对河流生态系统中巨脊椎动物和鱼类的大小谱进行建模
Summary
这是一个协议,用于对来自可涉水溪流和河流的鱼类和无脊椎动物数据的大小谱(个体质量与种群密度之间的缩放关系)进行建模。方法包括:采集定量鱼类和无脊椎动物样品的田间技术;实验室方法标准化现场数据;和统计数据分析。
Abstract
大小谱是平均身体质量(M)与生态社区或食物网内个体密度(D)之间的反向、均等缩放关系。重要的是,大小谱假定个体大小,而不是物种的行为或生命历史特征,是生态系统内丰度的主要决定因素。因此,与侧重于物种级数据(例如,平均物种的体型与种群密度)的传统等值关系不同,大小光谱分析是”非正数”-单个标本仅按其大小进行识别,而不考虑分类标识。尺寸光谱模型是传统复杂食品网的有效表示形式,可用于描述性和预测性上下文(例如,预测大型消费者对基础资源变化的反应)。来自不同水生生态系统的实证研究也报告,大小光谱斜率的中度到高水平相似程度,表明共同过程可能调节在非常不同的环境中小型和大型生物体的丰度。这是一种在可涉水流中模拟社区级大小频谱的协议。该协议由三个主要步骤组成。首先,收集定量底栖鱼类和无脊椎动物样本,用于估计局部密度。其次,通过将所有个体转换为非塔克单位(即按大小识别的个人,而不考虑分类身份),并在日志2大小箱内对个体进行求和,使鱼类和无脊椎动物数据标准化。第三,使用线性回归来建模非税M和D估计值之间的关系。 此处提供了详细说明,以完成每个步骤,包括自定义软件,以方便D估计和大小光谱建模。
Introduction
体型缩放关系,如身体质量和代谢率之间的正关联,在个体生物体水平上是众所周知的,目前正在组织1,2,3的较高层次进行研究.这些等分关系通常是形式Y = aMb的功率定律函数,其中Y是感兴趣的变量(例如,新陈代谢、丰度或家庭范围大小),M 是单个或平均值的体质量单个,b 是缩放系数,a 是常量。 为了便于统计,Y 和M数据通常在分析之前进行对数转换,然后用形式日志 (Y) 的线性方程 + 日志 (a) = b日志 (M) 进行建模,其中b和日志 ( a) 分别成为线性模型斜率和截距。
大小谱是一种等值关系,用于预测密度(D,单位面积的个体数量)或生物量(B,单位面积的个体总质量)作为M的函数(参见第 4节,用于增加关于使用”标准化”D 或B估计的信息。与M和D之间或M和B之间的其他缩放关系一样,大小谱在基础生态学和应用生态学中起着核心作用。在种群一级,生物学家经常将负D 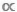 M关系解释为密度依赖生存的证据,或解释为生态系统承载能力的模型(即”自我稀释规则”)4, 5.在社区一级,B
M关系解释为密度依赖生存的证据,或解释为生态系统承载能力的模型(即”自我稀释规则”)4, 5.在社区一级,B 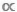 M关系可用于研究人为扰动的系统级影响,如大小选择性捕鱼6,7。D和B与M的均值缩放也是最近将人口、社区和生态系统生态统一到2、8、9的努力的核心。
M关系可用于研究人为扰动的系统级影响,如大小选择性捕鱼6,7。D和B与M的均值缩放也是最近将人口、社区和生态系统生态统一到2、8、9的努力的核心。
大小谱的一个特别重要的特点是它完全是无税的9,10。在比较D 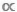 M或B
M或B 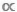 M数据的散点图时,很容易遗漏这一点,但分类模型和非税模型之间的区别至关重要。在分类模型中,单个M值用于表示给定物种或 taxa11中每个人的平均身体质量。在 ataxic 模型中,数据集中的所有个体都分区在一系列正文大小间隔或M bin 之间,而不管其分类标识12 。后者,无税方法在水生生态系统中是有利的,因为许多分类动物表现出不确定的生长,在喂养行为中经历一种或多次遗传变化;在这些情况下,单个物种级M平均值将掩盖一个事实,即一个物种可以在其生命史9、13、14中扮演不同的功能角色。
M数据的散点图时,很容易遗漏这一点,但分类模型和非税模型之间的区别至关重要。在分类模型中,单个M值用于表示给定物种或 taxa11中每个人的平均身体质量。在 ataxic 模型中,数据集中的所有个体都分区在一系列正文大小间隔或M bin 之间,而不管其分类标识12 。后者,无税方法在水生生态系统中是有利的,因为许多分类动物表现出不确定的生长,在喂养行为中经历一种或多次遗传变化;在这些情况下,单个物种级M平均值将掩盖一个事实,即一个物种可以在其生命史9、13、14中扮演不同的功能角色。
在这里,我们提出了一个完整的协议,以量化在可涉水溪流和河流的大小频谱。该协议从现场采样方法开始,以收集必要的鱼类和底栖巨脊椎动物数据。鱼将通过”三通耗竭”取样过程收集。然后,将从使用 Zippin 方法15的损耗数据中估计丰度。在消耗取样中,封闭研究范围内的单个鱼类(即,个人不能进入或离开封闭接触)通过连续三个样本从接触点中取出。因此,剩余的鱼类数量将逐渐枯竭。从这个消耗趋势,可以估计研究范围内的总丰度,然后根据研究范围的已知表面积转换为D(以每米2的鱼)。底栖巨脊椎动物将采用标准固定区域取样器收集,然后在实验室中识别和测量。
接下来,组合的鱼和宏无脊椎动物数据将在大小箱之间划分。传统上,倍频程或对数2刻度(即双倍间隔)用于设置大小 bin 边界16。建立大小箱列表后,将单个底栖宏脊椎动物划分到各自的大小箱中非常简单,因为无脊椎动物直接枚举为单位区域的个体数。然而,估计大小箱内的鱼类丰度更抽象,因为这些估计是从消耗数据中推断的。因此,提供了详细的说明,以便从损耗样本数据中估计大小箱内的鱼类丰度,而不论分类特征如何。
最后,线性回归将用于对大小频谱进行建模。该协议与克尔和Dickie16的原始一般方法完全兼容,与麦加维和柯克在西弗吉尼亚州溪流中鱼类和无脊椎动物大小光谱研究中所使用的方法相同。通过使用这个协议,研究人员可以确保他们的结果能够与基于克尔和Dickie16的其他研究直接比较,从而加速对淡水体尺寸缩放关系的广泛而有力的理解生态系统和推动他们的机制。
Protocol
Representative Results
Discussion
这种非税大小光谱协议可用于定量和建模流鱼和无脊椎动物群落内的大小结构。以往在河流生态系统中的大小光谱研究范围包括基本描述性研究39、40、纵向河流剖面41和不同生物地理区域42的比较。季节比较已经进行了43,44和最近,大小光谱参数的季节性变化已与水温和水文17。</…
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
这项工作的资金由美国国家科学基金会(DEB-1553111赠款)和埃普利科学研究基金会提供。本手稿是VCU赖斯河中心贡献#89。
Materials
| Chest waders | Multiple options | n/a | Personal protective equipment for use during electrofishing. Do NOT use 'breatheable' waders as electrical current will pass through them. |
| Rubber lineman's gloves | Multiple options | n/a | Personal protective equipment for use during electrofishing. |
| Dip nets with fiberglass poles | Multiple options | n/a | Used to capture stunned fishes during electrofishing. |
| Backpack electrofishing unit | Smith-Root; Halltech; Midwest Lake Management; Aqua Shock Solutions | www.smith-root.com; www.halltechaquatic.com; https://midwestlake.com; https://aquashocksolutions.com/ | Backpack electrofishers are currently manufactured and distributed by four independent companies in North America. Prices and warranty/technical support are the most important factors in choosing a vendor. |
| Block nets/seines (×2) | Duluth Nets | https://duluthfishnets.com/ | Necessary length will depend on stream width. 3/8 inch mesh is recommended. |
| Cam-action utility straps with 1 inch nylon webbing (×4) | Multiple options | n/a | Used to secure/anchor block nets. Available at auto supply, hardware, and department stores. |
| Large tent stakes (×4) | Multiple options | n/a | Used to secure/anchor block nets. Available at camping and department stores. |
| 5 gallon plastic buckets (×5) | Multiple options | n/a | Used to hold and transport fish during electrofishing. Available at hardware and paint supply stores. |
| 10-20 gallon totes (×3) | Multiple options | n/a | Used as livewells, sedation tanks, and recovery bins for captured fishes. Available at hardware and department stores. |
| Battery powered 'bait bucket' aeration pumps | Cabelas | IK-019008 | Used to aerate fish holding bins during field processing. |
| Fish anesthesia (Tricaine-S) | Syndel | www.syndel.com | Used to sedate fishes for field processing. Tricaine-S is regulated by the U.S. Food and Drug Administration. |
| Folding camp table and chairs | Cabelas | IK-518976; IK-552777 | Used to process fish samples. |
| Pop-up canopy | Multiple options | n/a | Used as necessary for sun and rain protection. |
| Fish measuring board | Wildco | 3-118-E40 | Used to measure fish lengths. |
| Battery powered field scale with weighing dish | Multiple options | n/a | Used to weigh fishes. Must weigh be accurate to 0.1 or 0.01 grams. |
| Clear plastic wind/rain baffle | Multiple options | n/a | Used to shield scale in rainy or windy conditions. Must be large enough to cover the scale and a weighing dish. |
| White plastic or enamel examination trays | Multiple options | n/a | Trays are essential for examining fishes in the field. |
| Stainless steel forceps | Multiple options | n/a | Forceps are helpful when examining small fishes and in transfering invertebrates to specimen jars. |
| Hand magnifiers | Multiple options | n/a | Magnification is often helpful when identifying fish specimens in the field. |
| Fish identification keys | n/a | n/a | Laminated keys that are custom prepared for specific locations are most effective. |
| Datasheets printed on waterproof paper | Rite in the Rain | n/a | Waterproof paper is essential when working with aquatic specimens. |
| Retractable fiberglass field tapes | Lufkin | n/a | Used to measure stream channel dimensions. |
| Surber sampler or Hess sampler | Wildco | 3-12-D56; 3-16-C52 | Either of these fixed-area benthic samplers will work well in shallow streams with gravel or pebble substrate. |
| 70% ethanol or isopropyl alcohol | Multiple options | n/a | Used as invertebrate preservative. |
| Widemouth invertebrate specimen jars (20-32 oz.) | U.S. Plastic Corp. | 67712 | Any widemouth plastic jars will work but these particular jars are durable and inexpensive. |
Referenzen
- Peters, R. H. . The ecological implications of body size. , (1983).
- Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., West, G. B. Toward a metabolic theory of ecology. Ecology. 85 (7), 1771-1789 (2004).
- Marquet, P. A., et al. Scaling and power-laws in ecological systems. Journal of Experimental Biology. 208 (9), 1749-1769 (2005).
- Bohlin, T., Dellefors, C., Faremo, U., Johlander, A. The energetic equivalence hypothesis and the relation between population-density and body-size in stream-living salmonids. The American Naturalist. 143 (3), 478-493 (1994).
- Dunham, J. B., Vinyard, G. L. Relationships between body mass, population density, and the self-thinning rule in stream-living salmonids. Canadian Journal of Fisheries and Aquatic Sciences. 54 (5), 1025-1030 (1997).
- Jennings, S., Blanchard, J. L. Fish abundance with no fishing: predictions based on macroecological theory. Journal of Animal Ecology. 73 (4), 632-642 (2004).
- Petchey, O. L., Belgrano, A. Body-size distributions and size-spectra: universal indicators of ecological status?. Biology Letters. 6 (4), 434-437 (2010).
- Woodward, G., et al. Body size in ecological networks. Trends in Ecology and Evolution. 20 (7), 402-409 (2005).
- Trebilco, R., Baum, J. K., Salomon, A. K., Dulvy, N. K. Ecosystem ecology: size-based constraints on the pyramids of life. Trends in Ecology and Evolution. 28 (7), 423-431 (2013).
- White, E. P., Ernest, S. K. M., Kerkhoff, A. J., Enquist, B. J. Relationships between body size and abundance in ecology. Trends in Ecology and Evolution. 22 (6), 323-330 (2007).
- Schmid, P. E., Tokeshi, M., Schmid-Araya, J. M. Relation between population density and body size in stream communities. Science. 289 (5484), 1557-1560 (2000).
- Morin, A., Nadon, D. Size distribution of epilithic lotic invertebrates and implications for community metabolism. Journal of the North American Benthological Society. 10 (3), 300-308 (1991).
- Mittelbach, G. G., Persson, L. The ontogeny of piscivory and its ecological consequences. Canadian Journal of Fisheries and Aquatic Sciences. 55 (6), 1454-1465 (1998).
- Woodward, G., Hildrew, A. G. Body-size determinants of niche overlap and intraguild predation within a complex food web. Journal of Animal Ecology. 71 (6), 1063-1074 (2002).
- Zippin, C. The removal method of population estimation. Journal of Wildlife Management. 22 (1), 82-90 (1958).
- Kerr, S. R., Dickie, L. M. . The biomass spectrum: a predator-prey theory of aquatic production. , (2001).
- McGarvey, D. J., Kirk, A. J. Seasonal comparison of community-level size-spectra in southern coalfield streams of West Virginia (USA). Hydrobiologia. 809 (1), 65-77 (2018).
- Reynolds, J. B., Kolz, A. L., Zale, A. V., Parrish, D. L., Sutton, T. M. Electrofishing. Fisheries techniques. 8, 305-361 (2012).
- Bowker, J., Trushenski, J. Fish drug questions answered by the FDA. Fisheries. 38 (12), 549-552 (2013).
- Topic Popovic, N., et al. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. Journal of Applied Ichthyology. 28 (4), 553-564 (2012).
- Trautman, M. B. . The fishes of Ohio. , (1981).
- Riley, S. C., Fausch, K. D. Underestimation of trout population size by maximum-likelihood removal estimates in small streams. North American Journal of Fisheries Management. 12 (4), 768-776 (1992).
- Merritt, R. W., Cummins, K. W., Resh, V. H., Batzer, D. P., Merritt, R. W., Cummins, K. W., Berg, M. B. Sampling aquatic insects: collection devices, statistical considerations, and rearing procedures. An introduction to the aquatic insects of North America. , 15-37 (2008).
- Hauer, F. R., Resh, V. H., Hauer, F. R., Lamberti, G. A. Macroinvertebrates. Methods in stream ecology. 1, 297-319 (2017).
- Thorp, J. H., Covich, A. P. . Ecology and classification of North American freshwater invertebrates. , (2010).
- Merritt, R. W., Cummins, K. W., Berg, M. B. . An introduction to the aquatic insects of North America. , (2008).
- Stewart, K. W., Stark, B. P. . Nymphs of North American stonefly genera (Plecoptera). , (2002).
- Wiggins, G. B. . Larvae of the North American caddisfly genera (Trichoptera). , (1998).
- Benke, A. C., Huryn, A. D., Smock, L. A., Wallace, J. B. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the Southeastern United States. Journal of the North American Benthological Society. 18 (3), 308-343 (1999).
- Smock, L. A. Relationships between body size and biomass of aquatic insects. Freshwater Biology. 10 (4), 375-383 (1980).
- Waters, T. F. Secondary production in inland waters. Adv. Ecol. Res. 10, 91-164 (1977).
- Carle, F. L., Strub, M. R. New method for estimating population-size from removal data. Biometrics. 34 (4), 621-630 (1978).
- Ogle, D. H., Wheeler, P., Dinno, A. FSA: fisheries stock analysis. R package version 0.8.22.9000. , (2018).
- Lockwood, R. N., Schneider, J. C., Schneider, J. C. Stream fish population estimates by mark-and-recapture and depletion methods. Manual of fisheries survey methods II: with periodic updates. 7, (2000).
- Blanco, J. M., Echevarría, F., García, C. M. Dealing with size-spectra: some conceptual and mathematical problems. Scientia Marina. 58 (1-2), 17-29 (1994).
- White, E. P., Enquist, B. J., Green, J. L. On estimating the exponent of power-law frequency distributions. Ecology. 89 (4), 905-912 (2008).
- Vidondo, B., Prairie, Y. T., Blanco, J. M., Duarte, C. M. Some aspects of the analysis of size spectra in aquatic ecology. Limnology and Oceanography. 42 (1), 184-192 (1997).
- Sprules, W. G., Barth, L. E. Surfing the biomass size spectrum: some remarks on history, theory, and application. Canadian Journal of Fisheries and Aquatic Sciences. 73 (4), 477-495 (2016).
- Poff, N. L., et al. Size structure of the metazoan community in a Piedmont stream. Oecologia. 95 (2), 202-209 (1993).
- Ramsay, P. M., et al. A rapid method for estimating biomass size spectra of benthic metazoan communities. Canadian Journal of Fisheries and Aquatic Sciences. 54 (8), 1716-1724 (1997).
- Solimini, A. G., Benvenuti, A., D’Olimpio, R., Cicco, M. D., Carchini, G. Size structure of benthic invertebrate assemblages in a Mediterranean river. Journal of the North American Benthological Society. 20 (3), 421-431 (2001).
- Huryn, A. D., Benke, A. C., Hildrew, A., Raffaelli, D., Edmonds-Brown, R. Relationship between biomass turnover and body size for stream communities. Body size: the structure and function of aquatic ecosystems. 4, 55-76 (2007).
- Gaedke, U. The size distribution of plankton biomass in a large lake and its seasonal variability. Limnology and Oceanography. 37 (6), 1202-1220 (1992).
- Stead, T. K., Schmid-Araya, J. M., Schmid, P. E., Hildrew, A. G. The distribution of body size in a stream community: one system, many patterns. Journal of Animal Ecology. 74 (3), 475-487 (2005).
- Brose, U., et al. Consumer-resource body-size relationships in natural food webs. Ecology. 87 (10), 2411-2417 (2006).
- Mehner, T., et al. Empirical correspondence between trophic transfer efficiency in freshwater food webs and the slope of their size spectra. Ecology. 99 (6), 1463-1472 (2018).
- Daan, N., Gislason, H. G., Pope, J. C., Rice, J. Changes in the North Sea fish community: evidence of indirect effects of fishing?. ICES Journal of Marine Science. 62 (2), 177-188 (2005).
- Murry, B. A., Farrell, J. M. Resistance of the size structure of the fish community to ecological perturbations in a large river ecosystem. Freshwater Biology. 59 (1), 155-167 (2014).
- Broadway, K. J., Pyron, M., Gammon, J. R., Murry, B. A. Shift in a large river fish assemblage: body-size and trophic structure dynamics. PLoS ONE. 10 (4), e0124954 (2015).
- Vila-Martínez, N., Caiola, N., Ibáñez, C., Benejam, L., Brucet, S. Normalized abundance spectra of fish community reflect hydro-peaking on a Mediterranean large river. Ecological Indicators. 97, 280-289 (2019).
- Brucet, S., et al. Size-based interactions across trophic levels in food webs of shallow Mediterranean lakes. Freshwater Biology. 62 (11), 1819-1830 (2017).
- Ersoy, Z., et al. Size-based interactions and trophic transfer efficiency are modified by fish predation and cyanobacteria blooms in Lake Mývatn, Iceland. Freshwater Biology. 62 (11), 1942-1952 (2017).
- Arranz, I., Hsieh, C. H., Mehner, T., Brucet, S. Systematic deviations from linear size spectra of lake fish communities are correlated with predator–prey interactions and lake-use intensity. Oikos. 128 (1), 33-44 (2019).
- Jennings, S., et al. Long-term trends in the trophic structure of the North Sea fish community: evidence from stable-isotope analysis, size-spectra and community metrics. Marine Biology. 141 (6), 1085-1097 (2002).
- Guiet, J., Poggiale, J. C., Maury, O. Modelling the community size-spectrum: recent developments and new directions. Ecological Modelling. 337, 4-14 (2016).
- Robinson, J. P. W., et al. Fishing degrades size structure of coral reef fish communities. Global Change Biology. 23 (3), 1009-1022 (2017).
- Reuman, D. C., Mulder, C., Raffaelli, D., Cohen, J. E. Three allometric relations of population density to body mass: theoretical integration and empirical tests in 149 food webs. Ecology Letters. 11 (11), 1216-1228 (2008).
- Huryn, A. D., Wallace, J. B., Anderson, N. H., Merritt, R. W., Cummins, K. W., Berg, M. B. Habitat, life history, secondary production, and behavioral adaptations of aquatic insects. An introduction to the aquatic insects of. 5, 55-103 (2008).
- Werner, E. E., Gilliam, J. F. The ontogenetic niche and species interactions in size-structured populations. Annual Review of Ecology and Systematics. 15 (1), 393-425 (1984).
- Edwards, A. M., Robinson, J. P. W., Plank, M. J., Baum, J. K., Blanchard, J. L. Testing and recommending methods for fitting size spectra to data. Methods in Ecology and Evolution. 8 (1), 57-67 (2017).
- Roell, M., Orth, D. Production of three crayfish populations in the New River of West Virginia, USA. Hydrobiologia. 228 (3), 185-194 (1992).
- Hawkins, C. P., Murphy, M. L., Anderson, N. H., Wilzbach, M. A. Density of fish and salamanders in relation to riparian canopy and physical habitat in streams of the northwestern United States. Canadian Journal of Fisheries and Aquatic Sciences. 40 (8), 1173-1185 (1983).
- Rabeni, C. F., Collier, K. J., Parkyn, S. M., Hicks, B. J. Evaluating techniques for sampling stream crayfish (Paranephrops planifrons). New Zealand Journal of Marine and Freshwater Research. 31 (5), 693-700 (1997).
- DiStefano, R. J., Gale, C. M., Wagner, B. A., Zweifel, R. D. A sampling method to assess lotic crayfish communities. Journal of Crustacean Biology. 23 (3), 678-690 (2003).
- Price, J. E., Welch, S. M. Semi-quantitative methods for crayfish sampling: sex, size, and habitat bias. Journal of Crustacean Biology. 29 (2), 208-216 (2009).
- Sheldon, R. W., Sutcliffe, W. H., Paranjape, A. M. Structure of pelagic food chain and relationship between plankton and fish production. Journal of the Fisheries Research Board of Canada. 34 (12), 2344-2353 (1977).
- Andersen, K., et al. Asymptotic size determines species abundance in the marine size spectrum. The American Naturalist. 168 (1), 54-61 (2006).

