et al. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol. 356, 541-552 (2011).
- Mucenski, M. L., et al. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 278, 40231-40238 (2003).
- Hyatt, B. A., Shangguan, X., Shannon, J. M. FGF-10 induces SP-C and Bmp4 and regulates proximal-distal patterning in embryonic tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 287, L1116-L1126 (2004).
- Del Moral, P. M., et al. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 290, 177-188 (2006).
- Ott, S. R. Confocal microscopy in large insect brains: zinc-formaldehyde fixation improves synapsin immunostaining and preservation of morphology in whole-mounts. J Neurosci Methods. 172, 220-230 (2008).
- Jahrling, N., Becker, K., Dodt, H. U. 3D-reconstruction of blood vessels by ultramicroscopy. Organogenesis. 5, 145-148 (2009).
- Park, J., et al. Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn. 239, 514-526 (2010).
- Elluru, R. G., Thompson, F., Reece, A. Fibroblast growth factor 18 gives growth and directional cues to airway cartilage. Laryngoscope. 119, 1153-1165 (2009).
- Ahnfelt-Ronne, J., et al. An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J Histochem Cytochem. 55, 925-930 (2007).
- Yang, B., et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell. 158, 945-958 (2014).
- Gillotte, D. M., Fox, P. L., Mjaatvedt, C. H., Hoffman, S., Capehart, A. A. An in vitro method for analysis of chondrogenesis in limb mesenchyme from individual transgenic (hdf) embryos. Methods Cell Sci. 25, 97-104 (2003).
- Cohen, E. D., et al. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 119, 2538-2549 (2009).
- Boucherat, O., et al. Partial functional redundancy between Hoxa5 and Hoxb5 paralog genes during lung morphogenesis. Am J Physiol Lung Cell Mol Physiol. 304, L817-L830 (2013).

