A Microcontroller Operated Device for the Generation of Liquid Extracts from Conventional Cigarette Smoke and Electronic Cigarette Aerosol
Summary
Here, we describe a programmable laboratory device that can be used to create extracts of conventional cigarette smoke and electronic cigarette aerosol. This method provides a useful tool for making direct comparisons between conventional cigarettes and electronic cigarettes, and is an accessible entry point into electronic cigarette research.
Abstract
Electronic cigarettes are the most popular tobacco product among middle and high schoolers and are the most popular alternative tobacco product among adults. High quality, reproducible research on the consequences of electronic cigarette use is essential for understanding emerging public health concerns and crafting evidence based regulatory policy. While a growing number of papers discuss electronic cigarettes, there is little consistency in methods across groups and very little consensus on results. Here, we describe a programmable laboratory device that can be used to create extracts of conventional cigarette smoke and electronic cigarette aerosol. This protocol details instructions for the assembly and operation of said device, and demonstrates the use of the generated extract in two sample applications: an in vitro cell viability assay and gas-chromatography mass-spectrometry. This method provides a tool for making direct comparisons between conventional cigarettes and electronic cigarettes, and is an accessible entry point into electronic cigarette research.
Introduction
Despite a concentrated effort by health organizations, tobacco product use remains the leading cause of preventable death worldwide, with the majority of these deaths attributed to cigarette smoking1. Since entering the market in 2003, electronic cigarettes have been growing in popularity among tobacco product users. Currently, electronic cigarettes are the most popular alternative to conventional cigarettes among American adults (~5%)2 and the most popular nicotine delivery system among middle (~5.3%) and high schoolers (~16%)3. If current trends continue, electronic cigarettes can be expected to replace conventional cigarettes for future generations. However, the health consequences of electronic cigarette use remain unclear.
Research on electronic cigarettes did not start in earnest until electronic cigarette popularity rapidly increased in 20133,4. Since that time, a number of different models have been employed to address the question of their toxicity. However, the results of many studies are conflicting, and while it seems that electronic cigarettes are generally less toxic than conventional cigarettes there is no current consensus on the health consequences of electronic cigarette use5,6,7. Our previous research indicates that electronic cigarettes are significantly less toxic to the vascular endothelium than conventional cigarettes, despite their ability to cause DNA damage and the induction of oxidative stress and cell death8. However, more research is necessary before we can draw firm conclusions about the health consequences of electronic cigarette use.
As conventional cigarettes are a leading cause of preventable vascular disease9, there is a growing interest in the vascular health risk of electronic cigarette use10,11,12. In order to study the effects of electronic cigarettes on the vascular system, our lab developed a microcontroller operated smoking/vaping device (Figure 1)8. This device is capable of generating liquid extracts of either conventional cigarette smoke or electronic cigarette aerosol in either aqueous or organic solvents. As airflow is controlled by the combination of an adjustable air flow regulator and a PBASIC timing program, the device can be used to generate extracts according to any number of user defined protocols. Here we detail the assembly and operation of this device as well as two potential applications: in vitro cell viability assessment and gas-chromatography mass-spectrometry.
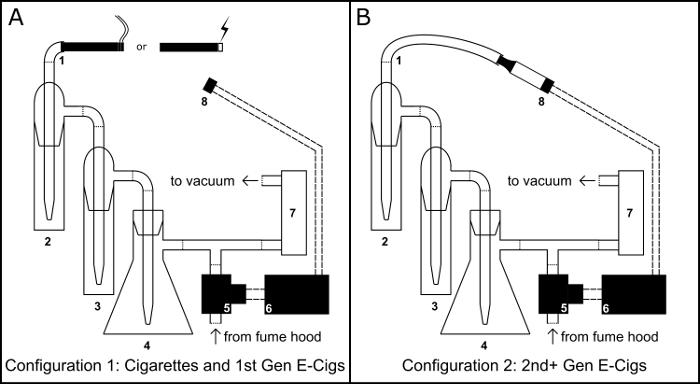
Figure 1: Smoking/Vaping Device. Schematic for the physical assembly of the smoking/vaping device in both the cigarette/cigarette like electronic cigarette (e-cig) configuration (A) and the tank electronic cigarette configuration (B). Component Key: 1) Inhalation port; 2) primary collection impinger; 3) overflow impinger; 4) Buchner flask vacuum trap; 5) normally open solenoid valve; 6) BS1 microcontroller; 7) air flow regulator; 8) 510 threaded electronic cigarette tank base. Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
The most critical elements of this protocol are ensuring the device is clean at the start and finish of each extraction, and ensuring that all seals are maintained so that air flow remains consistent. If the device is not properly cleaned, there is a risk of carry over between samples. Additionally, if the device is left unclean for an extended period of time condensed aerosol and dried solvent can block the system. Note that it is normal for there to be a pressure drop when puffing a conventional cigarette and the airfl…
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge the assistance of Dr. Robert Dotson of the Tulane University Department of Cell and Molecular Biology for his assistance in editing the manuscript and Dr. James Bollinger of the Tulane University Department of Chemistry for his assistance with mass spectrometry protocol design. The authors further acknowledge the Tulane University Department of Cell and Molecular Biology and the Tulane University Department of Chemistry for their support and the use of space and equipment. This work was supported by a Tobacco Product Regulatory Science Research Fellowship to C. Anderson from the Tulane University School of Science and Engineering.
Materials
| 12 V AC/DC Wall Mount Adaptor | Digi-Key | T1099-P5P-ND | |
| 2.2 Ohm Resistors | Digi-Key | A105635-ND | Used in tandem to generate the 4.4 Ohm resistance in Figure 2A |
| 330 Ohm Resistors | Digi-Key | 330QBK-ND | |
| 510 Threaded Base | NJoy | N/A | Recovered by dismantalling a second generation NJoy electronic cigarette |
| Acetic Acid, Glacial | Sigma-Aldritch | A6283 | |
| Acetone (Chromatography Grade) | Sigma-Aldritch | 34850 | |
| Basic Stamp Project Board | Digi-Key | 27112-ND | This board contains the BS1 Microcontroller, serial adaptor, power switch, and a barrel pin connector for the AC/DC Wall Mount Adaptor |
| Basic Stamp USB to Serial Adapter | Digi-Key | 28030-ND | An optional component to allow the BS1 serial adaptor to communicate through USB |
| Buchner Flask (Vacuum Flask) 250 mL | VWR | 10545-854 | |
| Clear Tape | 3M | S-9783 | |
| Clear Vinyl Tubing, 3/8" ID | Watts | 443064 | |
| EGM-2 Endothelial Cell Culture Medium | Lonza | CC-3162 | |
| Ethanol | Pharmco-Aaper | 111000200 | |
| Flow Regulator | Dwyer | VFA-23-BV | |
| Gas Chromatograph | Varian | 450-GC | |
| Glass Syringe, 10 mL | Sigma-Aldritch | Z314552 | |
| Glass Syringe, 10 µL | Hamilton | 80300 | |
| High Vacuum Silicon Grease | Dow Corning | 146355D | |
| Hose Clamp | Precision Brand | 35125 | |
| Human Umbilical Vein Endothelial Cells | ATCC | PCS-100-013 | |
| Mass Spectrometer | Varian | 300-MS | |
| Midget Impinger | Chemglass | CG-1820-01 | |
| Neutral Red | Sigma-Aldritch | N4638 | |
| Paraffin Film | 3M | PM-992 | |
| Plate Seal Roller | BioRad | MSR0001 | |
| Plate Seal; Foil | Thermo | 276014 | |
| Ring Stand 20" | American Educational Products | 7-G15-A | |
| Solenoid Valve (normally open) | US Solid | USS2-00081 | |
| Solid State Relay | Digi-Key | CLA279-ND | |
| Stand Clamp | Eisco | CH0688 | |
| Syringe Filter, PES, 0.22 um | Millipore | SLGP033RS | |
| Syringe, 10 mL | BD Syringe | 309604 | |
| Through Hole Stopper, Size 6 | VWR | 59581-287 | |
| Vacuum Pump | KNF Neuberger | N86KTP |
Referenzen
- World Health Organization. . WHO Report on the Global Tobacco Epidemic, 2011. , (2011).
- Weaver, S. R., Majeed, B. A., Pechacek, T. F., Nyman, A. L., Gregory, K. R., Eriksen, M. P. Use of electronic nicotine delivery systems and other tobacco products among USA adults, 2014: results from a national survey. Int. J. Public Health. 61 (2), 177-188 (2016).
- Singh, T., et al. Tobacco Use Among Middle and High School Students – United States, 2011–2015. MMWR Morb. Mortal. Wkly. Rep. 65 (14), 361-367 (2016).
- Corey, C. G., Ambrose, B. K., Apelberg, B. J., King, B. A. Flavored Tobacco Product Use Among Middle and High School Students–United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 64 (38), 1066-1070 (2015).
- Pisinger, C., Døssing, M. A systematic review of health effects of electronic cigarettes. Prev. Med. 69, 248-260 (2014).
- Callahan-Lyon, P. Electronic cigarettes: human health effects. Tob. Control. 23 (Suppl 2), ii36-ii40 (2014).
- Dinakar, C., O’Connor, G. T. The Health Effects of Electronic Cigarettes. N. Engl. J. Med. 375 (14), 1372-1381 (2016).
- Anderson, C., Majeste, A., Hanus, J., Wang, S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol. Sci. Off. J. Soc. Toxicol. , (2016).
- U.S. Department of Health and Human Services. . The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. , (2014).
- Farsalinos, K., et al. Comparison of the Cytotoxic Potential of Cigarette Smoke and Electronic Cigarette Vapour Extract on Cultured Myocardial Cells. Int. J. Environ. Res. Public. Health. 10 (10), 5146-5162 (2013).
- Schweitzer, K. S., et al. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am. J. Physiol. – Lung Cell. Mol. Physiol. 309 (2), L175-L187 (2015).
- Putzhammer, R., et al. Vapours of US and EU Market Leader Electronic Cigarette Brands and Liquids Are Cytotoxic for Human Vascular Endothelial Cells. PLOS ONE. 11 (6), e0157337 (2016).
- Crooks, I., Dillon, D. M., Scott, J. K., Ballantyne, M., Meredith, C. The effect of long term storage on tobacco smoke particulate matter in in vitro genotoxicity and cytotoxicity assays. Regul. Toxicol. Pharmacol. 65 (2), 196-200 (2013).
- Roemer, E., et al. Mainstream Smoke Chemistry and in Vitro and In Vivo Toxicity of the Reference Cigarettes 3R4F and 2R4F. Beitr. Zur Tab. Contrib. Tob. Res. 25 (1), (2014).
- International Organization for Standards. . ISO 3088:2012 Routine analytical cigarette smoking machine – Definitions and standard conditions. , (2012).
- World Health Organization. . Standard Operating Procedure for Intense Smoking of Cigarettes. , (2012).
- Brown, C. J., Cheng, J. M. Electronic cigarettes: product characterisation and design considerations. Tob. Control. 23 (Suppl 2), ii4-ii10 (2014).
- Cooperation Centre for Scientific Research Relative to Tobacco. . CRM No. 81 – Routine Analytical Machine for E-Cigarette Aerosol Generation and Collection – Definitions and Standard Conditions. , (2015).
- Thorne, D., Adamson, J. A review of in vitro cigarette smoke exposure systems. Exp. Toxicol. Pathol. 65 (7-8), 1183-1193 (2013).
- Klus, H., Boenke-Nimphius, B., Müller, L. Cigarette Mainstream Smoke: The Evolution of Methods and Devices for Generation, Exposure and Collection. Beitr. Zur Tab. Contrib. Tob. Res. 27 (4), (2016).
- Baker, R. The Development and Significance of Standards for Smoking-Machine Methodology. Beitr. Zur Tab. Contrib. Tob. Res. 20 (1), (2014).
- Thorne, D., Crooks, I., Hollings, M., Seymour, A., Meredith, C., Gaca, M. The mutagenic assessment of an electronic-cigarette and reference cigarette smoke using the Ames assay in strains TA98 and TA100. Mutat. Res. Toxicol. Environ. Mutagen. 812, 29-38 (2016).
- Thorne, D., Larard, S., Baxter, A., Meredith, C., Gaҫa, M. The comparative in vitro assessment of e-cigarette and cigarette smoke aerosols using the γH2AX assay and applied dose measurements. Toxicol. Lett. 265, 170-178 (2017).
- Herrington, J. S., Myers, C. Electronic cigarette solutions and resultant aerosol profiles. J. Chromatogr. A. 1418, 192-199 (2015).
- Yu, V., et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 52, 58-65 (2016).
- Ji, E. H., et al. Characterization of Electronic Cigarette Aerosol and Its Induction of Oxidative Stress Response in Oral Keratinocytes. PLOS ONE. 11 (5), e0154447 (2016).
- Morgan, D. L., et al. Chemical Reactivity and Respiratory Toxicity of the -Diketone Flavoring Agents: 2,3-Butanedione, 2,3-Pentanedione, and 2,3-Hexanedione. Toxicol. Pathol. 44 (5), 763-783 (2016).
- Cooperation Centre for Scientific Research Relative to Tobacco. . CRM No. 84 – Determination of Glycerin, Propylene Glycol, Water, and Nicotine in the Aerosol of E-Cigarettes by Gas Chromatographic Analysis. , (2017).

