Functional Manipulation of Maternal Gene Products Using In Vitro Oocyte Maturation in Zebrafish
Summary
An optimized protocol for the in vitro maturation of zebrafish oocytes used for the manipulation of maternal gene products is presented here.
Abstract
Cellular events that take place during the earliest stages of animal embryonic development are driven by maternally derived gene products deposited into the developing oocyte. Because these events rely on maternal products which typically act very soon after fertilization-that preexist inside the egg, standard approaches for expression and functional reduction involving the injection of reagents into the fertilized egg are typically ineffective. Instead, such manipulations must be performed during oogenesis, prior to or during the accumulation of maternal products. This article describes in detail a protocol for the in vitro maturation of immature zebrafish oocytes and their subsequent in vitro fertilization, yielding viable embryos that survive to adulthood. This method allows the functional manipulation of maternal products during oogenesis, such as the expression of products for phenotypic rescue and tagged construct visualization, as well as the reduction of gene function through reverse-genetics agents.
Introduction
During animal development, the mother deposits gene products (e.g., RNAs, proteins, and other biomolecules) into the egg; these products are important for early cellular processes immediately following fertilization1,2. The manipulation of the expression and function of maternal products is typically ineffective when using a standard approach for the injection of reagents into fertilized eggs3. This is because most RNAs and proteins are produced by the oocyte during oogenesis, so pre-loaded maternal products are already present in the mature egg. Such preexisting products are impervious to functional knockdown with gene targeting agents, such as Morpholino antisense oligos (MOs), because MOs target the mRNA, not the preexisting protein already present in the egg at fertilization. In addition, many early embryonic processes happen too soon after fertilization to be influenced by protein products derived from RNA injected into the fertilized egg, as the RNA may not be produced fast enough to influence the first events of embryogenesis. For the same reason, tagged protein fusions expressed through an mRNA injection into the fertilized egg may not be produced in time for visualization during their active role in the early embryo. Injection into extruded mature oocytes prior to egg activation is possible but is associated with similar technical issues: such mature eggs are already pre-loaded with maternal protein, and they will not become translationally active (i.e., produce protein from exogenous transcripts) until after egg activation. For these reasons, the manipulation of maternal gene products acting in early embryogenesis typically needs to be carried out during oogenesis in the maturing oocyte.
As one approach to overcoming these hurdles, in vitro maturation methods, which allow for the maturation of oocytes from stage IV to egg formation, have been established in zebrafish. Early methods allowed in vitro maturation, but the resulting mature eggs were not competent for fertilization4. Subsequently, the manipulation of culture conditions from neutral to pH 9.0, mimicking the alkaline pH of the ovarian fluid found in fish species5,6, allowed for reliable in vitro fertilization (IVF) after in vitro maturation7,8. Indeed, in vitro-matured oocytes can yield viable embryos that survive to adulthood and that are fertile3,8. This improved method has been further adapted to include the functional manipulation of maternal genes, the expression of tagged proteins during zebrafish oogenesis through exogenous expression, and MO-mediated functional knock down in maturing stage IV oocytes3 (Figure 1).
Zebrafish oogenesis exhibits a number of characteristic stages leading to the formation of mature oocytes9 (see Table 1 for a quick guide to the various stages in oogenesis). Briefly, oocyte development is initiated by stage I oocytes and is arrested at the diplotene stage of prophase I of meiosis. These oocytes undergo growth through active transcription (initiated during stages IA and IB), the formation of cortical alveoli (also known as cortical granules, initiated during stage II), and vitellogenesis (initiated during stage III). Oocyte growth is completed during stage IV, when meiosis I resumes, resulting in the disassembly of the oocyte nucleus, referred to as the germinal vesicle (GV). Subsequently, meiosis is arrested again at metaphase II. The completion of oocyte growth and meiotic arrest during stage IV leads to a mature stage V egg9. The removal of the follicular membrane occurs during the release of the oocyte into the lumen of the ovary10 and is essential for fertilization and proper egg activation. Once the eggs are extruded from the mother during natural mating, they become activated. In zebrafish, exposure to water is sufficient for full egg activation, regardless of the presence of sperm11.
In vitro conditions currently allow for the maturation of oocytes from early stage IV-which can be recognized by their size (690-730 µm), the presence of a large GV in an asymmetric position, and a fully opaque appearance due to the accumulation of yolk proteins (Figure 2B)-to the mature stage V egg, characterized by a fully disassembled GV and a translucent appearance due to yolk protein processing12 (Figure 2C). In this approach, whole ovaries containing oocytes at different stages of development are removed from females. The oocytes are allowed to develop in 17α-20β-dihydroxy-4 pregnen-3-one (DHP), an effective maturation-inducing hormone8. During this period, maturing oocytes can be manipulated through the injection of expression (e.g., capped, in vitro-transcribed mRNAs) or reverse-genetics agents (e.g., MOs). The follicular layer does not spontaneously shed towards the end of the maturation period, so it must be manually removed. After defolliculation, in vitro fertilization is achieved by triggering egg activation through the exposure of the eggs to water (present in embryonic (E3) medium)13 and a sperm solution. The resulting zygotes undergo embryonic development and allow for the evaluation of the ability of treatments to manipulate maternal gene function and for the visualization and analysis of tagged maternal products (Figure 3).
Protocol
All zebrafish were handled in strict accordance with good animal practice, as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the appropriate committee (University of Wisconsin-Madison assurance number A3368-01). Animals were maintained under standard conditions at 26.5 °C.
1. Pre-selection of Females
NOTE: Oocytes in adult females typically span a range of developmental stages, from stage I to V (Figure 2). Purging the females of preexisting oocytes through a successful natural mating increases the synchronization of oocyte staging, as newly developing oocytes appear as a cohort3,14. Within about 8 days post-purge, most oocytes are in early stage IV, which is optimal for the initiation of in vitro maturation. Such synchronization increases the yield of oocytes that can go through the full process of in vitro maturation, facilitating experimental manipulation. See previous descriptions13 for details on setting up fish in paired matings and on fish water composition (here, 14 g of ocean salt and 150 g of NaHCO3 per 1,000 L of reverse-osmosis water, pH 6.5-8.5 (preferred range: 6.8-7.5) and a conductivity of 180-360 μS). See Brand et al.13 for additional recipes.
- Eight to ten days prior to the in vitro culture manipulation, use a fish net to transfer a single male and a single female fish of the desired strain to a mating tank. Pair multiple sets. Leave them in the mating tank overnight. Allow them to mate during the evening and through the afternoon of the following day.
- Use a fish net to separate the females that yield eggs during mating and place them into a separate tank. Feed these females twice daily with a food mixture containing approximately an equal amount of brine shrimp and fish food flakes. The amount of food should be enough to provide approximately 20 min, but not more, of feeding time.
2. Preparation of Maturation Medium
NOTE: Prepare maturation medium on the day of the in vitro maturation experiment, within 1 h of removing the oocytes from the female (see section 3).
- Add 20 mL of Leibovitz's L-15 medium with L-glutamine, pH 7.0, to a 50 mL conical tube under sterile conditions. Bring it to pH 9.0 with 10 N NaOH.
- Add 9 mL of Leibovitz's L-15 medium, pH 9.0, to 2 separate 50 mL conical tubes. Label 1 tube "+DHP" and the other "-DHP."
- In the +DHP tube, add 10 µL of 17α-20β-dihydroxy-4 pregnen-3-one (DHP), 490 µL of dH2O, and 500 µL of 10% bovine serum albumin (BSA).
- In the -DHP tube, add 100 µL of 10 mg/mL gentamycin, 400 µL of dH2O, and 500 µL of 10% BSA.
3. Dissection of the Oocytes and Initiation of the In Vitro Culture
NOTE: The in vitro culture experiment is carried out with pre-selected females 8-10 days after they release eggs through natural mating. Use maturation medium made the same day (see section 2). Oocytes mature appropriately if the dissection is begun near the end of the fish diurnal cycle (determined in the laboratory by pre-set artificial lighting in the fish facility)13, possibly mimicking processes occurring through natural mating. This implies that most steps in the protocol must be carried out in the evening if fish are housed in a facility with a standard light cycle (e.g., beginning step 3.2 at 6 pm in a facility with an 8 am to 10 pm light period), although other work time-periods are possible with an appropriately time-shifted light cycle.
- Prepare a 0.2% tricaine stock solution in dH2O, buffered to pH 7.0 with 1 M Tris, pH 9.0, and keep this solution at 4 °C. This can be prepared ahead of time.
- Initiate the experiment 0-4 h prior to the end of the daily light cycle in the facility. In a 250-mL beaker, add 20 mL of 0.2% tricaine stock solution, pH 7.0, to 80 mL of fish water and mix.
- Transfer the pre-selected females to tricaine solution and euthanize them by overexposure. Leave the females in the tricaine solution for 15 min after the cessation of gill movement.
- Using a spoon, collect the euthanized fish from the tricaine solution and rinse them briefly in fish water. Place the fish onto a paper towel to absorb excess water.
- Use a clean razor blade to decapitate the euthanized fish at the level of the pectoral fin. Using dissecting scissors, make a longitudinal incision on the ventral side of the fish, extending from the anterior end to the anal area.
- Place the fish on a petri dish under a dissecting microscope with incident light. Using a pair of dissecting forceps, transfer ovary portions to a 35 x 10 mm culture dish containing 4 mL of Leibovitz's L-15 medium +DHP.
NOTE: The ovaries, which contain developing oocytes, will appear as opaque and clumpy structures within the internal body cavity. - Using dissecting forceps, gently dissociate the oocytes from the follicular masses. Sort the early stage IV oocytes (Figure 2B; prior to GV breakdown, at near-maximal size and characterized by a dark, opaque cytoplasm and a readily apparent GV located asymmetrically within the oocyte). Discard oocytes at earlier stages (Figure 2A) and translucent, mature stage V eggs (similar to those in Figure 2C but present in the ovaries prior to DHP treatment).
NOTE: Oocytes are selected for maturation in early stage IV, the earliest stage that results in mature, stage V oocytes after in vitro culture conditions (Figure 2C and D)3. Because stage IV oocytes are actively producing products, manipulation at this stage allows for the expression of exogenous products through RNA injection or for the reduction of gene function via introduced MOs. - Use a glass Pasteur pipet to transfer the early stage IV oocytes to a second 35 x 10 mm plastic culture dish containing 4 mL of Leibovitz's L-15 medium +DHP. Transfer minimal amounts of -DHP medium to the dish containing the +DHP solution.
4. Oocyte Microinjections
NOTE: Isolated stage IV oocytes undergoing in vitro maturation can be microinjected to introduce reagents for functional manipulation or tagged protein expression. The microinjection of reagents, such as mRNA and MOs (see section 4), is typically carried out when the oocytes are undergoing in vitro maturation in +DHP medium, prior to defolliculation. If desired, in order to allow more time to conduct manipulations prior to oocyte maturation, the injections can also be carried out in -DHP medium, prior to maturation, for at least 2 h. They can subsequently be transferred to +DHP medium.
- Prepare mRNAs using a standard expression kit. Keep them at -80 °C as a 100-500 pg/µL stock for injection at a final concentration of 50-500 pg/µL (200 pg/µL is recommended) in RNA-grade water. Prepare MOs at a concentration of 4 ng/µL in water according to the manufacturer's instructions. Inject them at final concentrations of 2 ng/µL (or as empirically determined).
NOTE: Injections are typically carried out in +DHP medium prior to defolliculation (see the note in section 5). - If injecting mRNA, immediately prior to the injection, dilute the mRNA using RNA-grade reverse-osmosis water and 0.2 M KCl to achieve a final solution of 0.1 M KCl.
- If injecting MOs, immediately prior to the injection, dilute the MOs to the desired concentration using reverse-osmosis water and 0.2 M KCl to achieve a final solution of 0.1 M KCl.
- Manually hold oocytes with fine forceps and inject approximately 1 nL into wild-type stage IV oocytes using a needle made with a pulled-glass capillary pipette.
- Prepare the glass needles by pulling heated glass capillary tubes, loading the solution to be injected, and breaking the needle tip with forceps, as previously described in protocols for standard injection into zebrafish early embryos15.
NOTE: It is helpful if the needle has a gradual taper rather than an abrupt one; this allows more flexibility in terms of the location of the break in the needle tip and also helps to prevent damage to the injected embryo. Using the same needle and solution as for the embryo injections, adjust the injected volume by pre-determining the microinjector pressure settings that produce the desired volume. These settings can be determined by mock-injecting into a drop of mineral oil on a microscope stage calibration slide (0.01 mm) and adjusting the microinjector settings to obtain the desired diameter in the bolus of injected solution, as previously described15.
- Prepare the glass needles by pulling heated glass capillary tubes, loading the solution to be injected, and breaking the needle tip with forceps, as previously described in protocols for standard injection into zebrafish early embryos15.
5. Oocyte Maturation and Defolliculation
NOTE: During oocyte maturation, the oocytes will become progressively translucent, which allows for the assessment of successful culture conditions.
- Continue incubating the immature (and, if appropriate, injected) oocytes in +DHP medium at 26.5 °C, checking periodically (every 30 min) to ensure that the oocytes remain intact and are undergoing proper maturation by becoming progressively translucent (see Figure 2C).
- Remove any lysing oocytes with a Pasteur pipette and discard them in a lab waste beaker to maintain the quality of the medium. Exchange the culture medium with approximately a half-volume of fresh +DHP medium to maintain a clear solution, depending on the amount of oocyte lysis.
- Allow the in vitro maturation to proceed until a majority of the oocytes become translucent and have a GV that is no longer apparent (approximately 2 h in DHP treatment, see Figure 2C as compared to D). View them under a dissecting microscope with transmitted light optics.
- Remove the outermost follicular membrane from each matured oocyte. Use extra-fine forceps to make a tear in the follicular membrane in a region with increased space between the oocyte and the membrane. Peel off a portion of the membrane and roll the oocyte out of the membrane while holding down the peeled part.
NOTE: The underlying chorionic membrane will typically remain in close association with the egg during this process; after egg activation, it expands to form a protective layer for the embryo. - Transfer the defolliculated oocytes in a minimal volume of medium (typically, transfer about 5-10 mature oocytes in less than 20 µL) to a petri dish with a few drops of culture (+DHP) medium and proceed to fertilization.
6. Fertilization of In Vitro Cultured Oocytes
NOTE: The sperm solution on ice, prepared below, will maintain its potency for about 2 h.
- Prepare a sperm solution near the end of the oocyte maturation step and prior to defolliculation using testes from five males in 500 µL of Hanks' solution, as described previously16,17.
- Add 10-50 µL of sperm solution to the defolliculated oocytes in +DHP culture medium. Wait 10 s.
- Using a pipette, add a few drops of embryonic (E3) medium to the oocytes. Wait 1 min and then flood the plate with E3 medium.
NOTE: The composition of E3 medium is as follows: 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, and 1-5% Methylene Blue13. - Repeat steps 5.3, 6.2, and 6.3 to obtain greater numbers of fertilized embryos.
- Allow the fertilized embryos to develop. Use a dissecting microscope with transmitted light optics to observe the progression through the cleavage stages to ensure successful fertilization, as previously described for fertilization17 and staging18.
Representative Results
To determine if the procedure described above is successful, the embryos can be observed during the cleavage stages to confirm the appearance of the stereotypical early-embryonic cleavage pattern18, as well as at 24 h post-fertilization (hpf) to confirm the proper development of the basic body plan. This procedure allows for the manipulation of maternal products for functional studies through the injection of reagents, such as mRNAs and MOs, during oocyte development.
Manipulation of maternal products for functional studies:
mRNA coding for wild-type and mutated products can be injected into in vitro maturing oocytes to test the effect of the manipulation of maternal gene function during meiosis and the early embryonic stages. For example, wild-type embryos can be injected with wild-type mRNA to test for the effect of product overexpression, or with mutant RNA to test for potential dominant (e.g., gain-of-function and antimorphic) effects in these processes. Oocytes in in vitro culture are able to produce protein from exogenous mRNA throughout oocyte development, as shown by the expression of GFP from injected mRNA, although only oocytes that initiate culture conditions at stage IV of oogenesis can develop into mature stage V oocytes (Figure 2B-2D, see also Nair et al.3). The expression of wild-type product through injected mRNA is also instrumental for the rescue of maternal-effect mutations to confirm gene identity during positional cloning3,24. In this case, wild-type mRNA is injected into oocytes from homozygous mutant females to test whether wild-type products can rescue the mutant phenotype. This genetic rescue is illustrated in Figure 3A-3C, where injected AurB mRNA is shown to rescue the phenotypic effects of a mutation in its corresponding gene, cellular island (cei) (Figure 3A-3C). The embryos from a control group are also allowed to develop to show the corresponding mutant phenotype3. Mutant RNA can also be injected into mutant oocytes to test whether the mutated product retains partial function by comparing the extent of rescue to that caused by the wild-type product.
Protein expression from mRNA injected into developing oocytes appears to occur with little or no delay. Strong GFP expression is observed within 2 h of the injection of the corresponding mRNA, regardless of the developmental stage of the oocyte3 (Figure 2B-2D; see also Nair et al.3). Products such as mCherry:Sas6 and Birc5b(Motley):GFP can be observed immediately after fertilization and during the first embryonic cell cycle3,25. The injection of mRNA during oogenesis also leads to the production of protein that, regardless of the fused tagged moiety, is functional immediately after fertilization, as shown in the case of maternal products for cellular island/aurB3, futile cycle/lrmp24, and motley/bir5b25. Translation-blocking MOs also have an effect immediately after fertilization, as shown for futile cycle/Lrmp3 and at later stages of embryogenesis, as in the case of mission impossible/dhx163. Splice-blocking MOs, when injected in maturing oocytes, may not have an effect on maternal function, likely due to already-present mature maternal transcripts in stage IV oocytes3.
Generation of morphants:
When using a MO corresponding to a gene with an already-identified mutation, the morphant phenotype is expected to mimic the mutant phenotype. Morphants are compared to uninjected embryos and to those injected with a standard, control MO. The injection of a translation-blocking morpholino can successfully phenocopy the known mutant phenotype3, as shown by the injection of Lrmp MO into oocytes, which mimics the mutant phenotype of the corresponding mutation, futile cycle (Figure 3D-F). The specificity of MOs can be determined through the same approaches used when injecting MOs into embryos (reviewed previously)19,20.
Expression of fluorescently labeled fusion proteins:
mRNA coding for gene products of interest fused to fluorescent proteins (e.g., GFP and mCherry) can also be injected into either wild-type or mutant oocytes to visualize the corresponding products within the early embryo (i.e., reflecting a subcellular localization pattern; Figure 3G and 3H). mRNAs coding for similar fusions but involving the mutant allele can be similarly expressed to test whether the mutation affects any potential subcellular localizations.
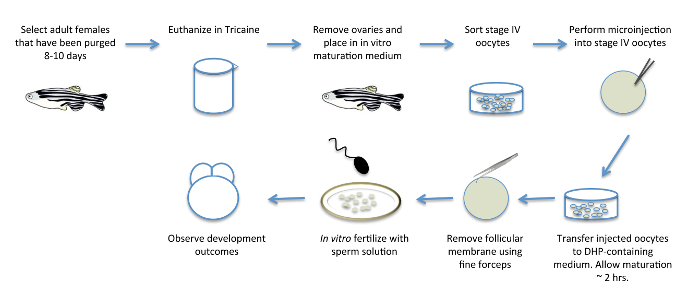
Figure 1: In Vitro Oocyte Maturation. Schematic diagram showing the various steps involved in in vitro oocyte maturation. Adult females are pre-selected for egg-laying 8 days prior to the procedure, to purge them of eggs that have already matured and to promote new oocyte development. The ovaries, containing developing oocytes, are removed from females and transferred to a medium containing the hormone DHP to induce oocyte maturation in vitro. After removal from females and immediately prior to or during oocyte maturation, oocytes can be injected with RNA products and other reagents for the functional manipulation of maternal factors. Mature oocytes are manually defolliculated and fertilized in vitro with a sperm solution to yield fertilized, viable embryos. Please click here to view a larger version of this figure.
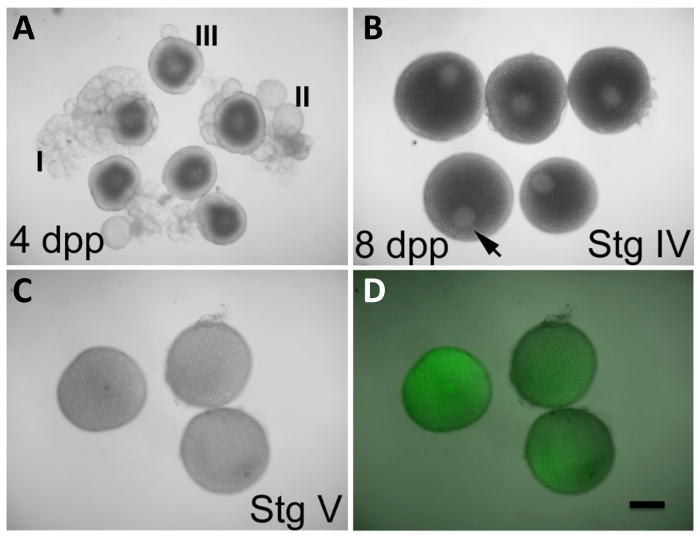
Figure 2: In Vitro Oocyte Maturation and Expression of Products from Injected mRNA. (A) Oocytes at stages I-III, observed in ovaries from females 4 days post-purging (dpp). (B) Oocytes at stage IV, observed in ovaries from females 8 dpp. The germinal vesicle (GV, arrowhead) is clearly visible and occupies an eccentric position. The stage III oocytes in (A) also exhibit a readily apparent GV, but it is found centered in the oocyte. Stage IV oocytes in (B) have a size that is near maximal compared to that of mature oocytes in (C). (C and D) Oocytes at stage V (mature oocytes) after 2 h in in vitro maturation conditions initiated at stage IV, as in (B), and injected during maturation with GFP-encoding, in vitro transcribed mRNA (C, visible light only; D, overlay of visible light and GFP fluorescence). The GV is no longer apparent in stage V oocytes, which are also less opaque than stage IV oocytes. Injected oocytes express GFP protein (D). Defolliculation of mature stage IV oocytes is described in the protocol section. Scale bar = 300 µm. All panels were reproduced, with permission, from Nair et al3. Please click here to view a larger version of this figure.
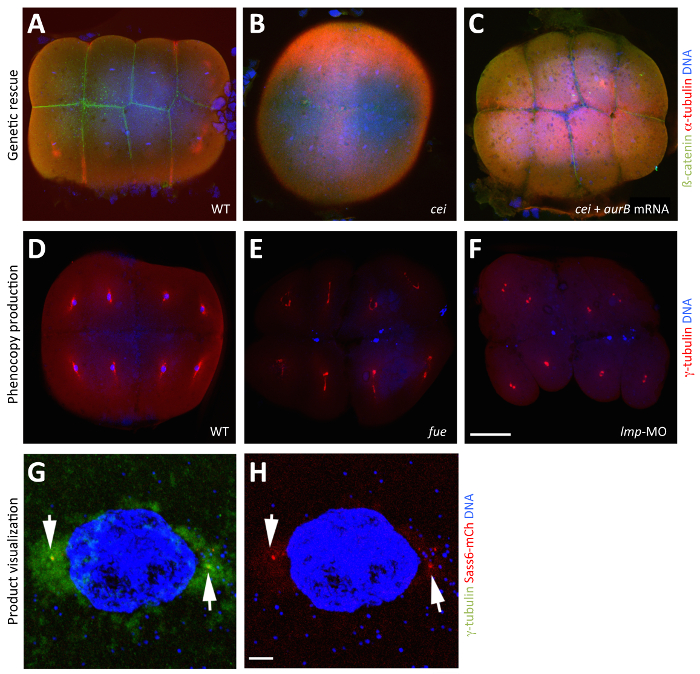
Figure 3: Manipulation and Visualization of Maternal Products through In Vitro Oocyte Maturation. (A–C) Rescue of the maternal-effect phenotype caused by a mutation in cellular island/aurora B through the injection of wild-type aurB mRNA into stage IV cei/aurB oocytes. Merged images showing animal views of 65 mpf fixed blastodiscs, visualized with anti-ß-catenin antibodies to highlight membranes (green), anti-α-tubulin antibodies to indicate microtubules (red), and DAPI to designate DNA (blue). (A) β-catenin accumulation in wild-type embryos, indicative of normal furrow maturation. (B) A cei/aurB embryo from an uninjected cei/aurB stage IV oocyte shows partial, rudimentary furrows that do not accumulate β-catenin. (C) A rescued cei/aurB embryo from a cei/aurB oocyte injected with wild-type aurB mRNA showing robust β-catenin accumulation at the furrows. (D–F) Phenocopy of the maternal-effect caused by a mutation in futile cycle/lrmp from the injection of Lrmp morpholino into stage IV oocytes. Merged images showing animal views of 70 mpf fixed blastodiscs, visualized with anti-γ-tubulin antibodies to indicate centrosomes (red) and DAPI to designate DNA (blue). (D) In wild-type embryos, each nucleus associates with γ-tubulin, a marker for centrosomal material. (E) In maternal-effect fue mutants, pronuclear fusion fails, resulting in two to three patches of DNA labels corresponding to unfused parental pro-nuclei and the polar body for meiosis II. (F) In Lrmp morphants, where maternal Lrmp function is inhibited, the nuclei similarly fail to divide and, in addition, fail to associate with γ-tubulin. (G and H) Visualization of maternally produced product in the early embryo. Sass6-mCherry protein from exogenous mRNA injected into stage IV oocytes localizes to the centrioles. Merged images showing animal views of 40 mpf fixed blastodiscs, visualized with anti-γ-tubulin antibodies to highlight centrosomes (green), mCherry fluorescence to indicate Sass6 (red), and DAPI to designate DNA (blue). (G) Expressed Sass6-mCherry protein localizes to foci labeled by the centrosome marker γ-tubulin at sites flanking the nucleus (arrows). (H) The same image, showing only Sass6-mCherry and DAPI for clarity. The embryos in (G and H) are fixed, but the fluorescence of expressed products, such as mCherry fusions, can also be observed in live embryos (not shown). Scale bar = 100 µm in A-F and 10 µm in G and H. All panels were reproduced, with permission, from Nair et al3. Please click here to view a larger version of this figure.
| Stages of Oogenesis | Oocyte diameter (μm) | Key landmark(s) |
| 1A – Prefollicle | 7 to 20 | Initiation of active transcription, accumulation of nucleoli |
| 1B – Follicle | 20 to 140 | Decondensation of chromosomes |
| II – Cortical alveolus | 140 to 340 | Cortical alveoli production |
| III – Vitellogenesis | 340 to 690 | Darkening of ooplasm by accumulation of yolk precursor protein and lipids |
| Early IV – Oocyte maturation | 690 to 730 (lower range) | Asymmetric localization of germinal vesicle |
| Early IV – Oocyte maturation | 690 to 730 (upper range) | Germinal vesicle disappears, arrest at metaphase II |
| V – Mature oocyte | ~750 | Ooplasm/yolk becomes translucent |
Table 1: Landmarks of Oocyte Development in Zebrafish.
Discussion
The above protocol is for the manipulation of gene products before fertilization, thus allowing for the study of maternal gene products in the early zebrafish embryo. Previous studies have been able to mature oocytes in vitro4; this protocol was modified to allow for the subsequent fertilization of in vitro matured oocytes8. This in turn allows for the injection of reagents for functional manipulation and visualization of maternally inherited products in the early embryo3. Embryos resulting from this method can be viable and can survive to become fertile adults. In preliminary experiments, approximately half of the fertilized embryos derived from this procedure were viable on day 5 of development, as assessed by swim bladder inflation at that stage, about half of which became healthy, fertile adults (unpublished observations).
There are a number of critical steps to consider in the protocol. Oocytes at the appropriate stage to initiate maturation (early stage IV) can be enriched if the female has been mated recently, but not before 8 dpp. Using fish that have not mated recently might result in degenerating eggs14, which will not undergo maturation. Females mated within less than 8 days will have a majority of eggs at stage III or less, and thus, the eggs will not mature properly in vitro. Ovaries in females that mated 8 days prior will have an optimal fraction of oocytes in early stage IV. These can be recognized by their opacity and the presence of the GV in an eccentric position (Figure 2B). Oocyte stage determination can also be aided by size measurement, with the oocytes placed onto a graduated micrometer slide under a microscope and compared to standard staging guidelines (Table 1). However, early stage IV oocytes have near-maximal size compared to mature oocytes (recognized by their relative transparency and lack of a GV; Figure 2C) and are easily recognized, as described above, which generally obviates the need of a direct oocyte size measurement.
In vitro maturation must begin within several hours of the end of the daylight cycle to which the female oocyte donors are acclimated. Oocytes will not mature properly, reflected in poor fertilization rates, if cultured during the morning period of the light cycle. The reason for the circadian dependency of the in vitro oocyte maturation method is not understood but likely reflects underlying circadian biases in in vivo egg maturation involving cycling gene expression during oogenesis21. A common cause for oocytes failing to undergo successful in vitro maturation in spite of proper oocyte staging is that the DHP has expired. DHP hormone generally expires after a year, and to insure effective maturation, the working batch should be replaced within 9 months of use.
The injection of oocytes is another critical step when the purpose of the method is the functional manipulation of maternal products. This procedure has requirements that differ from those for standard injections in the fertilized egg at 0-30 mpf. A key factor in oocyte injection is the need to carry out the injections under conditions that do not lead to premature egg activation, such as in Leibovitz's L-15 medium22,23. Because they are embedded in ovaries, maturing oocytes also pose particular challenges to injection. The protocol above suggests first dissociating oocytes from the ovaries and then holding each dissociated oocyte separately with forceps while injecting. This technique has worked well and allows pre-sorting oocytes at the appropriate stage with minimal handling. Alternative methods can also be used, such as: i) placing oocytes into standard injection agarose troughs15, where the vertical walls of the trough support the oocyte during injection (in this alternative method, the oocytes need to be transferred to injection plates while kept in in vitro maturation medium) and ii) allowing the oocytes to remain attached to the ovarian mass, which can be held with forceps during the injection to avoid contact with the injected oocyte (in this approach, the oocytes should be dissociated after injection to both facilitate DHP exposure and to allow their defolliculation, itself essential for IVF). In spite of this specific challenge, stage IV oocytes can be readily penetrated with an injection needle, likely because the chorionic membrane at this stage is not fully developed9.
One limitation of the current maturation protocol is that in vitro maturation conditions that promote proper oocyte development cannot be created when oocyte maturation is initiated at stages earlier than stage IV. Oocytes become competent to respond to DHP by undergoing maturation when they reach a diameter of 520 µm, which occurs during stage III (vitellogenesis, corresponding to the 340-to-690-µm range)9. However, the culture of stage III oocytes results in oocytes that are not competent for fertilization3. Only oocytes whose in vitro culture is initiated at stage IV (Figure 2B) can develop into mature, stage V oocytes (Figure 2C and D). This may be related to the fact that stage III is essential for vitellogenesis and oocyte growth9. Thus, the in vitro oocyte maturation procedure presented in this article should only be used with a starting population of stage IV oocytes (B), and not with oocytes at earlier stages (stages I – III; Figure 2A). For the same reason, this procedure is limited to genes that are expressed at stage IV or later. The functional manipulation of genes whose products are expressed earlier may instead have to rely on genetic methods (see below). Improvements to in vitro culture maturation methods may in the future allow for the initiation of oocyte culturing at earlier stages, thus expanding the power of the in vitro maturation approach.
Another limitation in the presented method is that defolliculation, which is essential for the subsequent steps involving fertilization, is currently a rate-limiting step in the procedure. The follicular membrane is a transparent layer surrounding the developing oocyte, and removal can be tedious. If this membrane is not fully removed, the egg will not become fully activated and fertilized. This is made evident by the failure of the chorion to expand and the development of irregularly placed, furrow-like structures (pseudocleavages), characteristic of unfertilized embryos11. Enzymatic defolliculation methods such as collagenase, as used in Xenopus, have been tried. However, in our hands, this technique has not been successful, and we therefore rely on manual defolliculation. Because manual defolliculation is labor-intensive and time-consuming, it is difficult to obtain large batches of fertilized eggs after in vitro oocyte maturation. Due to the temporal limitation imposed by manual defolliculation, we currently fertilize a few defolliculated, mature eggs at a time, in small batches of 5-10 eggs. The incorporation of enzymatic defolliculation with this procedure would likely significantly increase its yield and overall ease of use.
Although embryos produced after the fertilization of in vitro-matured oocytes can become viable adults, we note that after in vitro fertilization, a fraction of embryos do not divide normally or in a timely fashion. We are currently selecting for lines whose propagation includes rounds of in vitro oocyte maturation, which may result in more robust patterns of development in embryos derived through this method.
The procedure has allowed for the clear rescue or visualization of protein localization for a number of mutations24,25. However, for some genes, the regulation of product expression may be crucial, so the products expressed by injected mRNA may lead to variable results26. It is also important to be mindful of all the controls (e.g., embryos injected with reagents that have similar properties to those used in the experiment yet are predicted to not produce an effect, such as control MOs or RNAs coding for inert proteins only, such as GFP), as underlying variability may lead to improper conclusions.
An approach involving in vitro manipulation followed by fertilization is particularly useful in the case of maternally deposited products, where the standard method of injecting RNA, MOs, or protein into the fertilized egg may not effectively reduce products already accumulated in the egg. Other recently developed methods, such as CRISPR-mutant generation, are also effective at generating mutant conditions for maternally expressed genes26. However, this method requires the growth of several generations of fish. The in vitro oocyte maturation technique allows for rapid functional manipulation to study the function of maternally expressed genes, including the rescue and phenocopy of maternal-effect mutations, as well as the expression of RNA and proteins in the early embryo.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We would like to thank members of the Pelegri lab and fish facility managerial staff, who were instrumental in facilitating this project. Support for this project was provided by NIH R56 GM065303 and NIH 2 T32 GM007133 to E.L.W., NIH 5 F31 GM108449-02 and NIH 2 T32 GM007133 to C.C.E., and NIH RO1 GM065303 to F.P.
Materials
| Zebrafish mating boxes | Aqua Schwarz | SpawningBox1 | |
| NaCl | Sigma | S5886 | |
| KCl | Sigma | P5405 | |
| Na2HPO4 | Sigma | S3264 | |
| KH2PO4 | Sigma | P9791 | |
| CaCl2 | Sigma | C7902 | |
| MgSO4-7H2O | Sigma | 63138 | |
| NaHCO3 | Sigma | S5761 | |
| Tricaine | Western Chemical | Tricaine-D (MS 222) | FDA approved (ANADA 200-226) |
| Tris base | Sigma | 77-86-1 | to prepare 1 M Tris pH 9.0 |
| HCl | Sigma | 920-1 | to prepare 1 M Tris pH 9.0 |
| Fish net (fine mesh) (4-5 in) | PennPlax | (ThatFishThatPlace # 212370) | available in ThatFishThatPlace |
| Plastic spoon | available in most standard stores | ||
| Dissecting scissors | Fine Science Tools | 14091-09 | |
| Dissecting forceps | Dumont | SS | available from Fine Science Tools |
| Dissecting stereoscope (with transmitted light source) | Nikon | SMZ645 | or equivalent |
| Reflective light source (LED arms) | Fostec | KL1600 LED | or equivalent |
| Petri plates 10 cm diameter | any maker | ||
| Eppendorf tubes 1.5 ml | any maker | ||
| Ice bucket | any maker | ||
| Narrow spatula | Fisher | 14-374 | |
| Depression glass plate | Corning Inc | 722085 (Fisher cat. No 13-748B) | available from Fisher Scientific |
| Paper towels | any maker | ||
| Kimwipes | Kimberly-Clark | 06-666-11 | available from Fisher Scientific |
| Timer stop watch | any maker | ||
| Wash bottle | Thermo Scientific | 24020500 | available from Fisher Scientific |
| beakers, 250 ml (2) | Corning Inc. | 1000250 | available from Fisher Scientific |
| Leibovitz'z L-15 medium | Thermofisher | 11415064 | |
| NaOH | Sigma | 221465 | for pH'ing |
| BSA | Sigma | A2058 | |
| 17alph-20beta-dihyroxy-4-pregnen-3-one (DHP) | Sigma | P6285 | |
| gentamicin | Sigma | G1272 | |
| Injection Apparatus | Eppendorf | FemtoJet | or equivalent |
| Capillary Tubing for injection needles | FHC | 30-30-1 | or equivalent, Borosil 1.0 mm OD x 0.5 mm ID with fiber, 100 mm |
| Needle puller | Sutter Instruments | Model P-87 | any maker |
| Micropipetor (1-20 µl range) with tips | any maker | ||
| Micropipetor (20 – 200 µl range) with tips | any maker | ||
| Micropipetor (100 – 1000 µl range) with tips | any maker | ||
| Conical tubes 15ml | any maker | ||
| Conical tubes 50ml | any maker | ||
| plastic pipette 10 ml with bulb | any maker | ||
| plastic pipette 20 ml with bulb | any maker | ||
| Microscope stage Calibration Slide 0.01 mm | AmScope | MR095 | or equivalent |
| Reagents for fish water: | |||
| Instant Ocean Salt | Drs. Foster & Smith | CD-116528 | |
| Sodium bicarbonate (cell culture tested) | Sigma | S5761-1KG | |
| Reagents for E3 medium: | |||
| NaCl | Sigma | S5886-1KG | |
| KCl | Sigma | P5405-500G | |
| CaCl2, dihydrate | Sigma | C7902-500G | |
| MgSO4, heptahydrate | Sigma | 63138-250G | |
| Methylene Blue | Sigma | M9140-25G | |
| Fish Food: | |||
| Frozen brine shrimp | Brine Shrimp Direct | FBSFKG50 | |
| Tetramin Flakes | Drs. Foster & Smith | 16623 |
Referencias
- Lindeman, R., Pelegri, F. Vertebrate maternal-effect genes: insights into fertilization, early cleavage divisions, and germ cell determinant localization from studies in the zebrafish. Mol Rep Dev. 77 (4), 299-313 (2010).
- Abrams, E. W., Mullins, M. C. Early zebrafish development: it’s in the maternal genes. Curr Opin Genet Dev. 19 (4), 396-403 (2009).
- Nair, S., Lindeman, R. E., Pelegri, F. In vitro oocyte culture-based manipulation of zebrafish maternal genes. Dev Dyn. 242 (1), 44-52 (2013).
- Selman, K., Petrino, T. R., Wallace, R. A. Experimental conditions for oocyte maturation in the zebrafish, Brachydanio rerio. J Exp Zool. 269 (6), 538-550 (1994).
- Fauvel, C., Omnes, M. H., Suquet, M., Normant, Y. Reliable assessment of overripening in turbot (Scophtalmus maximus) by a simple pH measurement. Aquaculture. 117 (1-2), 107-113 (1993).
- Lahnsteiner, F., Weismann, T., Patzner, R. A. Composition of the ovarian fluid in 4 salmonid speices: Onchorhynchus mykiss, Salmo trutta flacustris, Salvelinus alpinus and Husho hucho. Reprod Nutr Dev. 35 (5), 465-474 (1995).
- Patiño, R., Bolamba, D., Thomas, P., Kumakura, N. Effects of external pH on hormonally regulated ovarian follicle maturation and ovulation in Atlantic croaker. Gen Comp Endocrinol. 141 (2), 126-134 (2005).
- Seki, S., et al. Development of a reliable in vitro maturation system for zebrafish oocytes. Reproduction. 135 (3), 285-292 (2008).
- Selman, K., Wallace, R. A., Sarka, A., Qi, X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol. 218 (2), 203-224 (1993).
- Clelland, E., Peng, C. Endocrine/paracrine control of zebrafish ovarian development. Mol Cell Endocrinol. 312 (1-2), 42-52 (2009).
- Kane, D. A., Kimmel, C. B. The zebrafish midblastula transition. Development. 119 (2), 447-456 (1993).
- Kanagaraj, P., et al. Souffle/Spastizin controls secretory vesicle maturation during zebrafish oogenesis. PLoS Genet. 10 (6), e1004449 (2014).
- Brand, M., Granato, M., Nüsslein-Volhard, C., Nüsslein-Volhard, C., Dahm, R. . Keeping and raising zebrafish,Zebrafish – A Practical Approach. , 7-37 (2002).
- Connoly, M. H., Dutkosky, R. M., Heah, T. P., Sayler, G. S., Henry, T. B. Temporal dynamics of oocyte growth and vitellogenin gene expression in zebrafish (Danio rerio). Zebrafish. 11 (2), 107-114 (2014).
- Rosen, J. N., Sweeney, M. F., Mably, J. D. Microinjection of zebrafish embryos to analyze gene function. J Vis Exp. (25), e1115 (2009).
- Pelegri, F., Mullins, M. Genetic screens for mutations affecting adult traits and parental-effect genes. Meth Cell Biol. 104, 83-120 (2011).
- Baars, D. L., Takle, K. A., Heier, J., Pelegri, F. Ploidy manipulation of zebrafish embryos with Heat Shock 2 treatment. J Vis Exp. , (2016).
- Kimmel, C., Ballard, W. W., Kimmel, S. R., Ullman, B., Schilling, T. F. Stages of embryonic development in the zebrafish. Dev Dyn. 203 (3), 253-310 (1995).
- Eisen, J. S., Smith, J. C. Controlling morpholino experiments: don’t stop making antisense. Development. 135 (10), 1735-1743 (2008).
- Schulte-Merker, S., Stainier, D. Y. R. Out with the old, in with the new: reassessing morpholino knockdowns in light of genome editing technology. Development. 141 (16), 3103-3104 (2014).
- Dekens, M. P. S., Santoriello, C., Vallone, D., Frassi, G., Whitmore, D., Foulkes, N. S. Light regulates the cell cycle in zebrafish. Curr Biol. 13 (23), 2051-2057 (2003).
- Sakai, N., Burgess, S., Hopkins, N. Delayed in vitro fertilization of zebrafish eggs in Hank’s saline containing bovine serum albumin. Mol Mar Biotechnol. 6 (2), 84-87 (1997).
- Pelegri, F., Mullins, M. C. Genetic screens for mutations affecting adult traits and parental-effect genes. Meth Cell Biol. 135, (2016).
- Lindeman, R. E., Pelegri, F. Localized products of futile cycle/lrmp promote centrosome-nucleus attachment in the zebrafish zygote. Curr Biol. 22 (10), 843-851 (2012).
- Nair, S., Marlow, F., Abrams, E., Kapp, L., Mullins, M., Pelegri, F. The chromosomal passenger protein Birc5b organizes microfilaments and germ plasm in the zebrafish embryo. PLoS Genetics. 9 (4), e1003448 (2013).
- Eno, C., Solanki, B., Pelegri, F. aura (mid1ip1l) regulates the cytoskeleton at the zebrafish egg-to-embryo transition. Development. 143 (9), 1585-1599 (2016).

