Probing the Limits of Egg Recognition Using Egg Rejection Experiments Along Phenotypic Gradients
Summary
This protocol provides guidelines for running egg rejection experiments: outlining techniques for painting experimental egg models to emulate the colors of natural bird eggs, conducting fieldwork, and analyzing the collected data. This protocol provides a uniform method for conducting comparable egg rejection experiments.
Abstract
Brood parasites lay their eggs in other females’ nests, leaving the host parents to hatch and rear their young. Studying how brood parasites manipulate hosts into raising their young and how hosts detect parasitism provide important insights in the field of coevolutionary biology. Brood parasites, such as cuckoos and cowbirds, gain an evolutionary advantage because they do not have to pay the costs of rearing their own young. However, these costs select for host defenses against all developmental stages of parasites, including eggs, their young, and adults. Egg rejection experiments are the most common method used to study host defenses. During these experiments, a researcher places an experimental egg in a host nest and monitors how hosts respond. Color is often manipulated, and the expectation is that the likelihood of egg discrimination and the degree of dissimilarity between the host and experimental egg are positively related. This paper serves as a guide for conducting egg rejection experiments from describing methods for creating consistent egg colors to analyzing the findings of such experiments. Special attention is given to a new method involving uniquely colored eggs along color gradients that has the potential to explore color biases in host recognition. Without standardization, it is not possible to compare findings between studies in a meaningful way; a standard protocol within this field will allow for increasingly accurate and comparable results for further experiments.
Introduction
Brood parasites lay their eggs in the nests of other species that may then raise their young and pay the costs associated with parental care1,2,3. This act of deception to outwit the host on the part of the parasite and sleuthing to detect the parasite on the part of the host provides strong selective pressures on both actors. In some cases of avian brood parasitism, the host's recognition of disparate parasitic eggs selects for parasites that mimic host eggs, which produces an evolutionary arms race between host and parasite4. Studying brood parasitism is important because it is a model system for investigating coevolutionary dynamics and decision-making in the wild5. Egg rejection experiments are one of the most common methods used for studying avian brood parasitism in the field and an important tool that ecologists use to investigate interspecific interactions6.
During the course of egg rejection experiments, researchers typically introduce natural or model eggs and assess the host's response to these experimental eggs over a standardized period. Such experiments can involve swapping real eggs (that vary in appearance) between nests7, or dyeing or painting the surfaces of real eggs (optionally adding patterns) and returning them to their original nests8, or generating model eggs that have manipulated traits such as color9, spotting10, size11, and/or shape12. The host response to eggs of varying appearance can provide valuable insight into the information content they use to reach an egg rejection decision13 and just how different that egg needs to be to elicit a response14. Optimal acceptance threshold theory15 states that hosts should balance the risks of mistakenly accepting a parasitic egg (acceptance error) or mistakenly removing their own egg (rejection error) by examining the difference between their own eggs (or an internal template of those eggs) and the parasitic eggs. As such, an acceptance threshold exists beyond which hosts decide a stimulus is too different to tolerate. When parasitism risk is low, the risk of acceptance errors is lower than when the risk of parasitism is high; thus, decisions are context specific and will shift appropriately as perceived risks change14,16,17.
Optimal acceptance threshold theory assumes that hosts base decisions upon continuous variation in host and parasite phenotypes. Therefore, measuring host responses to varying parasite phenotypes is necessary to establish how tolerant a host population (with its own phenotypic variation) is to a range of parasitic phenotypes. However, virtually all prior studies have relied on categorical egg color and maculation treatments (e.g., mimetic/non-mimetic). Only if host eggshell phenotypes do not vary, which is not a biologically practical expectation, would all responses be directly comparable (regardless of the degree of mimicry). Otherwise, a "mimetic" egg model will vary in how similar it is to host eggs within and between populations, which could potentially lead to confusion when comparing findings18. Theory suggests that host decisions are based upon the difference between the parasitic egg and their own14, not necessarily a particular parasitic egg color. Therefore, using a single egg model type is not an ideal approach to test hypotheses on host decision thresholds or discrimination abilities, unless the just noticeable difference (hereafter JND) between the egg model type and individual host egg color is the variable of interest. This also applies to experimental studies that swap or add natural eggs to test host responses to a natural range of colors19. However, while these studies do allow for variation in host and parasite phenotypes, they are limited by natural variation found in traits6, particularly when using conspecific eggs7.
By contrast, researchers that make artificial eggs of varied colors are free from the constraints of natural variation (e.g., they can investigate responses to superstimuli20), allowing them to probe the limits of host perception6. Recent research has used novel techniques to measure host responses across a phenotypic range, by painting experimental eggs designed to match and surpass the natural range of variation in eggshell9 and spot colors21. Studying host responses to eggs with colors along gradients can uncover underlying cognitive processes because theoretical predictions, such as acceptance thresholds15 or coevolved mimicry4, are based on continuous differences between traits. For example, by using this approach, Dainson et al.21 established that when chromatic contrast between eggshell ground coloration and spot coloration is higher, the American Robin Turdus migratorius tends to reject eggs more strongly. This finding provides valuable insights on how this host processes information, in this case through spotting, to decide whether to remove a parasitic egg. By customizing paint mixtures, researchers can precisely manipulate the similarity between an experimental egg's color and host's egg color, while standardizing other confounding factors such as spotting patterns10, egg size22 and egg shape23.
To encourage further replication and metareplication24 of classic and recent egg rejection work, it is important that scientists use methodologies that are standardized across phylogeny (different host species)7,22, space (different host populations)7,22,25,26 and time (different breeding seasons)7,22,25,26,27, which was done only rarely. Methodologies that were not standardized28 were later shown to lead to artefactual results29,30. This paper serves as a set of guidelines for researchers seeking to replicate this type of egg rejection experiment that examines responses to continuous variation and highlights a number of important methodological concepts: the importance of control nests, a priori hypotheses, metareplication, pseudoreplication, and color and spectral analysis. Despite egg rejection experiments dominating the field of avian host-parasite coevolution, no comprehensive protocol exists yet. Therefore, these guidelines will be a valuable resource to increase inter- and intra-lab repeatability as the true test of any hypothesis lies in metareplication, i.e., repeating whole studies across phylogeny, space and time24, which can only be meaningfully done when using consistent methods29,30,31.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of Long Island University-Post.
1. Mixing Acrylic Paints
- Mix the eggshell ground coloration, which is the color that will uniformly cover the entire eggshell surface. The following recipe will make 50 g of paint, which will fill a little more than two 22 mL aluminum paint tubes.
- Generate a blue-green color, representing a blue-green eggshell (e.g., an American Robin T. migratorius eggshell), using 18.24 g of cobalt turquoise Light, 20.77 g of titanium white 6.52 g of cobalt green, and 2.86 g of cobalt turquoise and 1.61 g of burnt umber.
- Generate a brown color, representing a brown eggshell (e.g., a chicken Gallus gallus domesticus eggshell), using 4.12 g of red iron oxide, 9.75 g of cadmium orange, 22.15 g of raw umber light, and 13.97 g of titanium white.
- Generate a beige color, representing a beige eggshell (e.g., a quail Coturnix japonica eggshell), using 10.60 g of brown egg color, 8.28 g of blue-green color, 18.51 g of titanium white, and 12.61 g of yellow ochre.
- Generate a white color using titanium white without mixing.
- Mix a dark brown spot color representing the spots found on quail C. japonica eggs, using 8.38 g of brown egg color, 26.04 g of burnt umber, and 15.59 g of mars black.
- Mix intermediate colors spanning the eggshell color gamut from blue-green to brown, by mixing blue-green and brown paints together and varying their contribution reciprocally (e.g., parts of blue-green to brown paint: 10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, and 0:10, see Figure 1E).
- To generate more intermediate colors, simply mix even quantities of these intermediate colors together and repeat until creating the desired number of unique colors. The even mixture of blue-green and brown will produce a neutral gray color, but this color can be adjusted using white or beige as necessary. If a more exact color (e.g., of a specific host egg) is required, use subtractive color mixing models to predict the spectral reflectance of unique combinations of paints and use the mixture producing lowest just noticeable difference (JND) to the desired color (see steps 3.3-3.7).
- Store paint in empty 22 mL aluminum paint tubes.
- Place the paint into a plastic sandwich bag with a small portion of one corner cut off. Place the end of the plastic bag into the tube and squeeze the paint into the tube, tapping the tube gently against the table.
- Seal tube using the adhesive edge and folding the end over itself at least 3-4 times.
2. Painting Experimental Egg Models
- Obtain experimental egg models.
- Print model eggs using a three dimensional (hereafter, 3D) printer or purchase from a distributor32. This simple approach is recommended because it generates eggs that are of consistent size and shape32.
NOTE: Model eggs can also be fashioned from plaster, clay, wood or other substances.
- Print model eggs using a three dimensional (hereafter, 3D) printer or purchase from a distributor32. This simple approach is recommended because it generates eggs that are of consistent size and shape32.
- Add an even coat of titanium white over each egg to obstruct the underlying color.
- Hold each egg using forceps, paint the desired color using high quality acrylic paints and a clean brush to color each egg uniquely.
- Use a hair dryer on a cool setting to speed up the drying process of each freshly painted egg.
- Use a sandpaper to sand down any bumps that may be on the egg once the egg is fully dry.
- Repeat step 2.3 until the egg has an even coating of paint without any lumps. Eggs require no fewer than two coats.
- Add any spots to model eggs by carefully applying these with a paintbrush and carefully spattering the paint with a toothbrush. Only a single coat is necessary.
CAUTION: If replication of ultraviolet (UV) reflectance is desired, apply an even coat of UV paint; however, this is not recommended unless permission to use these paints is obtained from institutional, state/provincial, and federal permitting offices.
3. Quantifying Color
- Turn on the spectrometer by pressing the power button.
- Insert SD card into SD card slot and link it to the system by pressing the red cancel button, select File System by pressing the green accept button, select Find SD Cards by pressing the menu up button. Afterwards, press the red cancel button two times or press the home button.
- Attach the fiber optic cables to the spectrometer and light source.
- Attach the end labelled Light Source to the light module and attach the end labelled Spectrometer to the spectrometer module.
- Insert the probe tip on the end of the fiber optic probe.
NOTE: An example probe tip printable on a 3D printer is available as a Supplemental Code File. This object will require threading a thumbscrew of your choice.- Establish a distance (e.g., 5 mm) between the sample and the measurement probe that maximizes the signal to noise ratio. Ensure a consistent measurement distance using a flexible ruler.
NOTE: The exact distance will vary with each individual spectrometer's unique combination of grating and slit width, optic width, and light source. Maintain the same distance for all measurements. A flexible rule is available for download as a Supplemental Code File. - Use a coincident normal measurement angle (90°), unless the natural host eggs or model eggs have a glossy surface, in which case use a 45° coincident oblique measurement angle. Measure all eggs, real and artificial, using the same angle.
- Wash the probe tip with 95% ethanol.
- Establish a distance (e.g., 5 mm) between the sample and the measurement probe that maximizes the signal to noise ratio. Ensure a consistent measurement distance using a flexible ruler.
- Turn on the light source by pressing the down button three times, select PX-Lamp by pressing the green accept button, select Setup by pressing the scroll right button, select Timing Controls by pressing the scroll right button, click the down button three times, and then select free running by pressing the Accept button.
- Wait for at least 15 min before taking any measurements.
- Calibrate and configure the spectrometer. To do this, press the home button, then select Tools by pressing the scroll left button, select Manual Control by pressing the menu up button, and select Acquire Parameters by pressing the menu up button.
- Set boxcar smoothing to five by pressing the scroll right button, moving the cursor to the right two spaces by pressing the scroll right button twice, and then increasing the boxcar setting by pressing the menu up button five times. Select Accept by pressing the green accept button once complete.
- Set averages to 10 by pressing the menu down button, then moving to the right two places by pressing the scroll right button twice and adjusting the value in the tens place by pressing the menu up button once and moving to the ones place by pressing the scroll right button once and reducing this to zero by pressing the menu down button once. Select Accept by pressing the green accept button once complete.
- Press the home button, select Reflectance by pressing the menu up button, and place the probe firmly on the white standard. Then store a reference white standard by pressing the menu up button. Store a dark standard by pressing the scroll right button and view the reflectance by pressing the menu down button.
- Measure each eggshell's reflectance six times by measuring twice near the blunt pole (wide end of egg), twice at the egg's equator, and twice near the sharp pole (narrower end). Be sure to report whether spots are avoided or not. Conduct this on both experimental eggs as well as the host's eggs.
- Use a locally weighted polynomial function with a 0.25 nm smoothing span to smooth noise in reflectance curves, using color analysis software33. If color scores, such as brightness, are not significantly repeatable between the regions of the egg model (step 3.7), repaint the egg (steps 2.2. – 2.6); otherwise, average the egg model coloration across the egg.
- Decide on the most appropriate set of relative photoreceptor sensitivities for the question.
NOTE: This may be a generic ultraviolet sensitive or violet sensitive bird, or once can choose to model relative sensitivities34,35,36. - Quantify quantum catches37 for each photoreceptor, both single38 and double cones39,40, by integrating the product of eggshell reflectance, photoreceptor sensitivities, and an appropriate irradiance spectrum across the avian visual spectrum (i.e., 300-700 nm).
- Use relative quantum catch estimates to generate coordinates within the avian tetrahedral color space37,41. Ensure that, unlike quantum catches, these relative quantum catches sums to 1.
- Use quantum catches (step 3.10) to estimate the discriminability, in JND, between host eggshell color (see step 2.6.1) and the perceived colors of each foreign egg using a neural noise-limited visual model36,42,43.
- Measure the host's own eggshell coloration using the same spectrometer and light source used to measure the model eggs (step 3.7), if possible.
NOTE: Practical or logistical considerations may make this impossible, in which case measure a subset of host eggs from different nests to establish an average host eggshell coloration. - Use a Weber fraction for the long-wave-sensitive cone of 0.144.
- Account for the relative abundance of cones and the principal member of the double cone34.
NOTE: If the egg colors used are only on a gradient corresponding to natural eggshell colors, the JND between the host and experimental egg can be multiplied by either 1 or −1 to differentiate differences on either extreme (e.g., blue-green or brown, see steps 1.1.1-1.1.2). If egg colors used fall across multiple gradients or fill the color space, summarize the perceivable color differences between eggs using perceptually uniform chromaticity diagrams45. The coordinates within this type of diagram describes both the direction and magnitude of perceived color differences between experimental eggs and host egg color and these can be used in further analyses.
- Measure the host's own eggshell coloration using the same spectrometer and light source used to measure the model eggs (step 3.7), if possible.
4. Field Work
- Determine the species to study.
NOTE: Factors to consider include (but are not limited to) the abundance of the host and/or parasite species and whether the host is a grasp46 or puncture47 rejecter, which will impact the type of egg to be used (e.g., do not use hard artificial model eggs for puncture ejectors48). - Systematically search for nests in the study area. Check previous nesting records that can provide a reasonable starting place in some species49.
NOTE: Visible markers or flagging can increase the risk of predation50; therefore, consider using a handheld GPS instead. - Monitor those nests daily using direct or video recording methods to record the presence of each host egg until the start of the experiment (step 4.4); for example, one day after clutch completion.
NOTE: This daily monitoring will continue until the experiment has concluded (step 4.6).- Listen for alarm calls made by adults and leave the area if they continue for more than 30 s. Do not approach a nest when any potential nest predator is present, especially if it is a visually oriented predator (e.g., corvid).
- Approach and leave nests from different locations, i.e., walk past nests, so mammalian predators cannot follow chemical cues directly to nests.
NOTE: This approach may be unfeasible in some habitats, namely dense reed-beds. - Always minimize the physical disturbance to the nest and the area around the nest.
- Do not get close to nests during nest-building period, because many birds will abandon the nests if they are disturbed prior to egg-laying50.
- Gently add an experimental egg to a host's nest by sliding it into the side of the nest's cup. Do not drop the experimental models as it can damage the host's eggs.
NOTE: Assign treatments randomly.- Record if the host parent remains nearby and thus has an opportunity to witness the act of artificial parasitism51. Record and statistically control for a variable indicating whether the host was flushed from the nest51. Conduct egg introductions while the parent is away.
- Collect a host egg if the parasite in the system removes a host egg.
NOTE: This may not be necessary in hosts where egg removal does not affect host responses to experimental eggs22.
- Employ a set of control nests (nests which are visited, checked and eggs handled but no experimental egg is added or swapped) to determine the natural nest desertion rates. Remove a host egg from the control nest only if they are removed from the experimental nests (see step 4.5.1).
NOTE: This is crucial because desertion may not be a response targeted to certain foreign eggs but may be a response for other egg types.- Choose the number of control nests a priori based upon the known or expected sample sizes and estimated effect. Assign every nth nest as a control nest until statistical determination of whether nest desertions are a host response to experimental eggs or not can be obtained (step 5.1).
NOTE: If a host egg is removed, remove the same number of host eggs as in the experimental treatment, hold one host egg in the hand for 5 s and then replace it, and remain at nest for the same length of time as treatment nests. If host eggs are not removed, hold one host egg in the hand for 5 s and then replace it, remain at the nest for the amount of time spent at experimental nests (e.g., 10 s).
- Choose the number of control nests a priori based upon the known or expected sample sizes and estimated effect. Assign every nth nest as a control nest until statistical determination of whether nest desertions are a host response to experimental eggs or not can be obtained (step 5.1).
- Revisit the nest enough to determine the response at each host's nest, including controls. Check the nest within a few hours of the experimental manipulation.
NOTE: In some species, rejections may occur on the same day as experimental parasitism; therefore, it is important to check the nest within a few hours of the experimental manipulation52.- Use a telescopic mirror when checking the nest to avoid the direct contact with either the nest or the clutch.
- Avoid observations in severe weather (rain, heat, or cold) because this can increase the danger to the nestlings and the eggs50.
- Perform daily checks until the host has responded to the introduced egg or a certain amount of time has passed.
NOTE: By convention, if the egg remains in the nest for 5-6 days, the host is considered an acceptor22; however, some host individuals respond even later53 and omission of such responses necessarily biases egg rejection rate estimates. Ideally, researchers should determine the upper 95% family-wise confidence interval of latency to rejection in their system and use this as their criterion.
5. Statistical Analyses
- Use a Fisher's exact test in a perfectly randomized study (i.e., experimental and control nests are perfectly interspersed and thus do not differ in laying date, clutch or any other parameter known to affect nest desertion) to compare the number of desertions between control (step 4.5) and treatments nests. Otherwise, use a generalized linear model (GLM) with potentially relevant covariates (see below), as a generally more cautious approach.
- If experimental nests have a significantly higher desertion rate than control nests, code both removed eggs and abandoned eggs as 'rejected'.
NOTE: By convention the host has 'rejected' the egg when it has recognized the parasitic egg and either removed it or abandoned it (with the whole nest). - If desertion rates do not differ between experimental and control nests, exclude desertions from the analysis, because they are not a host response to parasitism and code responses as 'ejected'.
NOTE: By convention, 'ejected' refers to when an egg has actually been removed from its nest. - Record the date and time when the host has rejected the egg. Recode the response variable depending on your finding on steps 5.1.1 – 5.1.2.
- If experimental nests have a significantly higher desertion rate than control nests, code both removed eggs and abandoned eggs as 'rejected'.
- Report the Fisher's exact test, its associated odds ratio, and its appropriate confidence interval.
- Decide on any meaningful covariates that will be added to predictive models (e.g., steps 5.4-5.5).
- Specify the coding (e.g., continuous, categorical, or ordinal) of each covariate.
- Code dates as ordinal days and center dates separately within years to remove any potential confound due to variation attributable to years or seasonality7,51,54,55.
- Center any covariates involved in an interaction to allow for easier interpretation of their lower order terms in model output.
NOTE: Scaling covariates allows straightforward comparison of effects between studies and sometimes can improve model convergence.
- Predict host responses (either eject or reject vs accept) using a generalized linear model (GLM) or a generalized linear mixed model (GLMM) with a binomial error distribution and logit link function.
NOTE: The choice between a GLM or GLMM depends on the data, and if including a random effect (e.g., nest ID, year). Random factors should have at least 5 levels otherwise variances are likely to be poorly estimated56.- Report the coefficient of determination (usually R2) to show what proportion of the variance was explained by a linear model57,58.
- Predict how long it takes the bird to respond to experimental parasitism using a GLM with a negative binomial error distribution (or a Poisson error distribution if the data are not overdispersed) and log link.
NOTE: Researchers refer to the length of time that it takes for a bird to respond as 'latency to response,' which is reported with precision to days, such that eggs rejected on the day of the experiment have a latency of zero. Model response variables with too many zeros (>50%) using zero-inflated or Hurdle models59. - Use diagnostic tools to check if model predicts the data satisfactorily and report model summary statistic to quantify what proportion of variance model explained60,61. Report coefficient of determination (usually R2), see step 5.4.2.
- Validate negative binomial models using graphical validation by producing a quantile-quantile plot and plotting Pearson residuals against the fitted values.
NOTE: A well-run model will have no outliers or unexpected patterns59. - Validate binomial models using goodness-of-fit tests such as Hosmer-Lemeshow tests, and other diagnostics available in the R package, 'binomTools'62 containing whole set of diagnostic tools.
- Validate negative binomial models using graphical validation by producing a quantile-quantile plot and plotting Pearson residuals against the fitted values.
- Consider controlling for consistent covariates for the purposes of consistency and comparability between studies.
NOTE: Common covariates would include clutch size22, laying date63, nest age at manipulation53, and whether the host was flushed from the nest or not51. Many, especially early, studies did not use any covariates. Authors should consider additionally analyzing the effects of egg types (or various gradients) without covariates to make their results quantitatively comparable to these studies lacking covariates.- Use an information-theoretical approach and report the result of averaging many potential models explaining host behavior64.
NOTE: Alternatively, use step-wise regression analysis as a model selection algorithm65. Researchers should use a predefined criterion (e.g., Adjusted R2, Mallows' Cp, Akaike's Information Criterion, Schwarz's BIC, or p-value) and provide both the full model (with common covariates) and a final reduced model.
- Use an information-theoretical approach and report the result of averaging many potential models explaining host behavior64.
Representative Results
Generating colorful egg models
Reflectance spectra of custom paint mixtures and natural eggs are shown in Figure 1A-1D. Paint mixtures used in brood parasitism studies should closely correspond with natural reflectance measurements in terms of spectral shape (color) and magnitude (brightness). If that is achieved, the color of the experimental egg should be perceived by the host as a natural egg color. To assess host recognition, these reflectances should be transformed into a relevant avian color space. To do this, the product of these reflectance spectra, solar irradiance, and photoreceptor sensitivity can be integrated to calculate quantum catches37. Avian color perception is different than in humans, because birds see colors using four, rather than three photoreceptors. The quantum catch from these four receptors can be transformed into coordinates within a tetrahedral color space (Figure 1E), where each vertex represents the relative stimulation of a specific photoreceptor: ultraviolet, short, medium, and long wavelength-sensitive photoreceptors41. Coordinates within this space provide a method for comparing colors and phenotypic diversity of colors, which is relatively limited with respect to the color of birds eggshells66. Plotting experimental egg color in a color space is important because their coordinates within that space will determine if these eggs would appear natural. Figure 1 illustrates the colors of custom paints (colorful dots, letters correspond with the reflectance spectra shown in Figure 1A-1D) that are described in this paper and how they compare to natural birds' eggshell colors. This novel approach provides opportunities for novel experimental designs and can provide new insights into host egg recognition.
Quantifying host responses to eggshell colors
Explicitly or implicitly, all but one9 previous study has assumed that hosts respond to trait dissimilarity, e.g., the difference between a parasitic egg and their own, in an absolute or symmetrical fashion (Figure 2, dashed). This difference usually varies from identical (0) to infinitely different; however, most traits vary along multiple dimensions and there is no a priori reason to assume their responses should be similar across the phenotypic space. Research that manipulates traits across their full phenotypic range (Figure 2, solid), can test this assumption. For two Turdus spp., Hanley et al.9 found that between a brown and blue-green egg, both equally different from the host's own, the brown egg was more likely to be rejected. By using egg models with known coordinates within a standard avian color space (Figure 1E), researchers can work within the natural phenotypic range or extend that phenotypic range (e.g., towards blue-green or brown) to explore host response and probe the perceptual limits of host recognition. Such an approach provides a context (based on phenotypic space) to understand hosts' responses.
Quantifying host responses to eggshell spot colors
A recent study21 showed that American robins are more likely to reject spotted experimental eggs when they perceive those spots as browner than the blue-green color of the eggshell (Figure 3). This host lays unspotted eggs, but their parasite the brown-headed cowbird Molothrus ater has brown spots and therefore, this decision rule seems adaptive. Such findings reinforce previous findings that have shown that American robins base decisions on both ground color and spots13; however, by measuring their responses across a color gradient Dainson et al. were able to establish that American robins use the chromatic contrast between ground and spot color in egg rejection decisions21. Experimental designs employing such continuous variation in coloration enable researchers to explore the role of host sensory and perceptual mechanisms in egg recognition more thoroughly.
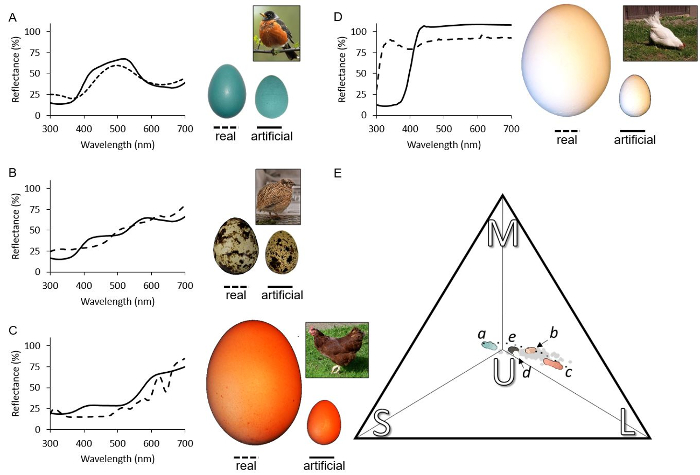
Figure 1. Representative variation in natural avian and artificial eggshell colors. The average of ten spectral reflectance measurements of (A) the blue-green, (B) beige, (C) brown, (D) white, and (E) dark brown paint mixtures (1.2.1 to 1.2.5, solid lines) alongside the reflectance of a real egg with a similar appearance: (A) American robin T. migratorius, (B) quail C. japonica, a (C) brown and (D) white domestic chicken egg Gallus g. domesticus (dashed lines). The peak in ultraviolet reflectance in (D) is due to the removal of the cuticle67. Inset photographs of real eggs on the left and artificial eggs to the left to scale (the bar above “artificial” represents 1 cm). The image of the real quail egg (inset B) was modified from a photograph taken by Roger Culos that is licensed under CC BY 4.0. We illustrate adult birds as inset images (photo credits of bird insets A-D respectively: Tomáš Grim, Ingrid Taylar under CC BY 2.0, Sherool, and Dejungen under CC-BY-SA-3.0). The avian perceived colors are also plotted within the (E) avian tetrahedral color space for the average ultraviolet-sensitive avian viewer. The vertices represent the relative stimulation of the ultraviolet (U), short (S), medium (M), and long (L) wavelength-sensitive photoreceptors. Gray dots represent the colors of natural avian eggs across the full phylogenetic diversity66, from previously published data68, while the colorful dots represent the colors of custom paint formulations here (steps 1.1.1 to 1.2), and small solid dots represent intermediate colors (step 1.3). Italic letters beside colorful dots reference spectral reflectances shown in this figure, while (e) references the dark spots from a quail egg. Please click here to view a larger version of this figure.
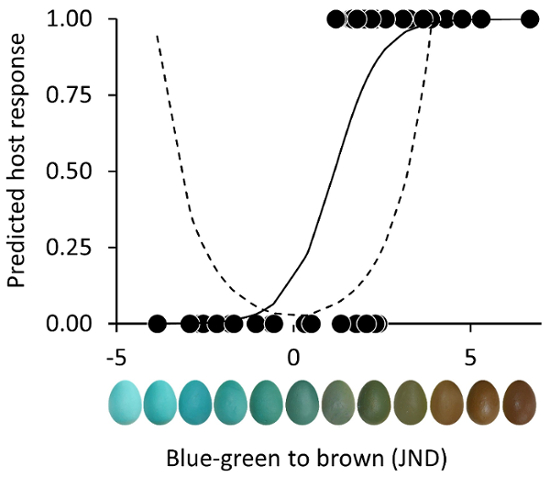
Figure 2. Representative host egg rejections of eggs with variable eggshell coloration. Traditionally, the predicted (dashed) rejection probability for a host is based upon the absolute perceived difference between the hosts' egg and foreign egg (i.e., as the foreign egg is more different responses to that egg are more likely, no matter the direction of the difference in the color space). This practice ignores natural variation in the host's own egg color. However, it is more likely that American robins (N = 52) will reject brown eggs than equally dissimilar blue-green eggs (solid line), which highlights the importance of examining host responses across a phenotypic gradient9.This figure was modified from Hanley et al.9 and these data69 are licensed under CC BY 4.0. Please click here to view a larger version of this figure.
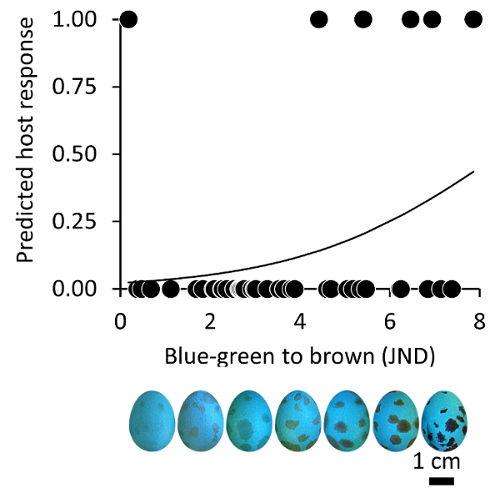
Figure 3. Representative host egg rejection of eggs with variable spot coloration. The chromatic contrast (JND) between the spot colors painted on experimental model eggs and the ground color of these models predicted host response (0 = acceptance, 1 = ejection) in the American robin. This figure has been modified from Dainson et al.21. Please click here to view a larger version of this figure.
Discussion
Although egg rejection experiments are the most common method to study brood parasite-host coevolution70, concerted efforts to standardize materials, techniques, or protocols are lacking. This is especially problematic for meta-analyses. No meta-analysis, to our knowledge, of host egg rejection so far has controlled for methodological discrepancies among studies71,72, including what is considered mimetic or non-mimetic. This represents a major problem because mimetic (by human standards) eggs can be rejected by hosts more often than seemingly non-mimetic ones73, which shows that human color classification is both inadequate and inappropriate for inferences regarding avian cognition18. Additionally, meta-analyses ignore the fact that some studies, even of the same host species, handled desertions differently, either counting them as responses22 or excluding them from analyses7,71,72. Moreover, misclassifying egg models as either non-mimetic or mimetic eggs, can lead to fallacious comparisons between studies that both used these classifications for different egg model types74. Differences between studies1,74 may reflect both differences in the study design (e.g., model egg types) or differences between populations, both to an unknown degree; this prevents clear interpretation of the differences and precludes rejecting null and alternative hypotheses. This protocol provides a standardized approach for egg rejection experiments, and particularly emphasizes the coloring and quantifying the color of egg models. Following or adapting (and appropriately reporting) this protocol should promote the methodological standardization necessary for productive scientific debate, inter-study comparisons, and future progresses in this field of research.
Since eggshell coloration is determined by only two pigments, protoporphyrin IX appearing brown, and biliverdin IXα appearing blue-green66,75,76, eggshell colors only occupy a small section of avian vision66. This variation can be replicated through carefully formulating acrylic paints that match natural eggshell colors, and this will lead to a better understanding of host recognition mechanisms. For example, Turdus hosts are more likely to reject brown eggs than blue-green eggs, despite the absolute perceived color difference between these foreign eggs and their own (Figure 2). Responses to eggs colored along color gradients vary considerably, highlighting the importance of accurately producing and reproducing the colors used in egg rejection experiments. Even variation in spot coloration can result in striking differences in host response (Figure 3)21. By using this approach, researchers can more systematically probe the limits of host recognition and uncover the relative importance of chromatic and luminance pathways in informing host decisions.
Despite the benefits that this approach provides for quantifying host responses across a phenotypic range, it is not suited for testing every hypothesis. When rejection rates are necessary for testing hypotheses, particularly for inter-population comparisons, using one or more consistent egg model types would be a less costly and demanding approach. For example, presenting specific egg model types representative of specific parasitic egg polymorphisms, can provide insight into historic and contemporary selection pressures77. Calculating rejection rates is impossible when each egg is a unique color; however, quantifying host responses across a range of potential parasitic egg colors can provide insight into questions related to decision thresholds and discrimination abilities. Specifically, this approach provides a tool for researchers to measure a host's egg discrimination abilities. This protocol outlines paint recipes to help standardize the color of egg models used for either approach. Moreover, regardless of the approach chosen, researchers should report the paints they used for coloring their egg models and should quantify those colors carefully. This should enhance inter-study comparisons and meta-analysis.
Egg rejection studies with continuous color, pattern, size, and/or shape traits have revolutionized the field of avian host-parasite arms-race studies in combination with the now standard usage of avian visual perception modelling73,78. There is now evidence that some hosts do not only use the absolute perceptual difference between own and foreign eggs in egg recognition, but instead base rejection decisions upon the direction of these difference along the phenotypic gradient of avian eggshell color9. Future research should use habituation/dishabituation or operant training studies to assess whether hosts of avian brood parasites can perceive and discriminate between natural and artificial egg colors in non-egg recognition contexts. Furthermore, these same experiments could reveal whether current-, historic-, and non-hosts can distinguish natural from artificial eggshell, which would highlight the role of sensory mechanisms in coevolutionary arms-races. Finally, adequately incorporating and replicating the UV components of avian egg coloration and maculation into egg rejection studies is a necessary challenge to overcome by future research79; this will be necessary to assess whether UV-based egg color signals represent a perceptually salient or unique cue for egg recognition and rejection in hosts of avian brood parasites. By using this consistent protocol, researchers can create new experiments and more easily interpret and compare their findings6,29,30,31.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
MEH was funded by the HJ Van Cleave Professorship at the University of Illinois, Urbana-Champaign. In addition, for funding we thank the Human Frontier Science Program (to M.E.H. and T.G.) and the European Social Fund and the state budget of the Czech Republic, project no. CZ.1.07/2.3.00/30.0041 (to T.G.). We thank Ocean Optics for covering publication costs.
Materials
| Replicator Mini + | Makerbot | ||
| Professional Acrylic Paint Cobalt Turquoise Light | Winsor & Newton | 28382 | |
| Professional Acrylic Paint Titanium White | Winsor & Newton | 28489 | |
| Professional Acrylic Paint Cobalt Green | Winsor & Newton | 28381 | |
| Professional Acrylic Paint Cobalt Turquoise | Winsor & Newton | 28449 | |
| Professional Acrylic Paint Burnt Umber | Winsor & Newton | 28433 | |
| Professional Acrylic Paint Red Iron Oxide | Winsor & Newton | 28486 | |
| Professional Acrylic Paint Cadmium Orange | Winsor & Newton | 28437 | |
| Professional Acrylic Paint Raw Umber Light | Winsor & Newton | 28391 | |
| Professional Acrylic Paint Yellow Ochre | Winsor & Newton | 28491 | |
| Professional Acrylic Paint Mars Black | Winsor & Newton | 28460 | |
| Paint Brush | Utrecht | 206-FB | Filbert brush |
| Paint Brush | Utrecht | 206-F | Flat brush |
| Hair Dryer | Oster | 202 | |
| Fiber optic cables | Ocean Optics Inc. | OCF-103813 | 1 m custom bifurcating fiber optic assembly with blue zip tube (PVDF), 3.8mm nominal OD jeacketing and 2 legs |
| Spectrometer | Ocean Optics Inc. | Jaz | Spectrometer unit with a 50 um slit width, installed with a 200-850 nm detector (DET2B-200-850), and grating option # 2. |
| Battery and SD card module for spectrometer | Ocean Optics Inc. | Jaz-B | |
| Light source | Ocean Optics Inc. | Jaz-PX | A pulsed xenon light source |
| White standard | Ocean Optics Inc. | WS-1-SL | made from Spectralon |
| OHAUS Adventurer Pro Scale | OHAUS | AV114C | A precision microbalance |
| Gemini-20 portable scale | AWS | Gemini-20 | A standard scale |
| Empty Aluminum Paint Tubes (22 ml) | Creative Mark | NA | |
| Telescopic mirror | SE | 8014TM | |
| GPS | Garmin | Oregon 600 | |
| 220-grit sandpaper | 3M | 21220-SBP-15 | very fine sandpaper |
| 400-grit sandpaper | 3M | 20400-SBP-5 | very fine sandpaper |
| color analysis software: ‘pavo’, an R package | for use in, R: A language and environment for statistical computing | v 1.3.1 | https://cran.r-project.org/web/packages/pavo/index.html |
| UV clear transparent | Flock off! | UV-001 | A transparent ultraviolet paint |
| Plastic sandwich bags | Ziploc | Regular plastic sandwich bags from Ziploc that can be purchased at the supermarket. | |
| Kimwipes | Kimberly-Clark Professional | 34120 | 11 x 21 cm kimwipes |
| Toothbrush | Colgate | Toothbrush |
Referencias
- Davies, N. B., Brooke, M. d. e. L. An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. Journal of Animal Ecology. 58 (1), 207-224 (1989).
- Feeney, W. E., Welbergen, J. A., Langmore, N. E. Advances in the study of coevolution between avian brood parasites and their hosts. Annu Rev Ecol Evol Syst. 45, 227-246 (2014).
- Kilner, R. M., Madden, J. R., Hauber, M. E. Brood parasitic cowbird nestlings use host young to procure resources. Science. 305 (5685), 877-879 (2004).
- Dawkins, R., Krebs, J. R. Arms races between and within species. Proc R Soc Lond B. 205, 489-511 (1979).
- Wyllie, I. . The cuckoo. , (1981).
- Hauber, M. E., et al. The value of artificial stimuli in behavioral research: making the case for egg rejection studies in avian brood parasitism. Ethology. 121 (6), 521-528 (2015).
- Samas, P., Hauber, M. E., Cassey, P., Grim, T. Host responses to interspecific brood parasitism: a by-product of adaptations to conspecific parasitism?. Front Zool. 11 (1), 34 (2014).
- Bán, M., Moskát, C., Barta, Z., Hauber, M. E. Simultaneous viewing of own and parasitic eggs is not required for egg rejection by a cuckoo host. Behav Ecol. 24 (4), 1014-1021 (2013).
- Hanley, D., et al. Egg discrimination along a gradient of natural variation in eggshell coloration. Proc R Soc B. 284 (1848), 20162592 (2017).
- Moskat, C., et al. Discordancy or template-based recognition? Dissecting the cognitive basis of the rejection of foreign eggs in hosts of avian brood parasites. J Exp Biol. 213 (11), 1976-1983 (2010).
- Marchetti, K. Egg rejection in a passerine bird: size does matter. Anim Behav. 59, 877-883 (2000).
- Zölei, A., Hauber, M. E., Geltsch, N., Moskát, C. Asymmetrical signal content of egg shape as predictor of egg rejection by great reed warblers, hosts of the common cuckoo. Behaviour. 149 (3-4), 391-406 (2012).
- Rothstein, S. I. Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species?. Behav Ecol Sociobiol. 11 (4), 229-239 (1982).
- Davies, N. B., Brooke, M. d. e. L., Kacelnik, A. Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic cuckoo eggs. Proc R Soc B. 263 (1), 925-931 (1996).
- Reeve, H. K. The evolution of conspecific acceptance thresholds. Am Nat. 133 (3), 407 (1989).
- Mermoz, M. E., Haupt, C., Fernández, G. J. Brown-and-yellow marshbirds reduce their acceptance threshold of mimetic brood parasite eggs in the presence of non-mimetic eggs. J Ethol. 34 (1), 65-71 (2015).
- Hauber, M. E., Moskát, C., Bán, M. Experimental shift in hosts’ acceptance threshold of inaccurate-mimic brood parasite eggs. Biol Lett. 2 (2), 177-180 (2006).
- Grim, T. Mimicry vs. similarity: which resemblances between brood parasites and their hosts are mimetic and which are not?. Biol J Linn Soc. 84 (1), 69-78 (2005).
- Lahti, D. C. The limits of artificial stimuli in behavioral research: the Umwelt gamble. Ethology. 121 (4), 529-537 (2015).
- Alvarez, F. Attractive non-mimetic stimuli in Cuckoo Cuculus canorus eggs. Ibis. 141, 142-144 (1999).
- Dainson, M., Hauber, M. E., López, A. V., Grim, T., Hanley, D. Does contrast between eggshell ground and spot coloration affect egg rejection?. Sci Nat. 104, 54 (2017).
- Grim, T., et al. Constraints on host choice: why do parasitic birds rarely exploit some common potential hosts?. J Anim Ecol. 80 (3), 508-518 (2011).
- Underwood, T. J., Sealy, S. G. Influence of shape on egg discrimination in American robins and gray catbirds. Ethology. 112, 164-173 (2006).
- Johnson, D. H. The importance of replication in wildlife research. J Wildl Manage. 66 (4), 919-932 (2002).
- Stokke, B. G., et al. Predictors of resistance to brood parasitism within and among reed warbler populations. Behav Ecol. 19 (3), 612-620 (2008).
- Soler, J. J., Martínez, J. G., Soler, M., Pape Møller, A. Coevolutionary interactions in a host-parasite system. Ecol Lett. 4, 470-476 (2001).
- Thorogood, R., Davies, N. B. Reed warbler hosts fine-tune their defenses to track three decades of cuckoo decline. Evolution. 67, 3545-3555 (2013).
- Robert, M., Sorci, G. Rapid increase of host defence against brood parasites in a recently parasitized area: the case of village weavers in Hispaniola. Proc R Soc B. 266, 941-946 (1999).
- Lahti, D. C. Evolution of bird eggs in the absence of cuckoo parasitism. Proc Natl Acad Sci U S A. 102 (50), 18057-18062 (2005).
- Lahti, D. C. Persistence of egg recognition in the absence of cuckoo brood parasitism: pattern and mechanism. Evolution. 60 (1), 157-168 (2006).
- Grim, T., Stokke, B. G., Weis, J. S., Sol, D. In the light of introduction: Importance of introduced populations for the study of brood parasite-host coevolution. Biological invasions and animal behaviour. , 133-157 (2016).
- Igic, B., et al. Using 3D printed eggs to examine the egg-rejection behaviour of wild birds. PeerJ. 3, e965 (2015).
- Maia, R., Eliason, C. M., Bitton, P., Doucet, S. M., Shawkey, M. D. pavo: an R package for the analysis, visualization and organization of spectral data. Methods Ecol Evol. 4 (10), 906-913 (2013).
- Hart, N. S., Vorobyev, M. Modelling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. J Comp Physiol A. 191 (4), 381-392 (2005).
- Govardovskii, V. I., Fyhrquist, N., Reuter, T., Kuzmin, D. G., Donner, K. In search of the visual pigment template. Vis Neurosci. 17, 509-528 (2000).
- Vorobyev, M., Osorio, D., Bennett, A. T. D., Marshall, N. J., Cuthill, I. C. Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol A. 183 (5), 621-633 (1998).
- Endler, J. A., Mielke, P. W. Comparing entire colour patterns as birds see them. Biol J Linn Soc. 86 (4), 405-431 (2005).
- Hart, N. S. The visual ecology of avian photoreceptors. Prog Retin Eye. 20 (5), 675-703 (2001).
- Hart, N. S. Vision in the peafowl (Aves: Pavo cristatus). J Exp Biol. 205 (24), 3925-3935 (2002).
- Hart, N. S., Partridge, J. C., Cuthill, I. C., Bennett, A. T. D. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L). J Comp Physiol A. 186 (4), 375-387 (2000).
- Stoddard, M. C., Prum, R. O. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of new world buntings. Am Nat. 171 (6), 755-776 (2008).
- Vorobyev, M., Osorio, D. Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B. 265 (1394), 351-358 (1998).
- Siddiqi, A., Cronin, T. W., Loew, E. R., Vorobyev, M., Summers, K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J Exp Biol. 207, 2471-2485 (2004).
- Olsson, P., Lind, O., Kelber, A. Bird colour vision: behavioural thresholds reveal receptor noise. J Exp Biol. 218, 184-193 (2015).
- Pike, T. W. Preserving perceptual distances in chromaticity diagrams. Behav Ecol. 23, 723-728 (2012).
- Underwood, T. J., Sealy, S. G. Behavior of warbling vireos ejecting real and artificial cowbird eggs. Wilson J Ornithol. 123 (2), 395-400 (2011).
- Rohwer, S., Spaw, C. D. Evolutionary lag versus bill-size constraints: a comparative study of the acceptance of cowbird eggs by old hosts. Evol Ecol. 2 (1), 27-36 (1988).
- Martin-Vivaldi, M., Soler, M., Møller, A. P. Unrealistically high costs of rejecting artificial model eggs in cuckoo Cuculus canorus hosts. J Avian Biol. 3 (2), 295-301 (2002).
- Samaš, P., Heryán, J., Grim, T. How does urbanization affect dispersal in Eurasian blackbirds (Turdus merula)?. Sylvia. 49, 21-38 (2013).
- Baicich, P. J., Harrison, C. J. O. . A guide to the nests, eggs, and nestlings of North American birds. , (1997).
- Hanley, D., Samaš, P., Heryán, J., Hauber, M. E., Grim, T. Now you see it, now you don’t: flushing hosts prior to experimentation can predict their responses to brood parasitism. Sci Rep. 5 (1), 9060 (2015).
- Grim, T., Samaš, P., Hauber, M. The repeatability of avian egg ejection behaviors across different temporal scales, breeding stages, female ages and experiences. Behav Ecol Sociobiol. 68 (5), 749-759 (2014).
- Hanley, D., et al. Dynamic egg color mimicry. Ecol Evol. 6 (12), 4192-4202 (2016).
- Hanley, D., Samaš, P., Hauber, M. E., Grim, T. Who moved my eggs? An experimental test of the egg arrangement hypothesis for the rejection of brood parasitic eggs. Anim Cogn. 18 (1), 299-305 (2015).
- Samaš, P., et al. Ecological predictors of reduced avian reproductive investment in the southern hemisphere. Ecography. 36 (7), 809-818 (2013).
- Bolker, B. M. Linear and generalized linear mixed models. Ecol Stat Contemp theory Appl. , 309-334 (2015).
- Nakagawa, S., Johnson, P. C. D., Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface. 14 (134), 20170213 (2017).
- Nagelkerke, N. J. D. A note on a general definition of the coefficient of determination. Biometrika. 78 (3), 691-692 (1991).
- Zuur, A. F., Ieno, E. N., Walker, N., Saveliev, A. A., Smith, G. M. . Mixed effects models and extensions in ecology with R. Stat Biol Heal. , (2009).
- Grafen, A., Hails, R. . Modern statistics for the life sciences. , (2002).
- Zuur, A. F., Ieno, E. N., Elphick, C. S. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 1 (1), 3-14 (2010).
- Christensen, R. H. B., Hansen, M. K. . binomTools: Performing diagnostics on binomial regression models. , (2011).
- Lotem, A., Nakamura, H., Zahavi, A. Constraints on egg discrimination and cuckoo-host co-evolution. Anim Behav. 49 (5), 1185-1209 (1995).
- Burnham, K. P., Anderson, D. R. . Model selection and multimodel inference: a practical information-theoretic approach. 60, (2002).
- Whittingham, M. J., Stephens, P. A., Bradbury, R. B., Freckleton, R. P. Why do we still use stepwise modelling in ecology and behaviour?. J Anim Ecol. 75 (5), 1182-1189 (2006).
- Hanley, D., Grim, T., Cassey, P., Hauber, M. E. Not so colourful after all: eggshell pigments constrain avian eggshell colour space. Biol Lett. 11 (5), 20150087 (2015).
- Fecheyr-Lippens, D. C., et al. The cuticle modulates ultraviolet reflectance of avian eggshells. Biol Open. 4, 753-759 (2015).
- Hanley, D., Grim, T., Cassey, P., Hauber, M. E. Data from: Not so colourful after all: eggshell pigments constrain avian eggshell colour space. Dryad Digital Repos. , (2015).
- Hanley, D., et al. Data from: Egg discrimination along a gradient of natural variation in eggshell coloration. Dryad Digital Repos. , (2017).
- Grim, T. Equal rights for chick brood parasites. Ann Zool Fennici. 44 (March), 1-7 (2007).
- Medina, I., Langmore, N. E. The costs of avian brood parasitism explain variation in egg rejection behaviour in hosts. Biol Lett. 11 (7), 20150296 (2015).
- Soler, M. Long-term coevolution between avian brood parasites and their hosts. Biol Rev. 89 (3), 688-704 (2014).
- Cassey, P., Honza, M., Grim, T., Hauber, M. E. The modelling of avian visual perception predicts behavioural rejection responses to foreign egg colours. Biol Lett. 4 (5), 515-517 (2008).
- Hale, K., Briskie, J. V. Response of introduced European birds in New Zealand to experimental brood parasitism. J Avian Biol. 38 (2), 198-204 (2007).
- Igic, B., et al. Detecting pigments from colourful eggshells of extinct birds. Chemoecology. 20, 43-48 (2010).
- Igic, B., et al. A shared chemical basis of avian host-parasite egg colour mimicry. Proc R Soc Lond B. 279 (1731), 1068-1076 (2012).
- Yang, C., et al. Coevolution in action: disruptive selection on egg colour in an avian brood parasite and its host. PLoS One. 5 (5), 1-8 (2010).
- Stoddard, M. C., Stevens, M. Avian vision and the evolution of egg color mimicry in the common cuckoo. Evolution. 65 (7), 2004-2013 (2011).
- Honza, M., Polačiková, L. Experimental reduction of ultraviolet wavelengths reflected from parasitic eggs affects rejection behaviour in the blackcap Sylvia atricapilla. J Exp Biol. 211 (15), 2519-2523 (2008).

