FLIM-FRET Imaging for Characterization of Protein-Protein Interactions in Live Bacteria
Abstract
Source: Manko, H. et al., FLIM-FRET Measurements of Protein-Protein Interactions in Live Bacteria. J. Vis. Exp. (2020)
The video describes the FLIM-FRET imaging technique to determine the protein-protein interaction in live bacteria expressing cytoplasmic proteins labeled with fluorescent proteins, a donor eGFP, and acceptor mCherry. The combined technique also allows the quantification of the interacting proteins.
Protocol
1. Preparation of agarose pad (Figure 1)
- Place a microscope glass slide on a flat horizontal surface. Arrange two glass slides topped with two layers of adhesive tape on each side of the initial slide.
NOTE: Keep a 1-2 mm space between the three aligned slides to prevent the melted agarose from eventually spreading on the slides with adhesive tape. - Pipette and pour a droplet of 70 µL of 1% melted agarose on the glass slide. Add a fourth slide on the top to flatten the agarose droplet and press down gently. Wait about a minute.
- Take off the upper slide and drop with a pipette about 3 µL of bacteria into 3 to 4 spots at different locations onto the agarose pad.
- Cover with a microscopy glass coverslip (for example a 22×22 mm #1.5 thickness).
NOTE: Flatness and thickness of the coverslips are important for working with two-photon excitations. Precision coverslips with controlled uniform flatness and low autofluorescence are usually a good choice. - Fix the coverslip with melted paraffin to seal the coverslip onto the glass slide. Start by fixing the four corners of the coverslip.
2. Imaging with a two-photon microscopy setup
NOTE: We are using a homemade two-photon excitation scanning inverted microscope with a 60x 1.2NA water immersion objective operating in de-scanned fluorescence collection mode. Two-photon excitation wavelength is set at 930 nm. It is provided by a Ti:Sapphire laser (80 MHz repetition rate, ≈ 70 fs pulse width) working at 10-20 mW. Fluorescence photons were collected through a 680 nm short pass filter and a 525/50 nm band-pass filter before being directed to a fiber-coupled avalanche photo-diode connected to a time-correlated single photon counting (TCSPC) module. The microscope is also equipped with a transmission fluorescence lamp. Several FLIM-FRET microscopes are now commercially available and many imaging facilities are equipped with setups able to perform FLIM-FRET measurements.
- Use the fluorescence lamp to focus the objective on the monolayer of bacteria in the sample and select regions of interest.
- Check that the excitation laser shutter is closed and that the infrared light coming from the laser is blocked and does not enter the microscope.
Caution: Careful attention and constant vigilance should be given working with IR pulsed lasers as the laser light cannot be seen by the eyes but any and even transient direct exposition or laser reflection can be extremely harmful and create irreversible eye damage. Please refer to the local laser safety procedures and training before using microscopy setups. - Place the microscopy slide on the stage with the coverslips facing the objective.
- Check that the fluorescence lamp is ON.
- Turn the filter cube turret to select the eGFP cube and open the fluorescence lamp shutter.
- Send the fluorescence light toward the eyepiece of the microscope.
Caution: Ensure appropriate filters are disposed of in the light path to discard direct excitation light coming from the fluorescence lamp that can damage the eyes. - Focus the objective on bacteria using the microscope knob.
- Select a region of interest in the sample by translating it using the joystick controlling the motorized stage
NOTE: Focusing is easier with highly fluorescent samples allowing the fluorescence to be seen directly with the eyes. - Switch the excitation for the 2PE laser for FLIM-FRET measurements.
- Send the fluorescence emission path back toward the detector.
- Turn back the filter cube turret to select the dichroic cube for the 930 nm laser.
- Set the laser power to 20 mW.
- Set the size of the region of interest to 30 µm. This operation adjusts the voltage operating the galvo-mirrors and defines the range of their movements (Figure 2).
- Turn on the detector and start scanning the sample – the start and stop buttons controlling the scanning also control the opening and closing of the laser shutter both for safety reasons and to limit the photobleaching of the sample (Figure 2).
- If necessary, adjust the focus by slightly moving the microscope fine focus knob.
- Choose the field of view for imaging by moving finely the stage from the computer interface. This can be done on the setup by moving the cross on the image in the microscope control software (Figure 2) that will define the new center of the image and pressing “Move Stage”. A good field of view for acquisition corresponds to an image with 10-30 immobile bacteria, all correctly focused (all bacteria are on the same plane). If interested in extracting single cells FLIM-FRET data, ensure that bacteria are well individualized (image segmentation will be much easier).
Representative Results
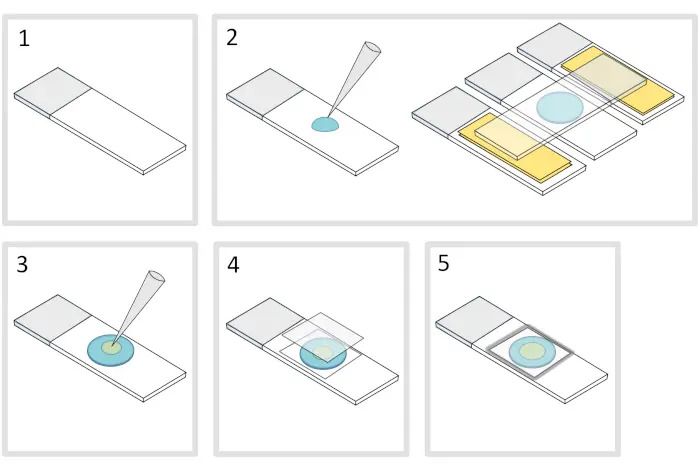
Figure 1. Agarose pad preparation.
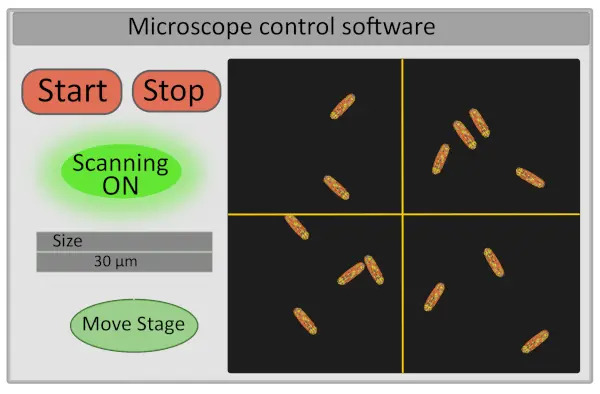
Figure 2. Schematic representation of the interface of microscope control software
Divulgaciones
The authors have nothing to disclose.
Materials
| 525/50 nm band-pass filter | F37-516, AHF, Germany | ||
| 680 nm short pass filter | F37-516, AHF, Germany | ||
| Agarose | Sigma-Aldrich | A9539 | |
| Fiber-coupled avalanche photo-diode | SPCM-AQR-14- FC, Perkin Elmer | ||
| Glass coverslips (Thickness No. 1.5, 20×20mm | Knitel glass | MS0011 | |
| Microscope slides (25×75mm) | Knitel glass | MS0057 | |
| TCSPC module | SPC830, Becker & Hickl, Germany | ||
| Ti:Sapphire laser | Insight DeepSee, Spectra Physics |

