Summary
The protocol describes efficient and reproducible tensile biomechanical testing methods for murine tendons through the use of custom-fit 3D printed fixtures.
Abstract
Tendon disorders are common, affect people of all ages, and are often debilitating. Standard treatments, such as anti-inflammatory drugs, rehabilitation, and surgical repair, often fail. In order to define tendon function and demonstrate efficacy of new treatments, the mechanical properties of tendons from animal models must be accurately determined. Murine animal models are now widely used to study tendon disorders and evaluate novel treatments for tendinopathies; however, determining the mechanical properties of mouse tendons has been challenging. In this study, a new system was developed for tendon mechanical testing that includes 3D-printed fixtures that exactly match the anatomies of the humerus and calcaneus to mechanically test supraspinatus tendons and Achilles tendons, respectively. These fixtures were developed using 3D reconstructions of native bone anatomy, solid modeling, and additive manufacturing. The new approach eliminated artifactual gripping failures (e.g., failure at the growth plate failure rather than in the tendon), decreased overall testing time, and increased reproducibility. Furthermore, this new method is readily adaptable for testing other murine tendons and tendons from other animals.
Introduction
Tendon disorders are common and highly prevalent among the aging, athletic, and active populations1,2,3. In the United States, 16.4 million connective tissue injuries are reported each year4 and account for 30% of all injury-related physician office visits3,5,6,7,8. The most commonly affected sites include the rotator cuff, Achilles tendon, and patellar tendon9. Although a variety of non-operative and operative treatments have been explored, including anti-inflammatory drugs, rehabilitation, and surgical repair, outcomes remain poor, with limited return to function and high rates of failure5,6. These poor clinical outcomes have motivated basic and translational studies seeking to understand tendinopathy and to develop novel treatment approaches.
Tensile biomechanical properties are the primary quantitative outcomes defining tendon function. Therefore, laboratory characterization of tendinopathy and treatment efficacy must include a rigorous testing of tendon tensile properties. Numerous studies have described methods to determine the biomechanical properties of tendons from animal models such as rats, sheep, dogs, and rabbits10,11,12. However, few studies have tested the biomechanical properties of murine tendons, primarily due to the difficulties in gripping the small tissues for tensile testing. As murine models have numerous advantages for mechanistically studying tendinopathy, including genetic manipulation, extensive reagent options, and low cost, development of accurate and efficient methods to biomechanically test murine tissues is needed.
In order to properly test the mechanical properties of tendons, the tissue must be gripped effectively, without slipping or artifactual tearing at the grip interface or fracturing of the growth plate. In many cases, particularly for short tendons, the bone is gripped on one end and the tendon is gripped on the other end. Bones are typically secured by embedding them in materials such as epoxy resin13 and polymethylmethacrylate14,15. Tendons are often placed between two layers of sandpaper, glued with cyanoacrylate, and secured using compression clamps (if the cross section is flat) or in a frozen medium (if the cross section is large)15,16,17. These methods have been applied to biomechanically test murine tendons, but challenges arise due to the small size of the specimens and the compliance of the growth plate, which never ossifies18. For example, the diameter of the murine humeral head is only a few millimeters, thus making gripping of the bone difficult. Specifically, tensile testing of murine supraspinatus tendon-to-bone samples often results in failure at the growth plate rather than in the tendon or at the tendon enthesis. Similarly, biomechanical testing of the Achilles tendon is challenging. Although the Achilles tendon is larger than other murine tendons, the calcaneus is small, making gripping of this bone difficult. The bone can be removed, followed by gripping the two tendon ends; however, this precludes the testing of the tendon-to-bone attachment. Other groups report gripping the calcaneus bone using custom-made fixtures19,20, anchoring by clamps21, fixing in self curing plastic cement22 or using a conical shape slot22, yet these prior methods remain limited by low reproducibility, high gripping failure rates, and tedious preparation requirements.
The objective of the current study was to develop an accurate and efficient method for tensile biomechanical testing of murine tendons, focusing on the supraspinatus and Achilles tendons as examples. Using a combination of 3D reconstructions from native bone anatomy, solid modeling, and additive manufacturing, a novel method was developed to grip the bones. These fixtures effectively secured the bones, prevented growth plate failure, decreased specimen preparation time, and increased testing reproducibility. The new method is readily adaptable to test other murine tendons as well as tendons in rats and other animals.
Protocol
Animal studies were approved by Columbia University Institutional Animal Care and Use Committee. Mice used in this study were of a C57BL/6J background and were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). They were housed in pathogen-free barrier conditions and were provided food and water ad libitum.
1. Development of custom-fit 3D printed fixtures for gripping bone
- Bone image acquisition and 3D bone model construction
- Dissect the bone of interest in preparation for 3D model creation and 3D bone grip printing; the humerus and the calcaneus are used as examples in the current protocol.
NOTE: Detailed instructions to dissect bone-tendon-muscle specimens for mechanical testing are provided in step 2.1.1. The following steps should be followed to isolate bones for the purpose of creating 3D-printed bone grips.- Dissection of the humerus: Euthanize a mouse per IACUC-approved procedure. Remove upper extremity skin, remove all muscles over the humerus, disarticulate the elbow and glenohumeral joints, and carefully remove all connective tissues attached to the humerus.
- Dissection of the calcaneus: Euthanize a mouse per IACUC-approved procedure. Remove lower extremity skin, disarticulate the Achilles tendon-calcaneus joint and joints between calcaneus and other foot bones, and carefully remove all connective tissues attached to the calcaneus.
- Perform a microcomputed tomography scan of the entire bone, e.g., scan the humerus and calcaneus samples.
NOTE: Depending on the scanner used, the settings will be different. For the scanner used in the current study (Table of Materials), the recommended settings are: scan at an energy of 55 kVP, Al 0.25 filter, at a resolution of 6 μm.- Mix agarose powder in ultrapure water and microwave for 1-3 min until the agarose is completely dissolved. It is helpful to microwave for 30-45 s, stop and swirl, and then continue towards a boil. Fill cryotubes up to three-quarters full with agarose. Let the agarose cool for about 5-10 min.
- Insert bone into the agarose gel (this will prevent movement artifacts during scanning). Insert a cryotube with bone into the scanner.
NOTE: For the scanner used in the current study, a 16-position automatic sample changer was used for all scans. This scanner can automatically select magnification according to a sample’s size and shape.
- Reconstruct microcomputed tomography scan projection images into cross-section images. Use recommended parameters for the experimenter’s scanner/software combination.
NOTE: For the program used in the current study (Table of Materials) it is recommended to use the following reconstruction parameters: Smoothing: 0-2, Beam Hardening Correction: 45, Ring Artefact Reduction: 4-9 and to reconstruct slices in 16-bit TIFF format. - Create a 3D model and save into a standard STL format compatible with most 3D printers and rapid prototyping. For the program used in the current study (Table of Materials), do the following:
- Select the command File > Open to open the file dataset. Open the dialog File > Preferences and select the Advanced tab.
- Use the adaptive rendering algorithm to construct the 3D models. This algorithm minimizes the number of facet triangles and provides smoother surface detail. Use 10 as the locality parameter; this parameter defines the distance in pixels to the neighboring point used for finding the object border. Minimize tolerance to 0.1 to decrease file size.
NOTE: After opening the dataset, the images are shown in the “Raw Images” page. - To specify the volume of interest (VOI), manually select two images to set as the top and bottom of the selected VOI range.
- Move to the second page, Region of Interest. Manually select the region of interest on a single cross section image.
NOTE: The selected region will be highlighted in red (i.e., the humerus cross-sectional area). - Repeat the previous step every 10–15 cross-section images.
- Move to the third page Binary Selection. On the histogram menu, click From Dataset. The histogram distribution of brightness from all images of the dataset will be shown. Also on the histogram menu, click the Create a 3D Model file menu.
- Save a 3D model of the bone in STL file format.
- Refine the mesh: Manipulate the mesh to reduce the size of the STL file and make it compatible with any solid modeling computer-aided design program. For the program used in the current study (Table of Materials), follow the steps below:
- Import mesh and select all to edit. Choose Reduce from the toolset Edit. Then, select Triangle Budget from the toolset Reduce Target. Reduce the Tri Count and accept changes. Resave the newly reduced file in STL format by choosing Export as…
- Dissect the bone of interest in preparation for 3D model creation and 3D bone grip printing; the humerus and the calcaneus are used as examples in the current protocol.
- Design of custom-fit bone fixtures
- Supraspinatus tendon-humeral bone
- Use a solid modeling computer-aided design program to create a custom-fit model of humerus gripping fixture (Figure 1, Supplemental Files).
NOTE: The program used in the current study is listed in the Table of Materials. - Open the STL format file of the humerus bone in a solid modeling program and save as a part file.
NOTE: For the software used in the current study (Table of Materials), the 3D bone object was saved in SLDPRT format. - Open the part file and manually create three anatomically relevant planes (i.e., sagittal, coronal, transverse).
- Manually define the sagittal plane to cut through the supraspinatus tendon attachment at the greater tuberosity. Ensure that the 3D block contains the sagittal plane as a plane of symmetry. To achieve this, add or cut material from the block if needed.
NOTE: This plane of symmetry ensures that when the specimen is inserted into the fixtures the tendon and tendon attachment are located in the central axis of the fixture.
- Manually define the sagittal plane to cut through the supraspinatus tendon attachment at the greater tuberosity. Ensure that the 3D block contains the sagittal plane as a plane of symmetry. To achieve this, add or cut material from the block if needed.
- Measure dimensions of the bone along each of the three planes (i.e., height, width, length).
- Measure the dimensions of the mechanical testing grips where the 3D printed fixture will be attached.
- Begin by designing a solid block part (e.g., a solid cylinder).
- Ensure that each dimension of the block is at least 5 mm greater than the dimensions of the humerus.
- Account for design constraints from mechanical testing grips (i.e., ensure that the 3D printed fixture can be assembled and disassembled freely in the mechanical testing grips).
- Create an assembly model with two components: the solid block and either the right or left humerus bone. Define the orientation of the bone within the block (i.e., the angle between the tendon and bone). Ensure that the entire bone volume fits inside the block.
- Create a cavity in the block using the humerus bone as the mold. If using the software specified in the Table of Materials, follow the following steps:
- Insert the design part (humerus) and the mold base (cylinder block) into an interim assembly. In the assembly window, select the block, and click Edit Component from the Assembly toolbar.
- Click Insert > Features > Cavity. Select Uniform Scaling and enter 0% as the value to scale in all directions.
- Suppress the bone part and save the assembly as a part.
- Open part (cylinder with cavity). Cut the part along the sagittal plane to create two symmetrical components that fit the bone anteriorly and posteriorly (e.g., two half cylinders, as seen in Figure 1).
NOTE: Two components are designed that fit the bone anteriorly and posteriorly. The anterior component includes a half spherical-shaped cavity extended from the anterior side of the humeral head up to the supraspinatus tendon attachment. The posterior component cavity is shaped like the posterior part of the humerus (i.e., posterior side of the humeral head, deltoid tuberosity, and medial and lateral epicondyle). - Save each component as a separate file part.
- For the anterior component, ensure that the humeral head is embedded in the cavity of the part by defining appropriate tolerances.
NOTE: In the current study, using the software specified in the Table of Materials, it is suggested to follow the steps below:- Create a revolved cut to smooth the mesh geometry of the cavity. Create a sketch for the cut by emulating the cavity geometry and adding a locational clearance.
NOTE: The clearance allows for free assembly and disassembly between the bone and the anterior component.
- Create a revolved cut to smooth the mesh geometry of the cavity. Create a sketch for the cut by emulating the cavity geometry and adding a locational clearance.
- Modify the posterior component to imitate the cavity geometry to create a cut that adds clearance, as described above for the anterior component.
- Make a cut in the transverse plane starting from the top of the posterior component up to the crest of the greater/lesser tubercle.
NOTE: As seen in Figure 1 and Figure 2, the posterior component includes a cut that creates an opening at the tendon attachment. - Create a snug fit between the two components to allow for free assembly and disassembly.
NOTE: A hole-shaft fit with a loose running clearance was created for the fixtures in the current study. - Create 3D mirror models for each component of the fixture for the opposite limb (i.e., left or right).
- Add an etch on the bottom of the fixtures to distinguish between the left and right sides.
- Save all fixture parts in STL standard file format in preparation for 3D printing.
- Use a solid modeling computer-aided design program to create a custom-fit model of humerus gripping fixture (Figure 1, Supplemental Files).
- Achilles tendon-calcaneus bone
- Follow the same steps as described above for supraspinatus-humeral head fixture.
NOTE: Only one set of fixtures is necessary for the Achilles-calcaneal, since the anatomy of the left and right calcaneus bones is nearly symmetrical.
- Follow the same steps as described above for supraspinatus-humeral head fixture.
- Supraspinatus tendon-humeral bone
2. Biomechanical testing of murine tendons
- Specimen preparation and cross-sectional area measurement
- Dissect the muscle-tendon-bone of interest in preparation for tensile mechanical testing. In the current study, supraspinatus muscle – tendon – humerus bone specimens (N=10, 5 male, 5 female) and gastrocnemius muscle – Achilles tendon-calcaneus bone specimens (N=12, 6 male, 6 female) were isolated from 8 week old C57BL/6J mice.
- Dissection of the supraspinatus muscle – tendon – humerus bone specimen
- Euthanize a mouse per IACUC-approved procedure. Position the mouse in a prone position. Make an incision in the skin from above the elbow of the forepaw towards the shoulder.
- Carefully remove the skin with blunt dissection so that the musculature of the shoulder is visible. Remove the tissue surrounding the humerus until the bone is exposed and can be held securely with forceps.
- Hold the humerus with forceps and carefully remove the deltoid and trapezius muscles to expose the coracoacromial arch. Identify the acromioclavicular joint and carefully separate the clavicle from the acromion with a scalpel blade.
- Taking care not to damage the supraspinatus tendon and its bony attachment, remove the muscle from its scapular attachment using a scalpel blade. Taking care not to damage the supraspinatus tendon and its bony attachment, detach the humeral head from the glenoid; using a scalpel blade, lacerate the joint capsule and the infraspinatus, subscapularis, and teres minor tendons.
- Disarticulate the elbow joint to separate the humerus from the ulna and radius. Isolate the humerus – supraspinatus tendon – muscle specimen and clean off excess soft tissues on the humerus and humeral head.
- Dissection of the Achilles tendon – calcaneus bone sample
- Euthanize a mouse per IACUC-approved procedure. Position the mouse in a prone position. Taking care not to damage the Achilles tendon and its bony attachment, remove the skin with blunt dissection so that the musculature around the ankle and knee joints is exposed.
- Using a scalpel blade, starting at the Achilles tendon – calcaneus attachment, carefully detach the gastrocnemius muscle from its proximal attachments.
- Carefully disarticulate the calcaneus from the various adjacent bones. Isolate the Achilles tendon – calcaneus specimen and clean off excess soft tissues.
- Dissection of the supraspinatus muscle – tendon – humerus bone specimen
- Determine the cross-sectional area of the tendon using microcomputed tomography.
NOTE: For the scanner used in the current study (Table of Materials), the recommended settings are: scan at an energy of 55 kVP, Al 0.25 filter, at a resolution of 5 μm.- Mix agarose powder in ultrapure water and microwave for 1-3 min until the agarose is completely dissolved. It is helpful to microwave for 30-45 s, stop and swirl, and then continue towards a boil. Fill cryotubes up to three-quarters full with agarose. Let the agarose cool for about 5-10 min.
- Suspend the specimen in the cryotube by inserting the bone upside down.
NOTE: Only the bone should be in the agarose gel. The tendon and muscle should be suspended outside.
- After the scan, gently remove muscle from tendon using scalpel blade. Insert the specimen into the 3D-printed fixture.
NOTE: The grips are reusable for each test. Do not use glue or epoxy in the fixture; the bone is held in a press fit. - Insert and glue the tendon between a folded thin tissue paper (2 cm x 1 cm) and clamp the construct using thin film grips. Attach the 3D printed fixture with the specimen into the testing grips.
- Insert the sample and the grips into a testing bath of phosphate buffered saline (PBS) at 37 °C (i.e., mouse body temperature23).
- Dissect the muscle-tendon-bone of interest in preparation for tensile mechanical testing. In the current study, supraspinatus muscle – tendon – humerus bone specimens (N=10, 5 male, 5 female) and gastrocnemius muscle – Achilles tendon-calcaneus bone specimens (N=12, 6 male, 6 female) were isolated from 8 week old C57BL/6J mice.
- Tensile testing
- Perform tensile mechanical test on a material testing frame.
NOTE: For the testing frame used in the current study (Table of Materials), the recommended protocol is:- Define the gauge length as the distance from the tendon attachment to the upper grip.
- Precondition with 5 cycles between 0.05 N and 0.2 N.
- Hold for 120 s.
- Use a tension to failure of 0.2%/s.
- Collect load-deformation data.
- Calculate the strain as the displacement relative to the initial gauge length of the tendon.
- Calculate the stress as the force divided by the initial tendon cross-sectional area (as measured from microCT).
- If interested in viscoelastic behavior, perform a stress relaxation prior to the tension test to failure and use the data to calculate parameters such as A, B, C, tau1, and tau2 from the quasilinear viscoelastic model24.
- From the load deformation curve, calculate the stiffness (slope of linear portion of curve), the maximum force, and the work to yield (the area under the curve up to yield force).
- Identify the linear portion by choosing a window of points in the load-deformation curve that maximizes the R2 value for a linear least squares regression25.
- Determine the stiffness as the slope of the linear portion of the load-displacement curve25,26.
- From the stress strain curve, calculate the modulus (slope of linear portion of curve), the strength (maximum stress), and the resilience (area under the curve up to yield stress).
NOTE: Using the RANSAC algorithm, the yield strain (x-value) is defined as the first point when the y-fit has deviated more than 0.5% of the expected stress value (y-value). Yield stress is the corresponding y-value of the yield strain.
NOTE: In addition to the monotonic tensile loading to failure described in the current study, cyclic loading can provide important information about tendon fatigue and/or viscoelastic properties. For example, Freedman et al. reported fatigue properties of the murine Achilles tendons27. - After completion of tensile testing, perform a microcomputed tomography scan of the entire bone, e.g., scan the humerus and calcaneus samples.
NOTE: For the scanner used in the current study (Table of Materials), the recommended settings are: scan at an energy of 55 kVP, Al 0.25 filter, at a resolution of 6 μm.- Repeat steps 1.1.2.1–1.1.2.2.
- Repeat step 1.1.3.
- Use a 3D visualization program compatible with the scanner to create a volume-rendered 3D model of the scanned object.
NOTE: The program used in the current study is listed in the Table of Materials. - Determine the failure mode and failure site area by inspecting the 3D object.
- Perform tensile mechanical test on a material testing frame.
- Statistical analysis: Show all sample results as mean ± standard deviation (SD). Make comparisons between groups using student’s t-tests (two-tailed and unpaired). Set significance as p < 0.05.
NOTE: The statistical software used in the current study is listed in the Table of Materials.
Representative Results
3D-printed fixtures were used to test 8-week old murine supraspinatus and Achilles tendons. All mechanically tested samples failed at the enthesis, as characterized by microCT scans, visual inspection, and video analysis after tensile tests. A one-to-one comparison of the previous and current methods for supraspinatus tendon testing in our laboratory is shown in Figure 3. In the previous method28,29,30, the humerus bone was embedded in epoxy and a paper clip was placed over the humeral head in an effort to prevent growth plate fracture. 4-6 hours were necessary to allow for the epoxy to fully cure (Figure 3), allowing for only 6-8 specimens to be tested in a typical day. A further limitation of the approach was the user-dependent effectiveness of the paper clip placement for preventing growth plate fracture. The testing results using these prior methods were highly variable, with coefficients of variation on the order of 30% for most parameters and growth plate failure rates of approximately 10%–20%. As summarized in Figure 3, specimen preparation time using the new methods was decreased to 5–10 minutes, making it practical to test 16–20 samples per day. Furthermore, growth plate failures were eliminated.
Compared to methodology reported by others for testing murine tendons14,15,17,25,28,29,30,31,32,33, the new methods were more efficient and reproducible. For supraspinatus tendons, structural properties such as maximum load (3.8 ± 0.6 N) and stiffness (12.7 ± 1.8 N/mm), as well as normalized material properties such as maximum stress (8.7 ± 3.0 MPa), and modulus (51.7 ± 13.5 MPa) had considerably lower coefficients of variations compared to results from the literature (Table 1). For the Achilles tendon, mechanical properties such as maximum load (7.8 ± 1.1 N) and stiffness (13.2 ± 1.9 N/mm) had lower coefficients of variations compared to results from the literature19,21,22,32,33,34,35,36,37,38, whereas maximum stress (24.2 ± 5.4 MPa) and modulus (73.2 ± 22.1 MPa) had coefficients of variations similar to those reported in the literature (Table 2).
Animal sex had a significant effect on the mechanical properties of the supraspinatus and Achilles tendons (Figure 4). When comparing male and female supraspinatus tendons, there were significant increases in maximum force (p = 0.002) and work to yield (p = 0.008). There were trends between the two groups for stiffness (p = 0.057), stress (p = 0.068), modulus (p = 0.061) and resilience (p = 0.078). When comparing male and female Achilles tendons, there were significant increases in maximum stress (p = 0.0006) and resilience (p = 0.0019). There were trends between the two groups for work to yield (p = 0.079), and modulus (p = 0.074) and no difference for maximum force (p = 0.1880) and stiffness (p = 0.6759).
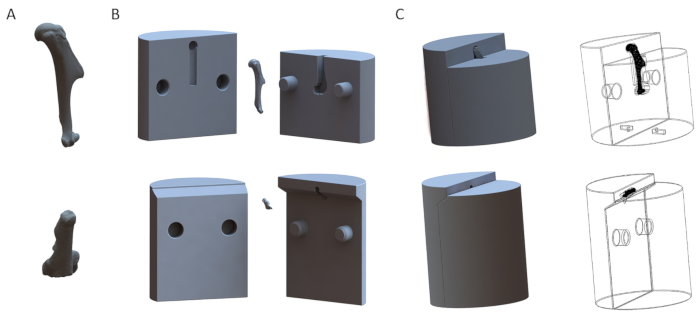
Figure 1: Representative 3D models of fixtures for the humerus (top row) and the calcaneus (bottom row). (A) 3D models of the bones. (B) Disassembled models of the fixtures. (C) Assembled models of the fixtures. Please click here to view a larger version of this figure.
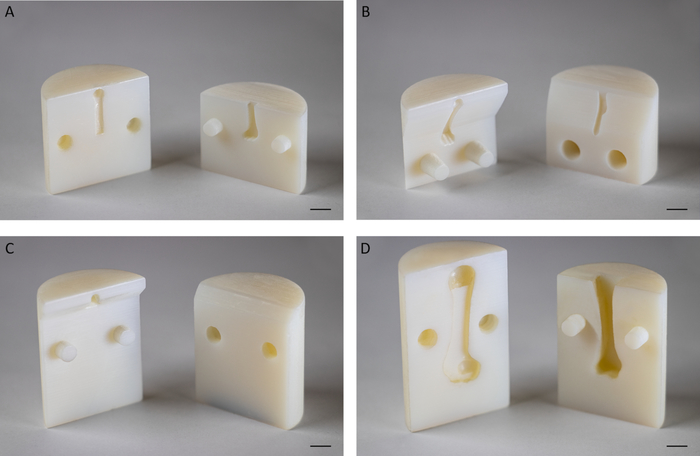
Figure 2: Representative 3D printed fixtures. (A) Fixture for biomechanical testing of supraspinatus tendons of 8-week old mice at an angle of 180° between the humerus and supraspinatus tendon. (B) Fixture for biomechanical testing of supraspinatus tendons of 8-week old mice at an angle of 135° between the humerus and supraspinatus tendon. (C) Fixture for biomechanical testing of murine Achilles tendons at an angle of 120° between the calcaneus and Achilles tendon. (D) Fixture for biomechanical testing of supraspinatus tendons of adult Sprague Dawley rats at an angle of 180° between the humerus and supraspinatus tendon. Scale bar: 5 mm. Please click here to view a larger version of this figure.
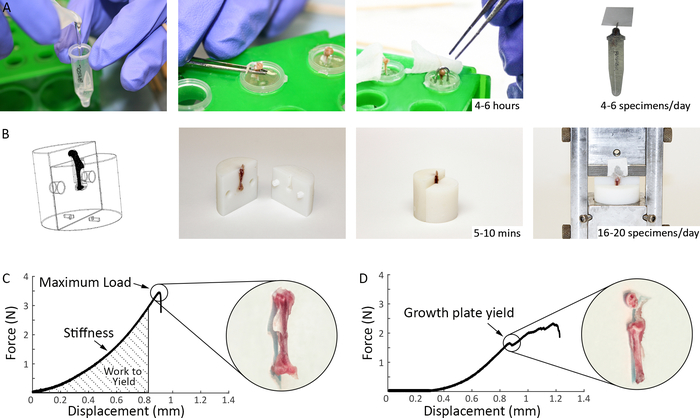
Figure 3: Comparison of previous and current methods for mechanical testing of murine supraspinatus tendons. (A) Previous specimen preparation methods used in our laboratory prior to mechanical testing: the humerus was potted in epoxy up to the humeral head to stabilize the bone, a paper clip was placed over the humeral head to prevent growth plate fracture, and, for the epoxy to cure, the specimens were left in room temperature for 4-6 hours prior to mechanical testing. (B) Specimen preparation methods used in the current study (Steps 1.2 and 2.1.4): Top left shows a 3D representation of the fixtures as produced by a solid modeling program. The 3D printed fixtures are reusable and easily assembled and disassembled. The bone end of the specimen is inserted into the fixtures, securing the growth plate and exposing the tendon for gripping and testing. The tendon end is glued between a folded thin tissue paper and inserted into the grips. Preparation time for each specimen is 10–15 minutes. (C) Representative load-deformation curves for tensile testing of supraspinatus tendon using current methods. (D) Representative load-deformation curve for tensile testing of supraspinatus tendon showing a growth plate failure. Please click here to view a larger version of this figure.
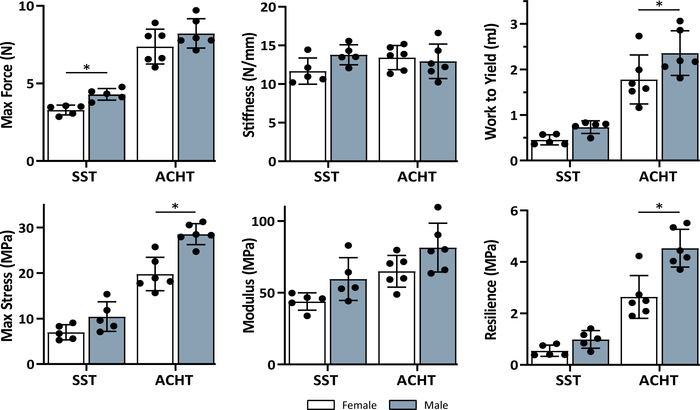
Figure 4: Sex effect on the mechanical properties of supraspinatus (SST) and Achilles (ACHT) tendons. There was a significant effect of sex on many of the mechanical properties based on unpaired t-tests (*sex effect, p < 0.05). Data shown as mean ± standard deviation. Please click here to view a larger version of this figure.
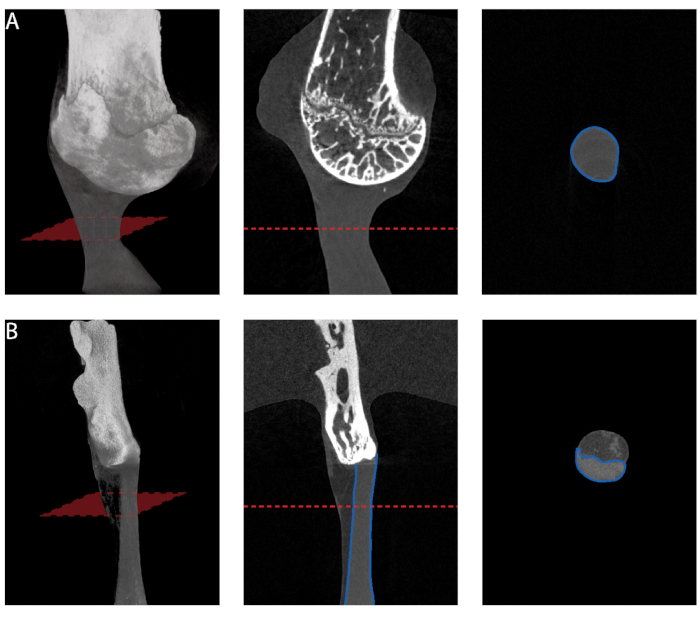
Figure 5: Cross-sectional area measurement from microCT. (A) Minimum cross-sectional area measurement along the length of supraspinatus tendon. (B) Minimum cross-sectional area measurement along the length of Achilles tendon. Only the tendon proper should be selected for measurement. Please click here to view a larger version of this figure.
| Structural Properties | Material Properties | |||||||||||
| Animals | Max Force (N) | Stiffness (N/mm) | Max Stress (Mpa) | Modulus (MPa) | ||||||||
| Autor | N | Background | Mean ± SD | COV(%) | Mean ± SD | COV (%) | Mean ± SD | COV (%) | Mean ± SD | COV (%) | ||
| Beason et al. Journal of Shoulder and Elbow Surgery (2013)15 | 10 | C57Bl/6 | 0.93±0.34 | 36.56 | 95.1±39.8† | 41.85 | 3.40±1.56 | 45.88 | 312.8±127.0 | 40.60 | ||
| Bell et al. Journal of Orthopaedic Research (2014)31 | 6 | C57Bl/6 | 1.22 ± 0.52 | 42.62 | 2.37 ± 1.6 | 67.51 | NR | NR | ||||
| Cong et al. Journal of Orthopaedic Research (2018)17 | 8 | C57Bl/6 | 5.38 ± 2.404# | 44.68 | 4.25 ± 1.67# | 39.29 | NR | NR | ||||
| Connizzo et al. Annals of Biomedical Engineering (2014)32 | 10 | NR (db/+) | NR | 84.44 ± 27.23*† | 32.25 | NR | 476 ± 186.27* | 39.13 | ||||
| Connizzo et al. Journal of Biomedical Engineering (2013)14 | NR | C57/BL6 | NR | NR | NR | 297 ± 148.90* | 50.13 | |||||
| Deymier et al. Acta Biomaterialia (2019)28 | 12 | CD-1 IGS Mouse (WT) | 5.0 ± 0.7 | 14 | 9.2 ± 2.9 | 31.52 | 33 ± 35 | 106.06 | NR | |||
| Eekhoff et al. Journal of Biomedical Engineering (2017)33 | 13 | Eln +/+ | NR | 8.50 ± 2.95 | 34.71 | 5.96 ± 3.23 | 54.19 | 101.2 ± 50.8 | 50.20 | |||
| Killian et al. FASEB Journal (2016)29 | 8 | C57BL/6 | NR | NR | 7.79 ± 2.61* | 33.50 | 58.32 ± 31.73* | 54.41 | ||||
| Schwartz et al. Bone (2014)25 | 20 | CD-1 IGS Mouse (WT) | 4.11 ± 0.79* | 19.22 | 8.58 ± 3.78* | 44.06 | 12.29 ± 5.95* | 48.41 | 133.80 ± 59.41* | 44.40 | ||
| Schwartz et al. Development (2015)30 | 12 | (Rosa-DTA (DTA) x Gli1-CreERT2 ) ScxCre;Smofl/fl (WT) | 4.16 ± 0.29* | 6.97 | 11.04 ± 1.98* | 17.93 | 26.24 ± 5.81 | 22.14 | 121.89 ± 44.18 | 36.25 | ||
| Average COV | 27.34 | Average COV | 38.64 | Average COV | 51.70 | Average COV | 45.02 | |||||
| New Method | 10 | C57BL/6J | 3.79 ± 0.62 | 16.41 | 12.73 ± 1.81 | 14.20 | 8.71 ± 3.04 | 34.91 | 51.67 ± 13.54 | 26.20 | ||
Table 1: Mechanical properties of supraspinatus tendons. Mean ± SD and coefficient of variation (COV) for structural and material properties estimated using new methods compared to ones reported in the literature. [NR: not reported, * estimated from figure(s), # standard deviation calculated from reported standard error, † measured deformation using optical stain lines].
| Structural Properties | Material Properties | |||||||||
| Animals | Max Force (N) | Stiffness (N/mm) | Max Stress (Mpa) | Young's modulus (MPa) | ||||||
| Autor | N | Background | Mean ± SD | COV(%) | Mean ± SD | COV (%) | Mean ± SD | COV (%) | Mean ± SD | COV (%) |
| Boivin et al. Muscles, Ligaments and Tendons Journal (2014)19 | 6 | Non-diabetic lean control mice | 8.1 ± 0.6 | 7.41 | 3.9 ± 0.7 | 17.95 | NR | 16 ± 3.7 | 23.13 | |
| Connizzo et al. Annals of Biomedical Engineering (2014)32 | 10 | db/+ | NR | 20.39 ± 2.43* | 11.92 | NR | 152.94 ± 44.12* | 28.85 | ||
| Eekhoff et al. Journal of Biomechanical Engineering (2017)33 | 8 | Eln +/+ | NR | 18.86 ± 3.37 | 17.87 | 10.55 ± 2.97 | 28.15 | 443.8 ± 131.7 | 29.68 | |
| Mikic et al. Journal of Orthopaedic Research (2006)34 | 20 | C57BL/6-J x 129SV/J | NR | NR | 18 ± 5 | 27.78 | 61 ± 20 | 32.79 | ||
| Probst et al. Journal of Investigative Surgery (2000)22 | 20 | BALB/c | 8.4 ± 1.1 | 13.10 | 6.3 ± 1.2 | 19.05 | NR | NR | ||
| Shu et al. Peer J (2018)21 | 9 | C57BL/6 | 9.6 ± 3.84 | 39.96 | 8.19 ± 3.63 | 44.32 | 27.55 ± 10.54 | 38.26 | NR | |
| Sikes et al. Journal of Orthopaedic Research (2018)35 | 7 | C57BL/6 | NR | NR | 19.53 ± 7.03 | 0.36 | 62.82 ± 20.20 | 32.16 | ||
| Wang et al. Journal of Orthopaedic Research (2006)36 | 9 | A/J | 8.4 ± 1.2 | 14.29 | 12.2 ± 2.8 | 22.95 | 78.2 ± 8.6 | 11.00 | 713.9 ± 203.7 | 28.53 |
| Wang et al. Journal of Orthopaedic Research (2006)36 | 8 | C57BL/6J | 10.2 ± 1.4 | 13.73 | 13.1 ± 2.5 | 19.08 | 97.4 ± 11.4 | 11.70 | 765.1 ± 179.6 | 23.47 |
| Wang et al. Journal of Orthopaedic Research (2006)36 | 7 | C3H/HeJ | 12.5 ± 1.7 | 13.60 | 14.1 ± 3.2 | 22.70 | 97.5 ± 10.9 | 11.18 | 708.6 ± 127.8 | 18.04 |
| Wang et al. Journal of Orthopaedic Research (2011)37 | 7 | C57BL/6 | 6.6 ± 1.7 | 25.76 | 8.2 ± 1.4 | 17.07 | 13.4 ± 3.7 | 27.61 | 86.8 ± 15.5 | 17.86 |
| Zhang et al. Matrix Biology (2016)38 | NR | CD-1 and C57BL/6J | 6.73 ± 3.74* | 55.57 | 12.03 ± 3.34* | 27.76 | 25.4 ± 15.14* | 59.61 | 632.31 ± 113.79* | 18.00 |
| Average COV | 22.93 | Average COV | 22.07 | Average COV | 23.96 | Average COV | 25.25 | |||
| New Method | 12 | C57BL/6J | 7.8 ± 1.08 | 13.91 | 13.19 ± 1.86 | 14.08 | 24.16 ± 5.42 | 22.45 | 73.17 ± 16.14 | 22.06 |
Table 2: Mechanical properties of Achilles tendons. Mean ± SD and COV for structural and material properties estimated using new methods compared to ones reported in the literature. [NR: not reported, * estimated from figure(s), # standard deviation calculated from reported standard error].
Supplemental Files. Please click here to download this file.
Discussion
Murine animal models are commonly used to study tendon disorders, but characterization of their mechanical properties is challenging and uncommon in the literature. The purpose of this protocol is to describe a time efficient and reproducible method for tensile testing of murine tendons. The new methods reduced the time required to test a sample from hours to minutes and eliminated a major gripping artifact that was a common problem in previous methods.
Several steps described in this protocol are critical to produce effective fixtures mechanically testing murine supraspinatus and Achilles tendons. First, step 1.1.4 is necessary to create a 3D model of the desired bone; however, due to the typically high resolution used for this scan, the file size may be too large to use with solid modeling programs. The software used in this protocol successfully reduced the size of the file (step 1.1.6) and preserved object geometry, although other softwares may also be effective to achieve this. Second, each anatomic site has specific design criteria to consider for effective gripping. For the design of the supraspinatus tendon fixture, it is critical to: (i) secure the humeral head to prevent growth plate failure (step 1.2.1.12), (ii) define a clearance fit that avoids disengaging of the humerus bone from the mold during testing (step 1.2.1.12.1) and (iii) orient the humerus bone to form a 180° angle with the long axis of the tendon (step 1.2.1.7). For the Achilles tendon fixture design, it is critical to: (i) define a clearance fit that grips the small calcaneus bone without slipping out from the fixture during testing and (ii) orient the calcaneus bone to form a 120° angle (30° plantar flexion) with the long axis of the tendon. Third, accurate measurement of the tendon cross-sectional area (step 2.1.2) is critical to properly calculate engineering stress for determination of material properties. To measure the cross-sectional area of the supraspinatus tendon, we recommend microcomputed tomography scans of the bone-tendon-muscle specimen suspended in a cryotube with a flat bottom, with the bone held upside down in the tube with agarose. Only the humerus bone should be inserted into the agarose gel, while the humeral head with the tendon and muscle attached should be scanned in air. As the supraspinatus tendon has a splayed geometry as it inserts into the bone, the most consistent way to measure the cross-sectional area is to determine the minimum cross-sectional area along the length of the tendon. A similar procedure should be followed to measure the cross-sectional area of the Achilles tendon. For the Achilles tendon, high resolution microcomputed tomography scans reveal two distinct tissues: the tendon proper and the surrounding sheath, which appears as a lighter shade. To consistently estimate the minimal cross-sectional area for the Achilles tendon, only the tendon proper should be selected for measurement (Figure 5). Lastly, the grips are reusable and small variations from sample to sample do not affect their effectiveness. Each bone should be scanned once (e.g., for the current study, left humerus, right humerus, and calcaneus) and one 3D model should be created for each bone. In addition, for animals of the same age, the bone geometry is nearly identical, thus the same fixture can be used for testing of all specimens. In this manuscript, 3D printed fixtures specific to 8-week old mice (skeletally mature adult mice) were used to test tendons. It was not necessary to create separate male and female fixtures. For other age groups (e.g., 4-week old mice) or mice with unique bone phenotypes, it is recommended that fixtures that fit the particular geometries of the bones are manufactured.
After design and 3D printing of the fixtures, to ensure reproducibility and efficiency of the approach, 10 tendon samples from mice of the same background and age of the planned study should typically be tested (the exact sample size may vary depending on the tissue and animal model). The mechanical properties of these tendons should be determined to ensure that coefficients of variation for structural and material properties are within the expected range, as described in Table 1 and Table 2. These pilot tests should also confirm that artifactual failures (e.g., growth plate failure) do not occur. Multiple cycles of design, prototyping, and validation may be needed to achieve the desired results for tendons other than the supraspinatus and Achilles tendons described in the current paper.
A number of groups have reported the mechanical properties of murine tendons. The coefficient of variations in these studies are typically high, often making it difficult to pick up differences among the comparison groups. Furthermore, methodological differences in tissue gripping among the various studies makes it difficult to determine whether failure properties are relevant to tendon or due to artifactual grip failures. To compare the new testing methods with existing methodologies, a literature review was performed and the results from 20 studies were summarized (Table 1 and Table 2). In the literature, for supraspinatus tendon mechanical testing, the average coefficients of variation for maximum force, stiffness, maximum stress, and modulus were 27%, 39%, 52%, and 45%, respectively. For Achilles tendon mechanical testing, the average coefficients of variation for maximum force, stiffness, maximum stress, and modulus were 23%, 22%, 24%, and 25%, respectively. In the current study, the new method for testing murine tendons resulted in a 32%–63% reduction of supraspinatus tendon coefficients of variation and 6%–39% reduction in Achilles tendon coefficients of variation.
There is no current standard methodology for gripping bones, thus it is unclear to what extent artifactual gripping issues has affected reported mechanical properties of murine tendons. Most groups report gripping the humerus bone by using epoxy resin13, polymethylmethacrylate (PMMA)14,15, or cyanoacrylate16 and securing the humeral head by applying a second coating of PMMA14, using custom fixture39 and/or inserting a paper clip25,28,30. Similarly, other groups report gripping of the much smaller calcaneus bone using custom-made fixtures19,20, anchoring by clamps21, fixing in self curing plastic cement22 or using a conical shape slot22. However, these methods remain limited by low reproducibility, high artifactual failure rates, and time-consuming preparation requirements. The new methods presented in this study have eliminated artifactual grip failures and have tripled the number of specimens that can be tested in a day. Furthermore, these methods are not limited to the supraspinatus and Achilles tendons, as they are easily adapted to testing other murine tendons and tendons from larger animal models. To test tendons from larger animals, however, the modulus of the 3D printed fixture material must be high enough that it is not compliant relative to the strength of the tendon being tested.
Several studies have shown sex-based differences in tendon disorders indicating that women have reduced function following treatment after tendon injury40,41,42. In the current study, sex had a significant effect on the mechanical properties of murine tendons. As guided by the National Institutes of Health (NIH), we recommend accounting for sex as a biological variable in the research design of animal models where tendon mechanical properties will be measured.
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
The study was supported by the NIH / NIAMS (R01 AR055580, R01 AR057836).
Materials
| Agarose | Fisher Scientific | BP160-100 | Dissovle 1g in 100 ml ultrapure water to make 1% agarose |
| Bruker microCT | Bruker BioSpin Corp | Skyscan 1272 | Used by authors |
| ElectroForce | TA Instruments | 3200 | Testing platform |
| Ethanol 200 Proof | Fisher Scientific | A4094 | Dilute to 70% and use as suggested in protocol |
| Fixture to attach grips | Custom made | Used by authors | |
| Kimwipes | Kimberly-Clark | S-8115 | As suggested in protocol |
| MicroCT CT-Analyser (Ctan) | Bruker BioSpin Corp | Used by authors for visualizing and analyzing micro-CT scans | |
| MilliQ water (Ultrapure water) | Millipore Sigma | QGARD00R1 (or related purifier) | 100 ml |
| Meshmixer | Autodesk | http://www.meshmixer.com/ | Free engineering software used by authors to refine mesh |
| Objet EDEN 260VS | Stratasys LTD | Precision Prototyping | |
| Objet Studio | Stratasys LTD | Used by authors with 3D printer | |
| PBS – Phosphate-Buffered Saline | ThermoFisher Scientific | 10010031 | 2.5 L of 10% PBS |
| S&T Forceps | Fine Science Tools | 00108-11 | Used by authors |
| Scalpel Blade – #11 | Fine Science Tools | 10011-00 | Used by authors |
| Scalpel Handle – #3 | Fine Science Tools | 10003-12 | Used by authors |
| SkyScan 1272 | Bruker BioSpin Corp | Used by authors for visualizing and analyzing micro-CT scans | |
| Skyscan CT-Vox | Bruker BioSpin Corp | Used by authors for visualizing and analyzing micro-CT scans | |
| SkyScan NRecon | Bruker BioSpin Corp | Used by authors for visualizing and analyzing micro-CT scans | |
| SolidWorks CAD | Dassault Systèmes | SolidWorks Research Subsription | Solid modeling computer-aided design used by authors |
| SuperGlue | Loctite | 234790 | As suggested in protocol |
| Testing bath | Custom made | Used by authors | |
| Thin film grips | Custom made | Used by authors | |
| VeroWhitePlus | Stratasys LTD | NA | 3D printing material used by authors |
| WinTest | WinTest Software | Used by authors to collect data |
Referenzen
- Girish, N., Ramachandra, K., Arun, G. M., Asha, K. Prevalence of Musculoskeletal Disorders Among Cashew Factory Workers. Archives of Environmental & Occupational Health. 67, 37-42 (2012).
- Thomopoulos, S., Parks, W. C., Rifkin, D. B., Derwin, K. A. Mechanisms of tendon injury and repair. Journal of Orthopaedic Research. 33, 832-839 (2016).
- Scott, A., Ashe, M. C. Common Tendinopathies in the Upper and Lower Extremities. Current Sports Medicine Reports. 5, 233-241 (2006).
- Praemer, A., Furner, S., Rice, D. P. Musculoskeletal Conditions in the United States. American Academy of Orthopaedic Surgeons. , (1992).
- Nourissat, G., Berenbaum, F., Duprez, D. Tendon injury: From biology to tendon repair. Nature Reviews Rheumatology. 11, 223-233 (2015).
- Galatz, L. M., Ball, C. M., Teefey, S. A., Middleton, W. D., Yamaguchi, K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. The Journal of Bone and Joint Surgery. 86, 219-224 (2004).
- Sher, J. S., Uribe, J. W., Posada, A., Murphy, B. J., Zlatkin, M. B. Abnormal findings on magnetic resonance images of asymptomatic shoulders. The Journal of Bone and Joint Surgery. 77, 10-15 (1995).
- Ker, R. F., Wang, X. T., Pike, A. V. Fatigue quality of mammalian tendons. The Journal of Experimental Biology. 203, 1317-1327 (2000).
- Wilson, J. J., Best, T. M. Common overuse tendon problems: A review and recommendations for treatment. American Family Physician. 72, 811-818 (2005).
- Fleischer, J., et al. Biomechanical strength and failure mechanism of different tubercula refixation methods within the framework of an arthroplasty for shoulder fracture. Orthopaedics & Traumatology: Surgery & Research. 103, 165-169 (2017).
- West, J. R., Juncosa, N., Galloway, M. T., Boivin, G. P., Butler, D. L. Characterization of in vivo Achilles tendon forces in rabbits during treadmill locomotion at varying speeds and inclinations. Journal of Biomechanics. 37, 1647-1653 (2004).
- Cavinatto, L., et al. Early versus late repair of rotator cuff tears in rats. Journal of Shoulder and Elbow Surgery. 27, 606-613 (2018).
- Potter, R., Havlioglu, N., Thomopoulos, S. The developing shoulder has a limited capacity to recover after a short duration of neonatal paralysis. Journal of Biomechanics. 47, 2314-2320 (2014).
- Connizzo, B. K., Sarver, J. J., Iozzo, R. V., Birk, D. E., Soslowsky, L. J. Effect of Age and Proteoglycan Deficiency on Collagen Fiber Re-Alignment and Mechanical Properties in Mouse Supraspinatus Tendon. Journal of Biomechanical Engineering. 135, 021019 (2013).
- Beason, D. P., et al. Hypercholesterolemia increases supraspinatus tendon stiffness and elastic modulus across multiple species. Journal of Shoulder and Elbow Surgery. 22, 681-686 (2013).
- Miller, K. S., Connizzo, B. K., Soslowsky, L. J. Collagen fiber re-alignment in a neonatal developmental mouse supraspinatus tendon model. Annals of Biomedical Engineering. 40, 1102-1110 (2012).
- Cong, G. T., et al. Evaluating the role of subacromial impingement in rotator cuff tendinopathy: Development and analysis of a novel murine model. Journal of Orthopaedic Research. 36, 2780-2788 (2018).
- Thomopoulos, S., Birman, V., Genin, G. M. Structural Interfaces and Attachments in Biology. Infection and Immunity. 35, (2013).
- Boivin, G. P., et al. Biomechanical properties and histology of db/db diabetic mouse Achilles tendon. Muscles, Ligaments and Tendons Journal. 4, 280-284 (2014).
- Ansorge, H. L., Adams, S., Birk, D. E., Soslowsky, L. J. Mechanical, Compositional, and Structural Properties of the Post-natal Mouse Achilles Tendon. Annals of Biomedical Engineering. 39, 1904-1913 (2011).
- Shu, C. C., Smith, M. M., Appleyard, R. C., Little, C. B., Melrose, J. Achilles and tail tendons of perlecan exon 3 null heparan sulphate deficient mice display surprising improvement in tendon tensile properties and altered collagen fibril organisation compared to C57BL/6 wild type mice. PeerJ. 6, 5120 (2018).
- Probst, A., et al. A new clamping technique for biomechanical testing of tendons in small animals. Journal of Investigative Surgery. 13, 313-318 (2000).
- Talan, M. Body temperature of C57BL/6J mice with age. Experimental Gerontology. 19, 25-29 (1984).
- Newton, M. D., et al. The influence of testing angle on the biomechanical properties of the rat supraspinatus tendon. Journal of Biomechanics. 49, 4159-4163 (2016).
- Schwartz, A. G., Lipner, J. H., Pasteris, J. D., Genin, G. M., Thomopoulos, S. Muscle loading is necessary for the formation of a functional tendon enthesis. Bone. 55, 44-51 (2014).
- Gimbel, J. A., Van Kleunen, J. P., Williams, G. R., Thomopoulos, S., Soslowsky, L. J. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon. Journal of Biomechanical Engineering. 129, 400-404 (2006).
- Freedman, B. R., Sarver, J. J., Buckley, M. R., Voleti, P. B., Soslowsky, L. J. Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury. Journal of Biomechanics. 47, 2028-2034 (2014).
- Deymier, A. C., et al. The multiscale structural and mechanical effects of mouse supraspinatus muscle unloading on the mature enthesis. Acta Biomaterialia. 83, 302-313 (2019).
- Killian, M. L., Thomopoulos, S. Scleraxis is required for the development of a functional tendon enthesis. FASEB Journal. 30, 301-311 (2016).
- Schwartz, A. G., Long, F., Thomopoulos, S. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development. 142, 196-206 (2015).
- Bell, R., Taub, P., Cagle, P., Flatow, E. L., Andarawis-Puri, N. Development of a mouse model of supraspinatus tendon insertion site healing. Journal of Orthopaedic Research. 33, 25-32 (2014).
- Connizzo, B. K., Bhatt, P. R., Liechty, K. W., Soslowsky, L. J. Diabetes Alters Mechanical Properties and Collagen Fiber Re-Alignment in Multiple Mouse Tendons. Annals of Biomedical Engineering. 42, 1880-1888 (2014).
- Eekhoff, J. D., et al. Functionally Distinct Tendons From Elastin Haploinsufficient Mice Exhibit Mild Stiffening and Tendon-Specific Structural Alteration. Journal of Biomechanical Engineering. 139, 111003 (2017).
- Mikic, B., Bierwert, L., Tsou, D. Achilles tendon characterization in GDF-7 deficient mice. Journal of Orthopaedic Research. 24, 831-841 (2006).
- Sikes, K. J., et al. Knockout of hyaluronan synthase 1, but not 3, impairs formation of the retrocalcaneal bursa. Journal of Orthopaedic Research. 36, 2622-2632 (2018).
- Wang, V. M., Banack, T. M., Tsai, C. W., Flatow, E. L., Jepsen, K. J. Variability in tendon and knee joint biomechanics among inbred mouse strains. Journal of Orthopaedic Research. 24, 1200-1207 (2006).
- Wang, V. M., et al. Murine tendon function is adversely affected by aggrecan accumulation due to the knockout of ADAMTS5. Journal of Orthopaedic Research. 30, 620-626 (2011).
- Zhang, K., et al. Tendon mineralization is progressive and associated with deterioration of tendon biomechanical properties, and requires BMP-Smad signaling in the mouse Achilles tendon injury model. Matrix Biology. 52-54, 315-324 (2016).
- Rooney, S. I., et al. Ibuprofen differentially affects supraspinatus muscle and tendon adaptations to exercise in a rat model. American Journal of Sports Medicine. 44, 2237-2245 (2016).
- Galasso, O., et al. Quality of Life and Functional Results of Arthroscopic Partial Repair of Irreparable Rotator Cuff Tears. Arthroscopy – Journal of Arthroscopic and Related Surgery. 33, 261-268 (2017).
- Sarver, D. C., et al. Sex differences in tendon structure and function. Journal of Orthopaedic Research. 35, 2117-2126 (2017).
- Razmjou, H., et al. Disability and satisfaction after Rotator Cuff decompression or repair: A sex and gender analysis. BMC Musculoskeletal Disorders. 12, 66 (2011).

