Determination of the Relative Cell Surface and Total Expression of Recombinant Ion Channels Using Flow Cytometry
Summary
Inherited cardiac arrhythmias are often caused by mutations that alter the surface delivery of one or more ion channels. Here, we adapt flow cytometry assays to provide a quantification of the relative total and cell surface protein expression of recombinant ion channels expressed in tsA-201 cells.
Abstract
Inherited or de novo mutations in cation-selective channels may lead to sudden cardiac death. Alteration in the plasma membrane trafficking of these multi-spanning transmembrane proteins, with or without change in channel gating, is often postulated to contribute significantly in this process. It has thus become critical to develop a method to quantify the change of the relative cell surface expression of cardiac ion channels on a large scale. Herein, a detailed protocol is provided to determine the relative total and cell surface expression of cardiac L-type calcium channels CaV1.2 and membrane-associated subunits in tsA-201 cells using two-color fluorescent cytometry assays. Compared with other microscopy-based or immunoblotting-based qualitative methods, flow cytometry experiments are fast, reproducible, and large-volume assays that deliver quantifiable end-points on large samples of live cells (ranging from 104 to 106 cells) with similar cellular characteristics in a single flow. Constructs were designed to constitutively express mCherry at the intracellular C-terminus (thus allowing a rapid assessment of the total protein expression) and express an extracellular-facing hemagglutinin (HA) epitope to estimate the cell surface expression of membrane proteins using an anti-HA fluorescence conjugated antibody. To avoid false negative, experiments were also conducted in permeabilized cells to confirm the accessibility and proper expression of the HA epitope. The detailed procedure provides: (1) design of tagged DNA (deoxyribonucleic acid) constructs, (2) lipid-mediated transfection of constructs in tsA-201 cells, (3) culture, harvest, and staining of non-permeabilized and permeabilized cells, and (4) acquisition and analysis of fluorescent signals. Additionally, the basic principles of flow cytometry are explained and the experimental design, including the choice of fluorophores, titration of the HA antibody and control experiments, is thoroughly discussed. This specific approach offers objective relative quantification of the total and cell surface expression of ion channels that can be extended to study ion pumps and plasma membrane transporters.
Introduction
This paper provides a reliable assay to report the relative cell surface expression of membrane proteins such as ion channels expressed in recombinant cells using the existing flow cytometry technology. Ion channels are pore-forming membrane proteins that are responsible for controlling electrical signals by gating the flow of ions across the cell membrane. They are classified by the activation mechanism, nature, and selectivity of ion species transiting through the pore where they are localized. At the cellular and tissue levels, the macroscopic ion fluxes through ion channels are the product of biophysical (gating and permeation), biochemical (phosphorylation), and biogenesis (synthesis, glycosylation, trafficking, and degradation) properties1. Each of these processes is unique to every type of ion channels and is optimized to fulfill the physiological role of the ion channel. Consequently, alterations in any of these fine-tuned processes through an inherited or a genetic modification, often referred to as "channelopathy", can be detrimental to cell homeostasis. It is important to stress that delivering the "right" amount of ion channels at the cell surface is critical to cell homeostasis. Even small increases (gain-of-function) and small decreases (loss-of-function) in ion channel activity have the potential to cause a serious pathology over a lifetime. Defects in the cell surface delivery of mature ion channels is an important determinant in numerous channelopathies, such as cystic fibrosis (CFTR ion channel)2 and cardiac arrhythmias of the long QT syndrome form (cardiac potassium channels)3.
Channelopathies are associated with cardiac sudden death4. The current worldwide prevalence of all cardiac channelopathies is thought to be at least 1:2,000-1:3,000 per individual5 and are responsible for about half of sudden arrhythmic cardiac death cases6. Dysfunction in cardiac voltage-gated sodium-, potassium-, and calcium- selective ion channels are known to play a key role in this process. The L-type CaV1.2 voltage-gated calcium channel is required to initiate synchronized heart muscle contraction. The cardiac L-type CaV1.2 channel is a multi-subunit protein complex composed of the main pore-forming CaVα1 subunit and CaVß and CaVα2δ1 auxiliary subunits7-12. Note that the full complement of auxiliary subunits is required to produce functional CaV1.2 channels at the plasma membrane and dynamic interactions between these subunits are essential to support the normal electric function of the heart13. CaVß promotes the cell surface expression of CaV1.2 channels through a non-covalent nanomolar hydrophobic interaction14. Co-expression of the CaVα2δ1 subunit with CaVß-bound CaVα1 stimulates peak current expression (5 to 10-fold) and promotes channel activation at more negative voltages. Gain-of-function mutations of the pore-forming subunit CaV1.2 have been associated with a form of ventricular arrhythmias called the long QT syndrome15 whereas a host of point mutations in the three main subunits forming the L-type CaV1.2 channel have been identified in subjects suffering from arrhythmias of the short QT syndrome form16,17. Ion channels are membrane proteins that can be investigated from a biochemical perspective (protein chemistry) or using electrophysiological tools (current-generating machines) and often using these complementary approaches. Electrophysiology, in particular whole-cell patch-clamping, is a suitable approach to elucidate the function of ion channels15 but cannot resolve modifications in protein trafficking from changes in their biophysical properties. Protein chemistry has, however, often limited use due to the relatively low expression of large membrane proteins relative to smaller soluble proteins. Robust high-throughput methods using fluorescence readout need to be developed in order to specifically address defects in protein biogenesis causing changes in the cell surface expression of ion channels.
Flow cytometry is a biophysical technology employed in cell counting, sorting, biomarker detection, and protein engineering18. When a sample solution of live cells or particles is injected into a flow cytometer, the cells are ordered into a single stream that can be probed by the machine's detection system (Figure 1). The first flow cytometer instrument produced in 195619 detected only one parameter but modern flow cytometers have multiple lasers and fluorescence detectors that allow the detection of more than 30 fluorescent parameters20,21. Filters and mirrors (emission optics) direct the light scatter or fluorescent light of cells to an electronic network (photodiode and detectors) that convert the light proportionally to its intensity. Digital data are analyzed using specialized software and the primary output is displayed as a dot plot21.
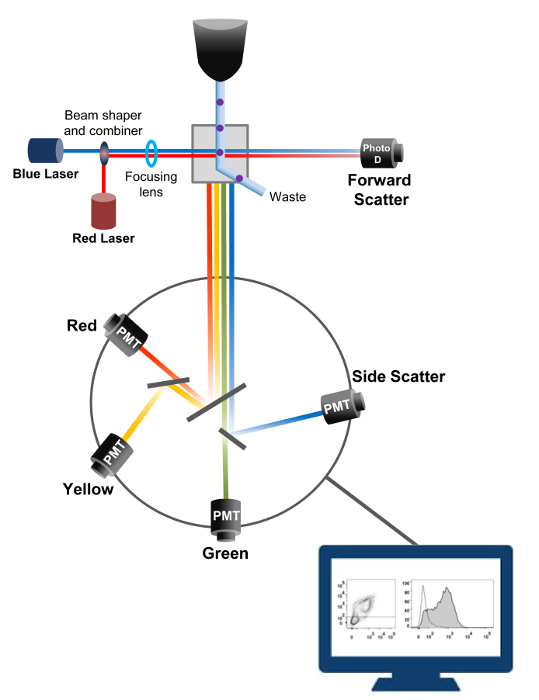
Figure 1: Biophysical principles of flow cytometry sorting. Single cells are pushed through a nozzle under high pressure within a stream of sheath fluid which moves them across one or more laser interrogation points. The light beam is deflected by the passing cells and the light collected in the forward direction (Forward Scatter, FCS) is sent to a photodiode that converts the light into a signal proportional to the size of the cell. The light is also collected at a 90° angle to the laser path and sent to detectors (also called photomultipliers (PMT)). This light is routed through dichroic mirrors that permit the detection of the side scatter signal (SSC), which reflects the granularity within the cells, and the fluorescent emissions if excited fluorochromes are present in the cell. Three detectors (Green, Yellow, and Red) are represented with different wavelength bandpass filters, allowing the simultaneous detection of different fluorochromes. The different signals are digitized by an external computer and converted into data that will be analyzed to quantify the characteristics of the cells. Please click here to view a larger version of this figure.
The high-throughput capacity of flow cytometers was exploited to quantify the relative membrane expression of recombinant wild-type and trafficking-deficient voltage-gated L-type CaV1.2 channels and associated subunits in live cells. cDNA constructs coding for the proteins were doubly tagged to simultaneously carry an extracellular non-fluorescent epitope that can be detected by an impermeable fluorescent conjugated antibody and an intracellular fluorophore that is constitutively fluorescent. Both the extracellular epitope, inserted in an extracellular loop of the protein, and the intracellular fluorophore, inserted after the C-terminus, are translated with the protein. In this series of experiments, the CaVα2δ1 protein was engineered to express an extracellular hemagglutinin (HA) epitope (YPYDVPDYA) detected by an impermeable FITC (Fluorescein isothiocyanate)-conjugated anti-HA and mCherry as the intrinsic intracellular fluorophore. To determine the relative cell surface expression level of the mCherry-CaVα2δ1 HA-tagged protein, recombinant cells expressing the fusion protein were harvested after transfection, and stained with the FITC-conjugated mouse monoclonal anti-HA epitope tag antibody (Figure 2). FITC is an organic fluorescent compound that is considerably smaller than enzyme reporters and therefore not as likely to interfere with biological function. mCherry- CaVα2δ1-HA overexpressed in tsA-201cells, produces a significant 3-log increase in the FITC fluorescence and mCherry fluorescence on two-dimensional plots22. Given that the HA epitope is located in the extracellular portion of the protein, the fluorescence intensity for FITC obtained in the presence of intact cells reflect the relative index of the cell surface expression of HA-tagged protein. The accessibility of the HA epitope in the constructs is systematically validated by measuring the FITC signal after cell permeabilization. This measure also serves to corroborate the normalized total protein expression since the relative fluorescence intensities for FITC estimated in permeabilized cells are qualitatively comparable to the relative fluorescence values for mCherry measured under permeabilized and non-permeabilized conditions22,23. It is important to note that the intrinsic fluorescence spectrum is shifted toward higher values after permeabilization but that the only value being reported is the change in fluorescence intensity as compared to the control construct. Relative changes in the fluorescence intensity for the test constructs are estimated using the ΔMean Fluorescence Intensity (ΔMFI) values for each fluorophore (mCherry or FITC). Experiments are designed to measure the fluorescence intensity of the test construct relative to the fluorescence intensity of the control construct expressed under the same conditions to limit experimental variations in the intrinsic fluorescence of the fluorophore-conjugated antibody. Two membrane proteins were successfully studied using this assay: the pore-forming subunit of the L-type voltage-gated calcium channel CaV1.214,22 and in a different series of experiments, the extracellular auxiliary CaVα2δ1 subunit22,23. The following protocol was used to determine the cell surface expression of the CaVα2δ1 subunit of the cardiac L-type CaV1.2 channel under control conditions and after mutations affecting the posttranslational modification of the ion channel. Under standardized experimental conditions, the cell surface fluorescence of FITC increases quasi-linearly with the expression of cDNA coding for the mCherry-CaVα2δ1-HA proteins (Figure 5 from reference22).
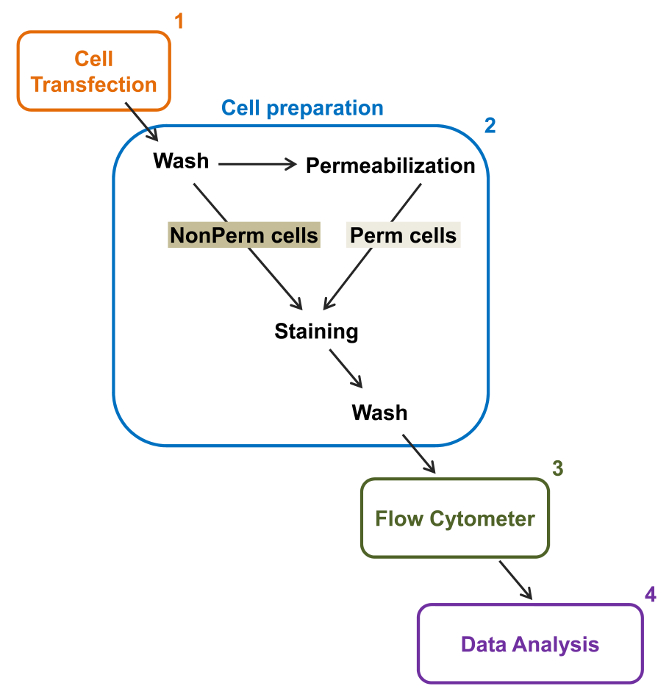
Figure 2: Schematic representation of total and membrane labeling in the flow cytometry experimental protocol. The scheme outlines some of the main steps necessary to quantify the relative total and cell surface expression of recombinant ion channels by flow cytometry. Cells are transfected with the double-tagged construction mCherry-CaVα2δ1-HA in tsA-201 cells (1) and stained before or after permeabilization (2). Multiparameter data are acquired in a flow cytometer (3) for multivariate analysis (4). Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
This flow cytometry-based assay was successfully applied to the measurement of relative total and cell surface levels of fluorescently-labelled pore-forming and associated subunits of voltage-gated calcium channels14,22,26. It is best used when investigating the impact of genetic mutations and thus requires that the intrinsic fluorescence intensity of the fluorescently-labelled tagged wild-type construct be at least 10 to 100-fold larger than for the fluorescence intensity of the fluorescently-labelled untagge…
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank Mr. Serge Sénéchal and Dr. Jacques Thibodeau for sharing their expertise and granting us access to their flow cytometry and cell sorting platform. This work was completed with the operating grant 130256 from the Canadian Institutes of Health Research, a grant-in-aid from the Canadian Heart and Stroke Foundation, and support from the “Fondation de l’Institut de Cardiologie de Montréal” to L.P.
Materials
| Q5 Site-Directed Mutagenesis Kit | New England Biolabs | E0554S | Can be substitute with QuickChange site-directed mutagenesis Kit (Agilent, #200523). |
| Tubes 1,5 mL | Sarstedt | 72-690-001 | |
| Tubes 15 mL | Sarstedt | 62-554-002 | |
| Disposable graduated Tranfer Pipets | VWR | 160001-192 | |
| 100-mm culture dish | Corning | 430167 | For standard culture of HEKT cells. |
| 35-mm culture dish | Falcon | 353001 | For standard culture of HEKT cells. |
| Serological pipette 1 ml | Sarstedt | 86.1251.001 | |
| Serological pipette 5 ml | Sarstedt | 86.1253.001 | |
| Serological pipette 10 ml | Sarstedt | 86.1254.001 | |
| Serological pipette 25ml | Sarstedt | 86.1285.001 | |
| Dulbecco's high-glucose medium | Life Technologies | 12100-046 | Warm in 37°C water bath before use. |
| Fetal Bovine Serum, qualified, heat inactivated, US origin | Life Technologies | 16140-071 | |
| Penicillin-Streptomycin (10,000 U/mL) | Life Technologies | 15140-122 | |
| Lipofectamine 2000 | Life Technologies | 11668-019 | For liposomal transfection. Can be substituted with calcium phosphate transfection. |
| Opti-MEM I Reduced Serum Medium | Life Technologies | 31985-070 | Warm in 37°C water bath before use. |
| Trypsin-EDTA (1X) 0.05%, phenol red | Life Technologies | 25300-062 | |
| 1.5 mL microtubes | Sarstedt | 72.690.001 | |
| Phosphate Buffered Saline 1X | Fisher | BP661-10 | Can be "home-made". |
| Anti-HA FITC conjugated antibody | Sigma | H7411 | |
| IgG1−FITC Isotype Control antibody | Sigma | F6397 | |
| BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit | BD Biosciences | 554714 | Fixation/Permeabilization. Permeabilization/Wash solution, store at 4 °C. |
| Hemacytometer | Fisher | 49105161 | |
| Trypan Blue | Fisher | 15250061 | To access cell viability. |
| Refrigerated Microcentrifuge, 5430R | Eppendorf | A14H172200 | |
| Forma Steri-Cycle CO2 Incubator | Fisher | 370 | |
| Laboratory Platform Rocker | Fisher | 545034 | |
| Water Bath | VWR | 89032-216 | |
| BD FACSARIA III | BD Biosciences | 648282 | Flow cytometer. |
| FlowJo Software v10 | FlowJo | FlowJo v10 Dongle | For data analysis. |
Referenzen
- Delisle, B. P., Anson, B. D., Rajamani, S., January, C. T. Biology of Cardiac Arrhythmias: Ion Channel Protein Trafficking. Circ. Res. 94, 1418-1428 (2004).
- Birault, V., Solari, R., Hanrahan, J., Thomas, D. Y. Correctors of the basic trafficking defect of the mutant F508del-CFTR that causes cystic fibrosis. Curr Opin Chem Biol. 17, 353-360 (2013).
- Balijepalli, S. Y., Anderson, C. L., Lin, E. C., January, C. T. Rescue of Mutated Cardiac Ion Channels in Inherited Arrhythmia Syndromes. J. Cardiovas Pharm. 56, 113-122 (2010).
- Gargus, J. J. Unraveling Monogenic Channelopathies and Their Implications for Complex Polygenic Disease. Am. J. Hum. Genet. 72, 785-803 (2003).
- Abriel, H., Zaklyazminskaya, E. V. Cardiac channelopathies: Genetic and molecular mechanisms. Gene. 517, 1-11 (2013).
- Behr, E. R., et al. Sudden arrhythmic death syndrome: familial evaluation identifies inheritable heart disease in the majority of families. Eur Heart J. 29, 1670-1680 (2008).
- Catterall, W. A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev.Biol. 16, 521-555 (2000).
- Peterson, B. Z., DeMaria, C. D., Adelman, J. P., Yue, D. T. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L- type calcium channels. Neuron. 22, 549-558 (1999).
- Dolphin, A. C. Calcium channel diversity: multiple roles of calcium channel subunits. Curr.Opin.Neurobiol. 19, 237-244 (2009).
- Dai, S., Hall, D. D., Hell, J. W. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 89, 411-452 (2009).
- Gao, T., et al. Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J. Biol. Chem. 272, 19401-19407 (1997).
- Carl, S. L., et al. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J. Cell Biol. 129, 673-682 (1995).
- Abriel, H., Rougier, J. S., Jalife, J. Ion Channel Macromolecular Complexes in Cardiomyocytes: Roles in Sudden Cardiac Death. Circ. Res. 116, 1971-1988 (2015).
- Bourdin, B., et al. Molecular Determinants of the Cavb-induced Plasma Membrane Targeting of the Cav1.2 Channel. J. Biol. Chem. 285, 22853-22863 (2010).
- Raybaud, A., et al. The Role of the GX9GX3G Motif in the Gating of High Voltage-activated Calcium Channels. J. Biol. Chem. 281, 39424-39436 (2006).
- Burashnikov, E., et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 7, 1872-1882 (2010).
- Hennessey, J. A., et al. A CACNA1C Variant Associated with Reduced Voltage-Dependent Inactivation, Increased Cav1.2 Channel Window Current, and Arrhythmogenesis. PLoS ONE. 9, e106982 (2014).
- Adan, A., Alizada, G., Kiraz, Y., Baran, Y., Nalbant, A. Flow cytometry: basic principles and applications. Crit Rev Biotechnol. , 1-14 (2016).
- Graham, M. D. The Coulter Principle: Foundation of an Industry. J. Lab. Autom. 8, 72-81 (2003).
- Baumgarth, N., Roederer, M. A practical approach to multicolor flow cytometry for immunophenotyping. J. Immunol. Methods. 243, 77-97 (2000).
- Rothe, G., Sack, U., Tarnok, A., Rothe, G. . Cellular Diagnostics. Basics, Methods and Clinical Applications of Flow Cytometry. , 53-88 (2009).
- Bourdin, B., et al. Functional Characterization of Cavalpha2delta Mutations Associated with Sudden Cardiac Death. J. Biol. Chem. 290, 2854-2869 (2015).
- Tetreault, M. P., et al. Identification of glycosylation sites essential for surface expression of the Cavalpha2delta1 subunit and modulation of the cardiac Cav1.2 channel activity. J. Biol. Chem. 291, 4826-4843 (2016).
- Senatore, A., Boone, A. N., Spafford, J. D. Optimized Transfection Strategy for Expression and Electrophysiological Recording of Recombinant Voltage-Gated Ion Channels in HEK-293T Cells. J Vis Exp. (47), (2011).
- Herzenberg, L. A., Tung, J., Moore, W. A., Herzenberg, L. A., Parks, D. R. Interpreting flow cytometry data: a guide for the perplexed. Nat.Immunol. 7, 681-685 (2006).
- Shakeri, B., Bourdin, B., Demers-Giroux, P. O., Sauve, R., Parent, L. A quartet of Leucine residues in the Guanylate Kinase domain of Cavbeta determines the plasma membrane density of the Cav2.3 channel. J Biol Chem. 287, 32835-32847 (2012).
- Morton, R. A., Baptista-Hon, D. T., Hales, T. G., Lovinger, D. M. Agonist- and antagonist-induced up-regulation of surface 5-HT3A receptors. Br. J. Pharmacol. 172, 4066-4077 (2015).
- Hoffmann, C., et al. Fluorescent labeling of tetracysteine-tagged proteins in intact cells. Nat. Protocols. 5, 1666-1677 (2010).
- Cockcroft, C. J., Gamper, N. . Ion Channels: Methods and Protocols. , 233-241 (2013).
- Gonzalez-Gutierrez, G., Miranda-Laferte, E., Neely, A., Hidalgo, P. The Src Homology 3 Domain of the beta-Subunit of Voltage-gated Calcium Channels Promotes Endocytosis via Dynamin Interaction. J. Biol.Chem. 282, 2156-2162 (2007).
- Galizzi, J. P., Borsotto, M., Barhanin, J., Fosset, M., Lazdunski, M. Characterization and photoaffinity labeling of receptor sites for the Calcium channel inhibitors d-cis-diltiazem, (+/-)-bepridil, desmethoxyverapamil, and (+)-PN 200-110 in skeletal muscle transverse tubule membranes. J. Biol.Chem. 261, 1393-1397 (1986).
- Bezanilla, F. The voltage sensor in voltage-dependent ion channels. Physiol.Rev. 80, 555-592 (2000).
- Sigworth, F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 307, 97-129 (1980).
- Bailey, M. A., Grabe, M., Devor, D. C. Characterization of the PCMBS-dependent modification of KCa3.1 channel gating. J. Gen. Physiol. 136, 367-387 (2010).
- Fletcher, P. A., Scriven, D. R., Schulson, M. N., Moore, E. D. Multi-Image Colocalization and Its Statistical Significance. Biophys. J. 99, 1996-2005 (2010).
- Lizotte, E., Tremblay, A., Allen, B. G., Fiset, C. Isolation and characterization of subcellular protein fractions from mouse heart. Anal. Biochem. 345, 47-54 (2005).
- Mattheyses, A. L., Simon, S. M., Rappoport, J. Z. Imaging with total internal reflection fluorescence microscopy for the cell biologist. J. Cell Sci. 123, 3621-3628 (2010).
- Yamamura, H., Suzuki, Y., Imaizumi, Y. New light on ion channel imaging by total internal reflection fluorescence (TIRF) microscopy. J. Pharmacol. Sci. 128, 1-7 (2015).
- Wible, B. A., et al. HERG-Lite-R: A novel comprehensive high-throughput screen for drug-induced hERG risk. J. Pharmacol. Toxicol. Methods. 52, 136-145 (2005).
- Wilde, A. A. M., Brugada, R. Phenotypical Manifestations of Mutations in the Genes Encoding Subunits of the Cardiac Sodium Channel. Circ. Res. 108, 884-887 (2011).
- Milano, A., et al. Sudden Cardiac Arrest and Rare Genetic Variants in the Community. Circ Cardiovasc Genet. , (2016).
- Schnell, U., Dijk, F., Sjollema, K. A., Giepmans, B. N. G. Immunolabeling artifacts and the need for live-cell imaging. Nat. Meth. 9, 152-158 (2012).

