Tree Core Analysis with X-ray Computed Tomography
Summary
Here we show how to process tree cores with an X-ray computed tomography toolchain. Except for chemical extraction for some purposes, no further physical lab treatment is needed. The toolchain can be used for biomass estimations, for obtaining MXD/tree-ring width data as well as for obtaining quantitative wood anatomy data.
Abstract
An X-ray computed tomography (CT) toolchain is presented to obtain tree-ring width (TRW), maximum latewood density (MXD), other density parameters, and quantitative wood anatomy (QWA) data without the need for labor-intensive surface treatment or any physical sample preparation. The focus here is on increment cores and scanning procedures at resolutions ranging from 60 µm down to 4 µm. Three scales are defined at which wood should be looked at: (i) inter-ring scale, (ii) ring scale, i.e., tree-ring analysis and densitometry scale, as well as (iii) anatomical scale, the latter approaching the conventional thin-section quality. Custom-designed sample holders for each of these scales enable high-throughput scanning of multiple increment cores. A series of software routines were specifically developed to efficiently treat three-dimensional X-ray CT images of the tree cores for TRW and densitometry. This work briefly explains the basic principles of CT, which are needed for a proper understanding of the protocol. The protocol is presented for some known species that are commonly used in dendrochronology. The combination of rough density estimates, TRW and MXD data, as well as quantitative anatomy data, allows us to broaden and deepen current analyses for climate reconstructions or tree response, as well as further develop the field of dendroecology/climatology and archeology.
Introduction
Wood density is an easy-to-measure variable1 that reflects both the anatomical and chemical properties of the wood2. In biomass estimations of aboveground biomass, wood density is an important weighing variable 3,4,5, that is multiplied with the dimensions of the tree and a factor representing the carbon content of the wood. Wood density is tightly linked to the mechanical properties of timber6 and reflects the life history of a tree7.
Cell wall density is measured as being approximately 1500 kg/m³ and is considered fairly constant8, however intra-ring cell wall density variations should be considered as well8,9.Woody cells (in general tracheids in conifers, vessels, parenchyma and fibers in hardwoods) are oriented/shaped in different ways and cell wall thickness and lumen size of these cells varies10. Therefore, wood density varies between trees, within a tree (axial and transversal) and within short intervals within a tree ring11,12. In many cases the wood density variation at the ring scale also delimits the tree ring boundary13. Wood density and ultimately tissue fractions are generated and in this paper are broadly put into three categories (i.e., three different resolution scales), depending on the study goal (Figure 1) as described below.
Inter-ring scale: By measuring pieces of wood, a single value is obtained for that sample. This can be done through water immersion or geometrically14. This way, general biomass or wood technological variables can be obtained. To include pith-to-bark variation, these pieces of wood can be further divided into blocks that are measured manually to obtain information on the life history strategy15. When switching to low-resolution X-ray CT such as in medical scanners17,18, TRW data on medium-to-wide rings can be made in an efficient way on many samples18,19,20. This is also the scale that can be used to assess biomass from pith-to-bark from both temperate and tropical trees4,22, typically ranging in resolutions from 50 µm to 200 µm.
Ring scale: Wood is a recorder of past environmental conditions. The best known parameter is tree-ring width (TRW), but for global temperature reconstructions, maximum latewood density (MXD) records are proven to be a better proxy for temperature22. MXD is an easy-to-measure variable23, and a proxy for cell wall thickness and cell size on the last cells of a tree ring, and are at tree line and boreal sites positively linked to seasonal air temperature24: the warmer and longer the summers, more cell wall lignification occurs which thus increases the density of these last cells. Traditional measurements such as immersion and geometry are less accurate to determine this ring-level density. A previous work developed a toolchain for using X-ray film on thin-cut samples25. This sparked a revolution in both forestry and later paleoclimatology15,18, defining maximum latewood density (MXD), i.e., the peak density value often at the end of a ring, as a proxy for summer temperature. The basic principle is that the samples are sawn (approximately 1.2 mm to 7 mm13) to be perfectly parallel to the axial direction, and the sample is put on a sensitive film exposed to an X-ray source. Then these radiography films are read out through a light source that detects the intensity and saves the profiles and the annual tree ring parameters. These tools, however, require a significant amount of sample preparation and manual work. Recently this has been developed for X-ray CT in a more standardized way or based on mounted cores26. Resolution here ranges between 10 µm and 20 µm. TRW is measured on this scale as well, especially when dealing with smaller rings.
Anatomical scale: At this scale (resolution < 4 µm), the average density levels become less relevant as the main anatomical features are visualized and their width and proportions can be measured. Typically, this is done through making microsections or high-resolution optical scans or µ-CT scans. When the ultrastructure of the cell walls needs to be visualized, scanning electron microscopy is the most commonly used method27. At the anatomical scale, the individual tissue fractions become visible so that physiological parameters can be derived from the images. Based on the individual anatomical parameters and the cell wall density of wood, anatomical density can be derived for comparison with conventional estimators of wood density24.
Due to improved sectioning techniques and image software29,30, dendro-anatomy30 has been developed to have a more accurate record of the wood, both to have a closer estimate of the MXD in conifers and to measure several anatomical variables from broadleaf trees. On this scale, actual anatomical parameters are measured and related to environmental parameters31 . With µCT this level can be obtained as well32,33.
As wood is inherently hygroscopic and anisotropic, wood density needs to be carefully defined and the measurement conditions need to be specified, either as oven-dry, conditioned (typically at 12% moisture content) or green (as felled in the forest)34. For large samples and technical purposes, wood density is defined as the weight divided by its volume at given conditions. However, the value of wood density is strongly dependent on the scale at which it is measured, for instance from pith-to-bark wood density can double, and on a ring scale (in conifers) the transition of earlywood to latewood results in a significant rise in wood density as well, with a peak at the ring boundary.
Here, an X-ray CT scanning protocol of increment cores is presented in order to measure features at the aforenoted 3 scales (Figure 1). Recent developments in X-ray CT can cover most of these scales, due to a flexible set-up. The research goals will determine the eventual protocol for scanning.
A crucial limiting factor (which is inherently connected to the scaled nature of wood density and wood in general) is the resolution and time necessary for scanning. Examples demonstrate how to: (i) obtain inter-ring tree scale wood density profiles for biomass estimations in Terminalia superba from the Congo Basin, (ii) obtain density records from Clanwilliam cedar (Widdringtonia cedarbergensis) based on helical scanning on a HECTOR system35, and (iii) measure vessel parameters on sessile oak, on the Nanowood system. Both scanners are part of the suite of scanners at the UGent Center for X-ray Tomography (UGCT,
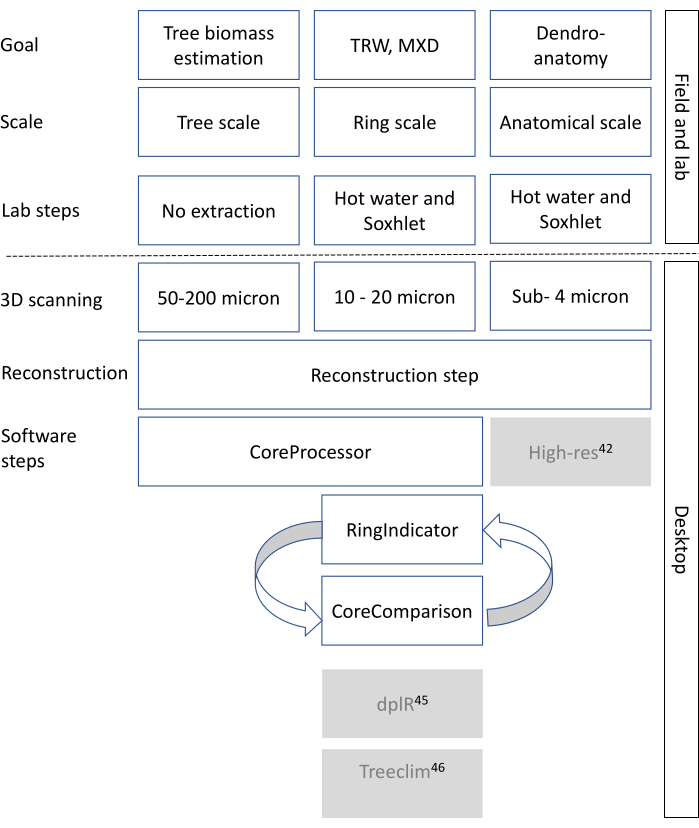
Figure 1: General methodological decision tree for X-ray CT scanning. The rows indicate the steps to take, starting from the research goal all the way to the final data format. White boxes are the steps that are relevant for this toolchain. Greyed-out boxes are steps that can be performed with other software or R packages, such as dplr47 and Treeclim48 for tree-ring analysis, and ROXAS44 as well as ImageJ42 or other (commercial) applications for deriving wood anatomical parameters based on the CT images. Please click here to view a larger version of this figure.
X-CT research on wood
Set-up of a scanner: A standard X-ray CT scanner consists of an X-ray tube, an X-ray detector, a rotation stage, and a set of motors to move the rotation stage, and in most cases also the detector, back and forth (Figure 2).
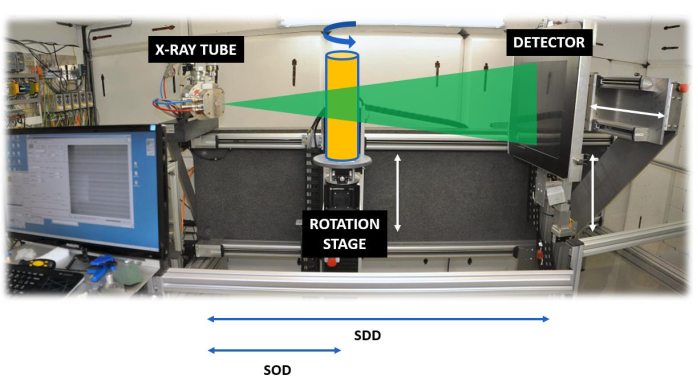
Figure 2. The HECTOR scanning system. The system35, showing the source detector distance (SDD) and the source object distance (SOD). Please click here to view a larger version of this figure.
Most lab-based systems have a cone-beam geometry, which means that the produced X-rays are distributed from the tube's exit window in a cone-beam shape, implying that by changing the distance between the object and the tube (SOD = Source-Object-Distance) and the detector and the tube (SDD = Source-Detector-Distance), the magnification is controlled (see the discussion on resolution). Due to the penetrating power of X-rays, they pass through the object, and the intensity of the attenuation beam is a function of the energy of the X-ray beam, the chemical composition of the object (the atomic number of the elements present) and the density of the material. Given a constant energy spectrum and a constant material composition of wood, the attenuation of the X-ray beam is highly dependent on the density of the material, which explains its use for densitometry. The attenuation (or transmission) can be expressed by the Beer-Lambert law:
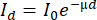
with I0 the incoming X-ray beam exponentially which decays to a transmitted X-ray beam Id when propagating through the material over a distance d. The linear attenuation coefficient µ depends on a series of interactions with the material of the object. The projections are thus recordings of the transmitted beam.
Practically, the object is mounted on the rotation stage, a proper SOD and SDD are selected, a certain power is selected as well (related to object size, density, and composition) and the object is rotated 360° and during that rotation multiple projections are taken. These projections are then used to reconstruct the interior structure of the object. There are several reconstruction algorithms available, of which the most used ones are still based on the analytical framework developed decades ago, relying on the Radon transformation and the Fourier slice theorem. For more details, the reader is referred to specialized literature36.
Conundrum of resolution, data volume and sample size: Resolution is key in X-ray CT scanning. In systems with inverse geometry, or parallel beam geometry such as synchrotron beamlines, other considerations play a role. This protocol only discusses standard lab-based X-ray CT scanning with cone-beam geometry. Here, the concept of magnification, detector pixel size and spot size are essential. Magnification is defined as the ratio of SDD/SOD. Next, the pixel size of the detector obviously also impacts the resolution: the smaller the pixel size, the higher the resolution, but in most cases the field of view (FoV) is also directly related to pixel size and size of the detector (smaller pixel size, smaller FoV for the same number of pixels). Further, the spot size of the X-ray beam is also important: the larger the spot size, the lower the resolution, which means that less details can be seen.
It is important to address that one could obtain a resolution that is higher than what is possible according to the abovementioned limits, therefore it is better to use the term voxel size (a voxel is a volume pixel) instead of resolution. Moreover, there are other factors at play, such as detector sharpness, which further limit the true resolution at which an object is scanned. Only a true calibration of the system, using established targets, supplies the truthful answer.
In most cases, however, the voxel size at which an object can be scanned is mostly limited by the size of the object. This means that the larger the object, the lower the voxel size will be. If the object does not fit within the FoV of the detector for a certain voxel size, then the voxel size can be reduced, e.g., by limiting the magnification.
Scan time and data volume are important to consider when deciding the desired voxel size. In general, the smaller the voxel size means the higher the detail one wants to see, the smaller the sample or the fewer samples that can be scanned at once, more time is required and larger the data volumes will be collected. Imagine the following theoretical example: one can scan a sample measuring 10 cm x 10 cm x 10 cm at 50 µm at once with a certain X-ray CT system and would like to scan that same volume at 10 µm, the volume that fits within the FoV would only be 2 cm x 2 cm x 2 cm, assuming that this is physically possible. This means that 125 scans are needed (5³ = 5 times higher resolution, scales to the power of 3 due to the volumetric nature of the imaging technique) to cover the entire volume, and that the data volume would increase likewise. Of course, this is only a thought experiment, and one needs to consider much more than only resolution. For more information, the reader is referred to an overview of the scanning possibilities37.
Flexibility of instruments for scanning of wooden objects: In the last decade, many companies have delivered X-ray CT systems with a similar assembly as HECTOR35. An overview of several CT systems, especially assessed for their temporal resolution, is given in38.
In all, the flexibility and user-friendliness of X-ray CT systems has improved considerably. Many systems allow scanning of a diverse range of objects, which is also the case with the systems at UGCT. The protocol below is demonstrated for the HECTOR system, which is suitable for tree-ring analysis. The protocol however is valid for any other available system if resolution and data format allows for it.
These systems allow the scanning of a variety of objects. A few pictures of different wooden objects scanned with the HECTOR system are given in Figure 3. It is this flexibility that comprises the three scales we present in Figure 1, ranging from a coarse resolution to a very fine resolution.
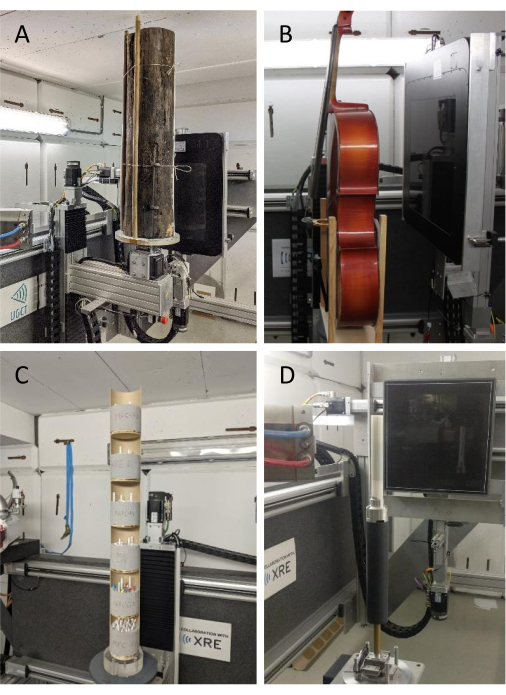
Figure 3. Scanning set-up examples. (A) A log, (B) a cello49, (C) sample holders (type 1) with tree cores for batch scanning and (D) sample holder type 2 with increment cores for helical scanning mounted on the rotation stage of HECTOR. Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
Critical steps within the protocol
Critical steps within the protocol include proper handling of the increment borer to obtain high-quality increment cores (step 1.1. and see39) to avoid bits and pieces. Next, it is essential that cores be left unmounted (but see26), both for insertion in the sample holder (Figure 5, see21) as well as for proper resin extraction50 and for possible future ana…
Offenlegungen
The authors have nothing to disclose.
Acknowledgements
We thank the three anonymous reviewers for their feedback and suggestions. This research was funded by the BOF Special Research Fund for JVdB (BOF Starting Grant BOF.STG.2018.0007.01), for the UGCT as a Center of Expertise (BOF.EXP.2017.0007) and as a Core Facility (BOF.COR.2022.008), The authors also acknowledge the Research Foundation Flanders (G019521N and G009720N), and the UGent Industrial Research Fund (IOF) for the financial support to the infrastructure through grant IOF.APP.2021.0005 (project FaCT F2021/IOF-Equip/021).
Materials
| 6 mm paper straws | http://artstraws.com/education/product/artstraws-thick-asst-cols/ | Coring | |
| Aluminium core holders | |||
| ASTM standard ASTM D 1107 – 96 | |||
| Cardboard postal tubes | https://www.rajapack.co.uk/envelopes-mailing-bags/postal-tubes/1-5-2mm-brown-cardboard-postal-tubes_PDT05623.html | ||
| Column drill | |||
| Computer hardware for reconstruction and analysis | X-ray CT scanning | ||
| Cooling | |||
| Drying oven | |||
| Ethanol | |||
| Flask for under soxhlet (2000 ml) | |||
| Floral foam | https://www.oasisfloral.eu/ | Sample holder type 1 | |
| Glass beads to fill void volume of Soxhlet to save solvent | |||
| Glue | |||
| Hot water bath | https://www.memmert.com/products/water-baths/water-bath/#!filters=%7B%7D | Soxhlet extraction | |
| Increment borer | https://haglofsweden.com/project/increment-borers/ | ||
| Plastic cylinder | Moonen et al. 2022 | Sample holder type 2 | |
| Plastic cylinders | |||
| Reservoir | |||
| Tailored soxhlet apparatus | |||
| Toluene | |||
| Water pump | |||
| X-ray CT scanner |
Referenzen
- Björklund, J., et al. The utility of bulk wood density for tree-ring research. Dendrochronologia. 69 (September), 125880 (2021).
- Lachenbruch, B., Mcculloh, K. A. Traits, properties, and performance: How woody plants combine hydraulic and mechanical functions in a cell, tissue, or whole plant. New Phytologist. 204 (4), 747-764 (2014).
- Baker, T. R., et al. Variation in wood density determines spatial patterns in Amazonian forest biomass. Global Change Biology. 10 (5), 545-562 (2004).
- Bastin, J. F., et al. Wood specific gravity variations and biomass of central African tree species: The simple choice of the outer wood. PLoS ONE. 10 (11), 1-16 (2015).
- Chave, J., et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Global Change Biology. 20 (10), 3177-3190 (2014).
- Chave, J., et al. Towards a worldwide wood economics spectrum. Ecology letters. 12, 351-366 (2009).
- Plourde, B. T., Boukili, V. K., Chazdon, R. L. Radial changes in wood specific gravity of tropical trees: inter- and intraspecific variation during secondary succession. Functional Ecology. 29 (1), 111-120 (2015).
- Decoux, V., Varcin, &. #. 2. 0. 1. ;., Leban, J. -. M. Relationships between the intra-ring wood density assessed by X-ray densitometry and optical anatomical measurements in conifers. Consequences for the cell wall apparent density determination. Annals of Forest Science. 61, 251-262 (2004).
- Rathgeber, C. B. K., Decoux, V., Leban, J. M. Linking intra-tree-ring wood density variations and tracheid anatomical characteristics in Douglas fir (Pseudotsuga menziesii (Mirb.) Franco). Annals of Forest Science. 63 (7), 699-706 (2006).
- Ziemińska, K., Butler, D. W., Gleason, S. M., Wright, I. J., Westoby, M. Fibre wall and lumen fractions drive wood density variation across 24 Australian angiosperms. AoB PLANTS. 5, plt046 (2013).
- Ziemińska, K., Westoby, M., Wright, I. J. Broad anatomical variation within a narrow wood density range – A study of twig wood across 69 Australian angiosperms. PLoS ONE. 10 (4), 1-25 (2015).
- De Mil, T., et al. Wood density profiles and their corresponding tissue fractions in tropical angiosperm trees. Forests. 9 (12), 763 (2018).
- Björklund, J., et al. Scientific Merits and Analytical Challenges of Tree-Ring Densitometry. Reviews of Geophysics. 57 (4), 1224-1264 (2019).
- Maniatis, D., Saint André, L., Temmerman, M., Malhi, Y., Beeckman, H. The potential of using xylarium wood samples for wood density calculations: A comparison of approaches for volume measurement. IForest. 4 (1), 150-159 (2011).
- Lehnebach, R., et al. Wood density variations of legume trees in French Guiana along the shade tolerance continuum: Heartwood effects on radial patterns and gradients. Forests. 10 (2), 1-22 (2019).
- Longuetaud, F., et al. Within-stem maps of wood density and water content for characterization of species: a case study on three hardwood and two softwood species. Annals of Forest Science. 73 (3), 601-614 (2016).
- Steffenrem, A., Kvaalen, H., Dalen, K. S., Høibø, O. A. A high-throughput X-ray-based method for measurements of relative wood density from unprepared increment cores from Picea abies. Scandinavian Journal of Forest Research. 29 (5), 506-514 (2014).
- Vannoppen, A., et al. Dendrochronologia Using X-ray CT based tree-ring width data for tree growth trend analysis. Dendrochronologia. 44, 66-75 (2017).
- Maes, S. L., et al. Evaluating the robustness of three ring-width measurement methods for growth release reconstruction. Dendrochronologia. 46 (May), 67-76 (2017).
- Van Den Berge, S., et al. Biomass increment and carbon sequestration in hedgerow-grown trees. Dendrochronologia. 70 (September), 125894 (2021).
- De Mil, T., Vannoppen, A., Beeckman, H., Van Acker, J., Van den Bulcke, J. A field-to-desktop toolchain for X-ray CT densitometry enables tree ring analysis. Annals of Botany. 117 (7), 1187-1196 (2016).
- St. George, S., Esper, J. Concord and discord among Northern Hemisphere paleotemperature reconstructions from tree rings. Quaternary Science Reviews. 203, 278-281 (2018).
- Schweingruber, F., Fritts, H., Braker, O., Drew, L., Schar, E. The X-ray technique as applied to dendroclimatology. Tree-Ring Bulletin. 38, 61-91 (1978).
- Björklund, J., et al. Cell size and wall dimensions drive distinct variability of earlywood and latewood density in Northern Hemisphere conifers. New Phytologist. 216 (3), 728-740 (2017).
- Polge, H. Applications dans les domaines Technologique et Physiologique. Annales des sciences forestières. 23 (1), 215 (1966).
- De Mil, T., et al. A lonely dot on the map: Exploring the climate signal in tree-ring density and stable isotopes of clanwilliam cedar, South Africa. Dendrochronologia. 69 (November 2020), 125879 (2021).
- Jansen, S., et al. Preparation of wood specimens for transmitted light microscopy and scanning electron microscopy. Belgian Journal of Botany. 131 (1), 41-49 (1998).
- Gärtner, H., Nievergelt, D. The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia. 28 (2), 85-92 (2010).
- von Arx, G., Crivellaro, A., Prendin, A. L., Čufar, K., Carrer, M. Quantitative Wood Anatomy-Practical Guidelines. Frontiers in Plant Science. 7 (June), 781 (2016).
- Seftigen, K., et al. Prospects for dendroanatomy in paleoclimatology — a case study on Picea engelmannii from the Canadian Rockies. Climate of the Past. 18 (5), 1151-1168 (2022).
- Castagneri, D., Regev, L., Boaretto, E., Carrer, M. Xylem anatomical traits reveal different strategies of two Mediterranean oaks to cope with drought and warming. Environmental and Experimental Botany. 133 (October), 128-138 (2017).
- Brodersen, C. R., et al. Automated analysis of three-dimensional xylem networks using high-resolution computed tomography. The New phytologist. 191 (4), 1168-1179 (2011).
- Van den Bulcke, J., et al. X-ray tomography as a tool for detailed anatomical analysis. Annals of Forest Science. 66 (5), 508 (2009).
- Williamson, G. B., Wiemann, M. C. Measuring wood specific gravity…Correctly. American journal of botany. 97 (3), 519-524 (2010).
- Masschaele, B., et al. HECTOR: A 240kV micro-CT setup optimized for research. Journal of Physics: Conference Series. 463 (1), 012012 (2013).
- Kak, A. C., Slaney, M. . Principles of Computerized Tomographic Imaging. , (2001).
- Van Den Bulcke, J., et al. Advanced X-ray CT scanning can boost tree ring research for earth system sciences. Annals of Botany. 124 (5), 837-847 (2019).
- Zwanenburg, E. A., Williams, M. A., Warnett, J. M. Review of high-speed imaging with lab-based x-ray computed tomography. Measurement Science and Technology. 33 (1), 012003 (2022).
- Gärtner, H., Cherubini, P., Schneider, L., Lucchinetti, S. Advanced Workflow for Taking High-Quality Increment Cores – New Techniques and Devices. JoVE. (193), e64747 (2023).
- Schweingruber, F. H., Fritts, H. C., Bräker, O. U. The X-ray technique as applied to dendroclimatology. Tree-Ring Bulletin. 38, (1978).
- Grabner, M., Wimmer, R., Gierlinger, N., Evans, R., Downes, G. M. Heartwood extractives in larch and effects on X-ray densitometry. Canadian Journal of Forest Research. 35 (12), 2781-2786 (2005).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 9 (7), 671-675 (2012).
- Arganda-Carreras, I., et al. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics. 33 (15), 2424-2426 (2017).
- von Arx, G., Carrer, M. ROXAS – A new tool to build centuries-long tracheid-lumen chronologies in conifers. Dendrochronologia. 32 (3), 290-293 (2014).
- Koubaa, A., Zhang, S. Y. T., Makni, S. Defining the transition from earlywood to latewood in black spruce based on intra-ring wood density profiles from X-ray densitometry. Annals of Forest Science. 59 (5-6), 511-518 (2002).
- Buras, A., Wilmking, M. Correcting the calculation of Gleichläufigkeit. Dendrochronologia. 34, 29-30 (2015).
- Bunn, A. G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia. 28 (4), 251-258 (2010).
- Zang, C., Biondi, F. Treeclim: an R package for the numerical calibration of proxy-climate relationships. Ecography. (November 2014), 1-6 (2014).
- Van den Bulcke, J., et al. Nondestructive research on wooden musical instruments: From macro- to microscale imaging with lab-based X-ray CT systems. Journal of Cultural Heritage. 27, S78-S87 (2017).
- Helama, S., Vartiainen, M., Kolström, T., Meriläinen, J. Dendrochronological investigation of wood extractives. Wood Science and Technology. 44 (2), 335-351 (2010).
- Black, B. A., et al. The value of crossdating to retain high-frequency variability, climate signals, and extreme events in environmental proxies. Global Change Biology. 22 (7), 2582-2595 (2016).
- Hubau, W., et al. The persistence of carbon in the African forest understory. Nature plants. 5 (2), 133-140 (2019).
- Stoffel, M., Klinkmüller, M. 3D analysis of anatomical reactions in conifers after mechanical wounding: First qualitative insights from X-ray computed tomography. Trees – Structure and Function. 27 (6), 1805-1811 (2013).
- Van den Bulcke, J., et al. Advanced X-ray CT scanning can boost tree-ring research for earth-system sciences. Annals of Botany. , 1-11 (2019).
- Ziaco, E., Biondi, F., Heinrich, I. Wood Cellular Dendroclimatology: Testing New Proxies in Great Basin Bristlecone Pine. Frontiers in Plant Science. 7 (October), 1-13 (2016).
- De Ridder, M., et al. High-resolution proxies for wood density variations in Terminalia superba. Annals of botany. 107 (2), 293-302 (2011).
- Resente, G., et al. Repeat! Artificial Intelligence for Quantitative Wood Anatomy. Frontiers in Plant Science. 12 (November), 1-14 (2021).

