A High-performance Liquid Chromatography Measurement of Kynurenine and Kynurenic Acid: Relating Biochemistry to Cognition and Sleep in Rats
Summary
Alterations in the kynurenine pathway (KP) neuroactive metabolites are implicated in psychiatric illnesses. Investigating the functional outcomes of an altered kynurenine pathway metabolism in vivo in rodents may help elucidate novel therapeutic approaches. The current protocol combines biochemical and behavioral approaches to investigate the impact of an acute kynurenine challenge in rats.
Abstract
The kynurenine pathway (KP) of tryptophan degradation has been implicated in psychiatric disorders. Specifically, the astrocyte-derived metabolite kynurenic acid (KYNA), an antagonist at both N-methyl-d-aspartate (NMDA) and α7 nicotinic acetylcholine (α7nACh) receptors, has been implicated in cognitive processes in health and disease. As KYNA levels are elevated in the brains of patients with schizophrenia, a malfunction at the glutamatergic and cholinergic receptors is believed to be causally related to cognitive dysfunction, a core domain of the psychopathology of the illness. KYNA may play a pathophysiologically significant role in individuals with schizophrenia. It is possible to elevate endogenous KYNA in the rodent brain by treating animals with the direct bioprecursor kynurenine, and preclinical studies in rats have demonstrated that acute elevations in KYNA may impact their learning and memory processes. The current protocol describes this experimental approach in detail and combines a) a biochemical analysis of blood kynurenine levels and brain KYNA formation (using high-performance liquid chromatography), b) behavioral testing to probe the hippocampal-dependent contextual memory (passive avoidance paradigm), and c) an assessment of sleep-wake behavior [telemetric recordings combining electroencephalogram (EEG) and electromyogram (EMG) signals] in rats. Taken together, a relationship between elevated KYNA, sleep, and cognition is studied, and this protocol describes in detail an experimental approach to understanding function outcomes of kynurenine elevation and KYNA formation in vivo in rats. Results obtained through variations of this protocol will test the hypothesis that the KP and KYNA serve pivotal roles in modulating sleep and cognition in health and disease states.
Introduction
The KP is responsible for degrading nearly 95% of the essential amino acid tryptophan1. In the mammalian brain, kynurenine taken into astrocytes is metabolized into the neuroactive small molecule KYNA primarily by the enzyme kynurenine aminotransferase (KAT) II2. KYNA acts as an antagonist at NMDA and α7nACh receptors in the brain2,3,4, and also targets signaling receptors including the aryl hydrocarbon receptor (AHR) and the G-protein coupled receptor 35 (GPR35)5,6. In experimental animals, elevations in brain KYNA have been shown to impair their cognitive performance in an array of behavioral assays2,7,8,9,10. An emerging hypothesis suggests that KYNA plays an integral role in modulating cognitive functions by impacting sleep-wake behavior11, thus further supporting the role of astrocyte-derived molecules in modulating the neurobiology of sleep and cognition12.
Clinically, elevations in KYNA have been found in cerebrospinal fluid and post-mortem brain tissue from patients with schizophrenia13,14,15,16, a debilitating psychiatric disorder characterized by cognitive impairments. Patients with schizophrenia are also often plagued by sleep disturbances that may exacerbate the illness17. Understanding the role of KP metabolism and KYNA in modulating a relationship between sleep and cognition, particularly between learning and memory, may lead to the development of novel therapies for treating these poor outcomes in schizophrenia and other psychiatric illnesses.
A reliable and consistent method for the measurement of KP metabolites is important to assure that the research emerging from various institutions can be integrated into the scientific understanding of KP biology. Presently, we describe the methodology to measure kynurenine in rat plasma and KYNA in the rat brain by high-performance liquid chromatography (HPLC). The present protocol, which makes use of a fluorimetric detection in the presence of Zn2+, was first developed by Shibata18 and more recently adapted and optimized to derivatize with 500 mM zinc acetate as the post-column reagent, allowing for the detection of endogenous, nanomolar amounts of KYNA in the brain11.
To stimulate the de novo endogenous KYNA production as described in the present protocol, the direct bioprecursor kynurenine is injected intraperitoneally (i.p.) in rats. In combination with biochemical assessments to determine the degree of KYNA production, the impacts of a kynurenine challenge on the hippocampal-dependent memory (passive avoidance paradigm) and the sleep-wake architecture (EEG and EMG signals) is also investigated11. A combination of these techniques allows for the study of the biochemical and functional impact of a kynurenine challenge in vivo in rats.
Protocol
Our experimental protocols were approved by the University of Maryland Institutional Animal Care and Use Committee and followed the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
NOTE: Adult male Wistar rats (250–350 g) were used in all experiments. Separate cohorts of animals were used for biochemical analysis, behavioral experiments, and sleep-wake recordings. The animals were housed in a temperature-controlled facility at the Maryland Psychiatric Research Center. They were kept on a 12/12 h light-dark cycle, with lights on at zeitgeber time (ZT) 0 and lights off at ZT 12. The animals received ad libitum access to food and water during the experiments. The facility was fully accredited by the American Association for the Accreditation of Laboratory Animal Care.
1. Intraperitoneal Kynurenine Administration to Rats
Note: In this protocol, kynurenine was administered at ZT 0 (the beginning of the light phase) and tissue was collected at ZT 2 and ZT 4 to determine a time course for the kynurenine metabolism. Saline-injected animals were used as a control. For instance, if a rat weighs 500 g and the desired dose is 100 mg/kg, the rat should receive a 5 mL injection of a 10 mg/mL solution of kynurenine.
- Weigh out L-kynurenine sulfate (“kynurenine”) and place it into a 20 mL glass vial. Make sure to keep the kynurenine on ice at all times.
- Add saline to achieve the desired concentration and adjust the pH to 7.6 using 1 N sodium hydroxide (NaOH). Vortex and sonicate the solution until the kynurenine has dissolved completely.
CAUTION: Follow all recommendation precautions while handling NaOH.- For example, to achieve a 10 mg/mL solution, weigh out 200 mg of kynurenine and place it in a glass vial. Add 15 mL of saline and vortex and sonicate (20 kHz for 5–10 s, 1/8 inch diameter microtip) it to dissolve. Using NaOH, adjust the pH of the solution. Lastly, use saline to adjust the volume to 20 mL.
- Weigh each experimental rat and calculate the volume of each injection based on the animal’s body weight and desired treatment dose. Carefully remove the animal from its home cage, inject the solution intraperitoneally, and return the animal to its cage.
2. Kynurenine Measurements Using High-performance Liquid Chromatography
- Tissue collection
NOTE: In this protocol, kynurenine was administered at ZT 0 (the beginning of the light phase) and tissue was collected at ZT 2 and ZT 4 to determine a time course for the kynurenine metabolism. Saline-injected animals were used as a control.- Use carbon dioxide to euthanize the animal. After the signs of respiration have ceased for 1 min, perform a secondary euthanasia method by decapitation.
- To collect whole trunk blood, place a disposable funnel into a 15 mL tube containing 20 μL of K3-EDTA (0.5 M). Allow the blood to drain from the body into the tube. Invert the tube repeatedly to ensure the EDTA is mixed throughout.
- Using scissors, cut the skin on the top of the head down the midline to expose the skull. Remove any excess neck muscle on the back of the skull and, at the opening for the spinal cord, cut through the skull from back to front.
- Gently pull the two sides of the skull open to expose the brain, and then carefully remove the brain with forceps so as not to damage it. Remove the olfactory bulbs with a razor blade and cut the brain in half down the midline. Using forceps, dissect the hemibrain into regions of interest (i.e., the hippocampus and the cortex).
- Place all dissected brain pieces on aluminum foil on dry ice. After the tissue has completely frozen, transfer it to a 20 mL plastic vial and store it at -80 °C.
- Centrifuge the whole trunk blood at 300 x g for 10 min. Remove the supernatant (plasma) and transfer it into new centrifuge tubes prior to storing them at -80 °C.
- Sample preparation
- Plasma: collected from treated animals
- Thaw the frozen tube with plasma (see step 2.1 for the tissue collection) on wet ice.
- In a new centrifuge tube, dilute the sample with ultrapure water (1:2 for kynurenine, 1:10 for KYNA). To 100 μL of the diluted sample, add 25 μL of 6% perchloric acid.
CAUTION: Follow all recommendation precautions while handling perchloric acid. - Vortex all samples, then centrifuge them at 12,000 x g for 10 min. Once finished, remove 100 μL of the supernatant (from each tube) and place them into a small 0.25 mL microcentrifuge tube for the kynurenine or KYNA determination.
- Brain: collected from treated animals
- Place tubes with frozen brain samples (see step 2.1 for the tissue collection) on dry ice. Individually weigh each brain sample on a precise analytical balance. Return it to the tube and place it back on dry ice.
- After all the samples have been weighed, move the tubes onto wet ice. Dilute each sample 1:10 w/v with ultrapure water and homogenize them using a sonicator (20 kHz for 5–10 s, 1/8 inch diameter microtip). For example, for 100 mg of brain tissue, add 900 μL of ultrapure water.
- Aliquot 100 μL of each diluted sample to a new tube and acidify it with 25 μL of 25% perchloric acid. Vortex, then centrifuge it at 12,000 x g for 10 min.
- After the centrifugation, remove 100 μL of supernatant and place it into small 0.25 mL microcentrifuge tubes for the KYNA determination.
- Plasma: collected from treated animals
- HPLC set-up and run
- Prepare 50 mM sodium acetate mobile phase. Dissolve 6.81 g of sodium acetate trihydrate in 1 L of ultrapure water. Adjust the pH to 6.2 using glacial acetic acid and then vacuum filter it into clean glassware. Transfer the sodium acetate mobile phase to a clean 1 L glass bottle.
- Prepare 500 mM zinc acetate. Dissolve 54.88 g of zinc acetate in 500 mL of ultrapure water, then vacuum filter it. Transfer it to a clean 500 mL glass bottle. Fill a 1 L bottle with ultrapure water and a 500 mL bottle with HPLC grade acetonitrile.
- Place the intake tubing from the HPLC machine separately into the mobile phase, the ultrapure water, and the 100% acetonitrile. Pump the zinc acetate solution separately through a peristaltic pump that combines with the mobile phase post-column, and prior to the derivatization.
- Using the control panel on the machine, program it to run 5% acetonitrile and 95% ultrapure water at 0.5 mL/min. Allow it to run for 20–30 min to achieve a stable pressure and baseline.
- Upon establishing a stable baseline, change the solution composition to 5% acetonitrile and 95% sodium acetate mobile phase. Equilibrate for 30 min.
- Turn on the lamp in the fluorescence detector and allow it to warm up.
- In the meantime, also turn on the post-column pump to a flow rate of 0.1 mL/min. Allow it to reach a stable pressure of about 500 psi after approximately 20 min.
- Prepare the standards.
- For KYNA, begin with a 1 mM stock solution and dilute it to prepare 5 standard concentrations (i.e., 10 nM, 5 nM, 2.5 nM, 1 nM, and 500 pM).
NOTE: This will produce a standard curve ranging from 200 fmoles to 10 fmoles per 20 μL injection. - For kynurenine, begin with a 1 mM stock solution and dilute it to prepare 5 standard concentrations (i.e., 10 μM, 5 μM, 2.5 μM, 1 μM, and 500 nM).
NOTE: This will produce a standard curve ranging from 200 pmoles to 10 pmoles per 20 μL injection.
- For KYNA, begin with a 1 mM stock solution and dilute it to prepare 5 standard concentrations (i.e., 10 nM, 5 nM, 2.5 nM, 1 nM, and 500 pM).
- Set up the HPLC system software to control the sample sequence parameters and allow for the autoinjections of multiple samples.
- When on the "Run Sample" screen, click "File" and drag down to "Create New Sample Set". When the new window opens, select "Empty". Individually list all standards (see step 2.3.8) or samples in the order they should be run.
- In the "Function" column, designate the standards and samples. The standards should be assayed at the beginning and end of the sequence. Inject water between every 5 samples.
- Set the run time for each sample to 15 min. Inject 20 μL of the sample from the standards and the plasma preparation or inject 30 μL of the sample from the brain homogenate preparation.
- Set the excitation and emission wavelengths (dependent on the compound being assessed) for kynurenine to excitation: 365 nm, emission: 480 nm, and retention time: ~6 min, and for KYNA to excitation: 344 nm, emission: 398 nm, and retention time: ~11 min.
- Once all parameters have been set and the machine has equilibrated, run the sequence.
- To quantify the data, navigate to the "Browse Project" screen. Under the "Sample Sets" tab, double-click on the sample set to be quantified. From the list of all injections, highlight all standards and right-click. In the new window, select "Process", then use the drop-down menu to select "Calibrate and Quantitate" next to "How":.
- Highlight all standards again, right-click and select "Review". When chromatograms open, use the buttons along the top to "Integrate" and then "Calibrate" each standard; this will automatically create the standard curve.
- Return to the "Sample Sets" tab and double-click on the sample set. Next, highlight all standards. Repeat the process stated above, but instead, selecting "Quantitate" from the drop-down menu. Highlight all samples again, then right-click and select "Review". When chromatograms open, use the buttons along the top to "Integrate" and then "Quantitate" each sample.
NOTE: This will output the concentration of each sample based on the previously created standard curve.
3. Passive Avoidance Paradigm
NOTE: These behavioral experiments were designed based on our biochemical findings with the acute kynurenine challenge. To maximize an increase in brain KYNA, kynurenine (100 mg/kg) was administered at ZT 0, 2 h prior to the training session in the passive avoidance paradigm to test hippocampal-mediated learning, that occurred at ZT 2. The apparatus consists of 2 equally sized compartments (21.3 cm high, 20.3 cm wide, and 15.9 cm deep) separated by a guillotine door and contained within a soundproof box. The two compartments of the testing apparatus are termed “light side” and “dark side”. The walls of the light side are clear and, during the trials, a light will turn on to further illuminate this compartment. The walls of the dark compartment are completely covered to maintain a black-out condition.
- Day 1: training trial
- Prior to the testing, clean the apparatus with 70% ethanol.
- Bring the animals to the testing room.
- Gently place the experimental animal into the light side of the apparatus and close and latch the apparatus door and the soundproof box.
- Using the associated software, begin the trial. From the home screen, select "File" and then "Open Session" from the drop-down menu. In the new window, ensure the correct procedure and apparatus are selected and click "Close". Next, select "Configure" and then "Signals". In the new window, select the correct apparatus and click "Issue".
NOTE: The software will initiate a light to turn on in the light side of the apparatus and open the guillotine door between the two compartments. A timer will be initiated. - When the animal fully enters the dark side of the apparatus, the guillotine door will close, and an inescapable foot shock (0.56 mA for 1 s) will be delivered.
- Record the time that has elapsed before the animal entered the dark compartment.
- After the animal has received the shock, allow 30 s of recovery time before removing the animal from the apparatus.
- Place the animal back in its home cage.
- Before beginning the trial with the next animal, wipe the apparatus clean with a wet towel and dispose of any fecal pellets.
- After all the animals have been trained, return them to the animal facility.
- Day 2: testing trial
- Bring animals back into the testing room 24 h after the training trial. Begin the testing trial for the first animal by placing it into the light side of the apparatus, closing and latching the door and the soundproof box.
- Initiate the testing trial using the same software commands as the previous day; the light will turn on in the light side of the apparatus and the guillotine door between the two compartments will open. A timer will be initiated.
- When the animal enters the dark compartment, the guillotine door will close. Record the time elapsed.
NOTE: The trial will be terminated via the software after a maximum of 300 s if the animal remains on the light side of the testing apparatus. - Place the animal back in their home cage and clean the apparatus (as described in step 3.1.9) before beginning the trial with the next animal.
4. Sleep Analysis
- Surgical implantation of a wireless EEG/EMG transmitter
NOTE: The telemetric transmitters should be sterilized and prepared prior to surgery.- Using a razor blade, gently remove a small length of the plastic coating to reveal the wire leads. Place the transmitters in a 50 mL conical tube filled with sterilizing solution [e.g., Wavecide (2.65% glutaraldehyde)].
- Leave the transmitters in the disinfecting solution for at least 12 h. Then, prior to the surgery, transfer the solution to a waste container. Rinse the transmitter with sterile saline.
- On the day of the surgery, weigh the animal prior to placing it in an anesthesia chamber. Induce 3–5% isoflurane with 100% O2.
- Upon a successful anesthesia, remove the animal and carefully shave, using an electric razor, the top of the head and neck, as well as a small patch of hair on the back of the body, just slightly left and below the ribcage.
- Administer carprofen (5 mg/kg) subcutaneously for postoperative analgesia.
- Place a thermostatically controlled warm-water heating pad set at 37 °C under the animal to maintain the animal’s body temperature during the surgery.
- In a stereotaxic frame, place the animal on a nose cone to maintain the anesthetic condition and place ear bars to stabilize the head. Sterilize the shaved skin by alternating swabs of betadine scrub and 70% ethanol. Apply opthalamic ointment to the eyes to lubricate them.
- To ensure adequate anesthesia prior to the first incision, use the toe-pinch test.
- Using a sterile scalpel, make an incision from just behind the eyes down to the back of the neck. The skull and the dorsal cervical neck muscle should be exposed.
- If necessary to expose the skull, use cotton-tipped applicators to remove any membranes. Micro-bulldog clamps can be used to hold the skin away from the skull. Locate and mark the position of the bregma. Drill 2 burr-holes and insert metal screws 2.0 mm anterior/+1.5 mm lateral and 7.0 mm posterior/-1.5 mm lateral relative to the bregma. Complete 2 revolutions to tighten the screws. Cover the exposed skull with a piece of gauze.
- Using sterile surgical scissors, make an incision into the body cavity, just below the ribs.
- On the surgical sheet, make sure to record the serial number of the transmitter to be implanted.
- Insert the sterile transmitter inside the body cavity. The wire leads should still be outside of the body.
- Use absorbable sutures to stitch the muscle wall, ensuring that all of the leads are gathered to one end of the incision.
- Remove the gauze from the head and lift up the skin just below the incision. Run a pair of long sterile hemostats under the skin from the head incision to the incision in the side. Keep pressure on the skin so as to minimize any injury.
- Once the hemostats are visible near the body incision, clip any membrane that may cover them with surgical scissors and grab the four transmitter leads with the hemostats. Slowly draw out the hemostats, so that the wires lay under the skin and run up to the skull.
- Designate which 2 leads will be connected to the skull. Using forceps, grasp the end of a wire with one hand and just below the end of the plastic sheath with the other. Pull on the wire until individual loops can just be made out.
- Tightly wrap the exposed wire around one of the metal screws 3–4x. Once secure, tighten the screw to prevent the wire from unraveling and clip off any extra wire. Repeat this with the second lead and screw.
- Pull both EEG leads from the body cavity so that they sit snug against the skull.
- Use dental cement to cover the screws and cortical leads, creating a cap over the exposed skull. Ensure that no dental cement sticks to skin or muscle and that no sharp edges are created. Allow the dental cement to dry.
Note: The cap should be small enough that skin can be sutured over top. - For the EMG leads, grasp the end of the wire and plastic sheath as described in step 4.1.15. Stretch the wire until the individual loops are clearly discernable.
- Run a 22 G needle under the neck muscle on the right side for 2–3 mm before allowing it to emerge. Using nonabsorbable sutures, place a single, loose suture around the embedded needle.
- Thread the EMG wire into the needle and pull the needle out, allowing the EMG wire to thread under the muscle. Tighten the suture to ensure the wire remains in place and clip the extra wire that may emerge from the muscle.
- Repeat steps 4.1.20 and 4.1.21 for the second EMG lead, about 1 mm down from the first lead.
- Pull both EMG leads from the body cavity so that they sit snug against the muscle.
- Use nonabsorbable stitches to close the head and neck incision. Tuck all extra wire under the skin of the body and use wound clips to close the body incision.
- Apply lidocaine ointment to both incision sites to assist the animal with postoperative pain.
- Return the animal to its home cage, allowing the cage to sit on the heating pad until the animal has recovered from the anesthesia.
Note: The animal should recover for 7–10 days before beginning the recordings.
- Using a razor blade, gently remove a small length of the plastic coating to reveal the wire leads. Place the transmitters in a 50 mL conical tube filled with sterilizing solution [e.g., Wavecide (2.65% glutaraldehyde)].
- EEG/EMG recording
NOTE: In this protocol, we administered saline or kynurenine at ZT 0 and then recorded the animal’s sleep-wake patterns for 24 h.- Complete all sleep recordings in a designated room where the animals will not be interrupted for the duration of the recording.
- Place the home cage of the animal (bearing the implanted EEG/EMG transmitter) on a receiver unit. Turn on the surgically implanted transmitter using a magnet.
NOTE: A light should appear on the receiver unit when the transmitter is activated. - Set up the recording of the EEG/EMG data using the associated software.
- Select "Experiment" and then "Create" from the drop-down menu. Name the experiment, and then save it. Next, select "Hardware" and then "Edit MX2 Configuration".
- In the new window, select all transmitters used in the experiment and indicate which receiving pad each is paired with. When ready, initiate the recording by pressing "Start All Continuous".
- At the termination of the experiments, select "Stop All Continuous" in the associated software and turn off the transmitters using the magnet.
- Euthanize the animals by CO2 asphyxiation followed by a cardiac puncture. Remove the transmitter carefully by reopening the incision along the top of the head with surgical scissors and cutting the leads free from the dental cement and neck muscle.
- Next, open the body cavity, locate the transmitter and slowly remove it from the body.
- EEG/EMG analysis
- Use the associated software to analyze the sleep-wake data. Open the program and select "Add New Location". In the new window, select the data to import it into the scoring software.
NOTE: This will create a new file within the software containing all raw waveforms collected during the experiment. - Manually score the vigilance states for the desired experiments. After scoring, analyze the parameters such as the total duration, the number of bouts, and the average bout duration of the various vigilance states [rapid eye movement (REM), non-REM (NREM), and wake].
NOTE: The analysis can be performed over the entire recording or shortened to specific time points of interest. Here, the analysis was performed for the recording period between ZT 0 to ZT 2.
- Use the associated software to analyze the sleep-wake data. Open the program and select "Add New Location". In the new window, select the data to import it into the scoring software.
Representative Results
To validate the use of an intraperitoneal kynurenine injection as a method to elevate the brain KYNA, an HPLC analysis of tissue was performed. Standard curves (Figure 1) were constructed using the associated software and allowed for the quantification of the tissue samples. Representative chromatograms for kynurenine and KYNA are presented in Figure 2. Kynurenine was observed at a retention time of 6 min, and KYNA had a retention time of 11 min. To observe the KP dynamics, 100 mg/kg of kynurenine was administered in this protocol, and tissue was collected immediately following the injection, or 2 or 4 h post-injection (Figure 3a). Plasma kynurenine (Figure 3b) and hippocampal KYNA (Figure 3c) were quantified. The specific parameters used in the HPLC determination of kynurenine metabolites are outlined in Table 1. The dose-response curve produced by this protocol supports its description of an acute kynurenine injection as a method to increase brain KYNA levels, with a peak achieved at 2 h post-injection and the levels of KYNA returned to their baseline 4 h post-injection.
Based on the aforementioned biochemical findings, the training in the passive avoidance paradigm was performed 2 h after the kynurenine injection (Figure 4a). No group differences were observed during the training trial (Figure 4b). During the testing trial, the control animals displayed a significant increase in latency to enter the dark side, indicating contextual learning. Animals injected with kynurenine on the previous day did not display the same increase in latency, demonstrating a deficit in learning. Based on these findings, we can conclude that acute kynurenine elevation, during the time of memory acquisition and consolidation, results in an impaired performance in a hippocampus-mediated task.
To investigate the impact of an acute kynurenine elevation during the light phase on sleep-wake architecture, animals were injected with saline on day 1 and with kynurenine on day 2 at ZT 0 (Figure 5a). EEG/EMG signals were recorded telemetrically for the entire 48 h period. Comparisons were made between the saline injection (vehicle) on day 1 and the kynurenine injection on day 2. The results indicated a reduction in total REM sleep duration (presented as a percentage change from the baseline) during the first 2 h (Figure 5b) after an injection. This was mirrored by an increase in wake duration during these time periods and a slight reduction in NREM sleep. These results demonstrate that an acute kynurenine elevation causes disturbances in sleep-wake dynamics.
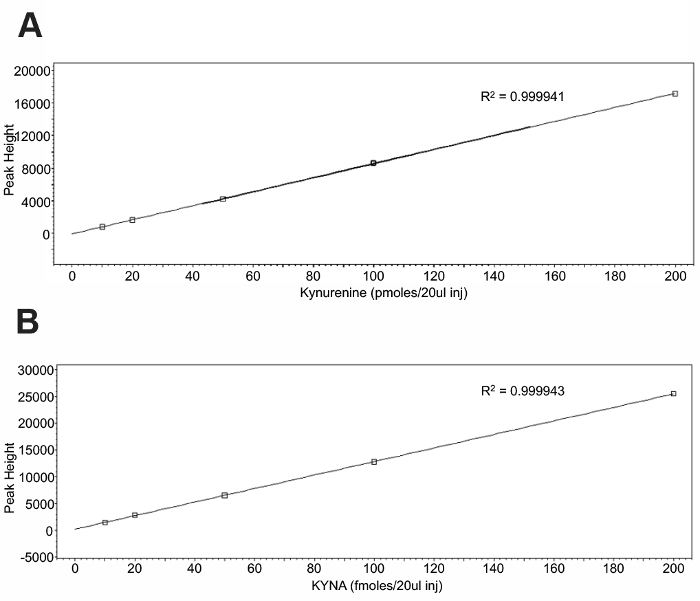
Figure 1: Standard curves for a kynurenine and KYNA injection. 20 µL of each standard was injected to create the standard curve for (A) kynurenine and (B) KYNA. Please click here to view a larger version of this figure.
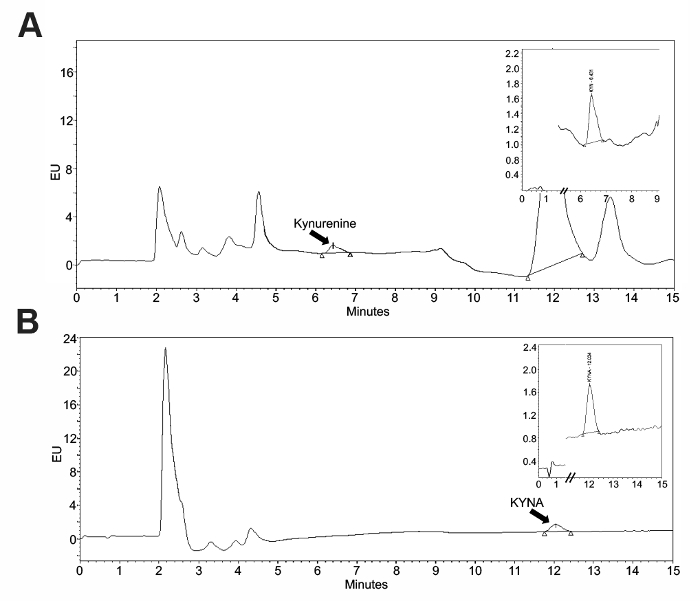
Figure 2: Representative chromatograms for kynurenine and KYNA. The standards were run prior to a sample to confirm their retention times. These panels show representative samples for (A) serum kynurenine and (B) brain KYNA. Please click here to view a larger version of this figure.
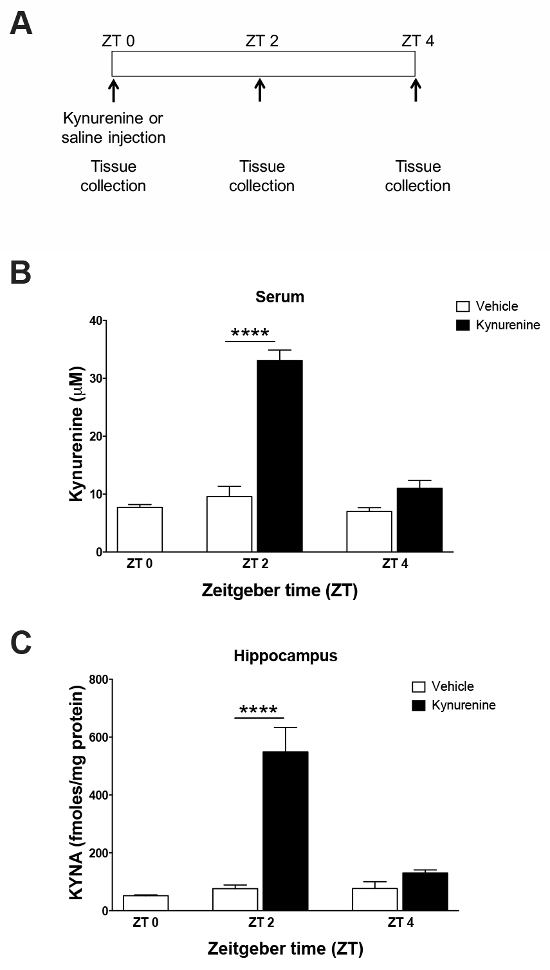
Figure 3: Analysis of serum and hippocampal tissue by HPLC following a kynurenine challenge. (A) Kynurenine (100 mg/kg) or saline were administered by an intraperitoneal injection at zeitgeber time (ZT) 0. Tissues were collected either 2 or 4 h post-injection. The following panels show exposure to (B) kynurenine-elevated serum kynurenine and (C) hippocampal KYNA levels 2 h after injection. **** P <0.001 indicates the significance by a two-way analysis of variance (ANOVA) followed by a Bonferroni t-test correction. N = 4–5 per group. All data are mean ± SEM. This figure has been modified from Pocivavsek et al.11. Please click here to view a larger version of this figure.
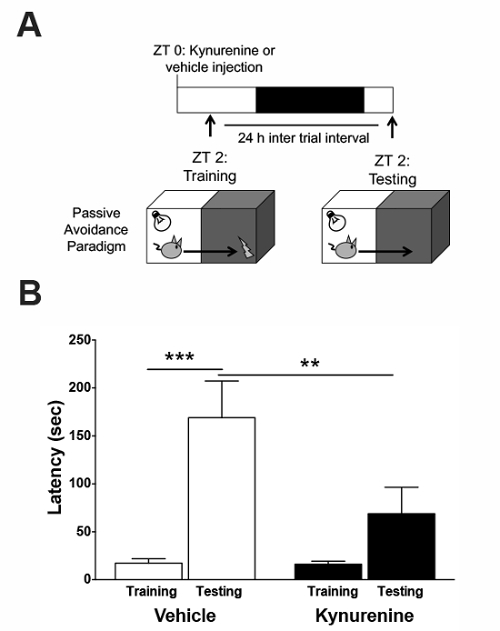
Figure 4: Effect of a kynurenine elevation on hippocampal-dependent memory in the passive avoidance paradigm. (A) Kynurenine (100 mg/kg) or saline were administered by intraperitoneal injection at zeitgeber time (ZT) 0. The animals were trained on the task at ZT 2. 24 h after the training, the animals were tested for memory formation. (B) An exposure to kynurenine prior to the training reduced the latency to avoid the aversive dark compartment on the testing day. ** P <0.01, *** P <0.001 indicate the significance by a two-way analysis of variance (ANOVA) followed by a Bonferroni t-test correction. N = 8–14 per group. All data are mean ± SEM. This figure has been modified from Pocivavsek et al.11. Please click here to view a larger version of this figure.
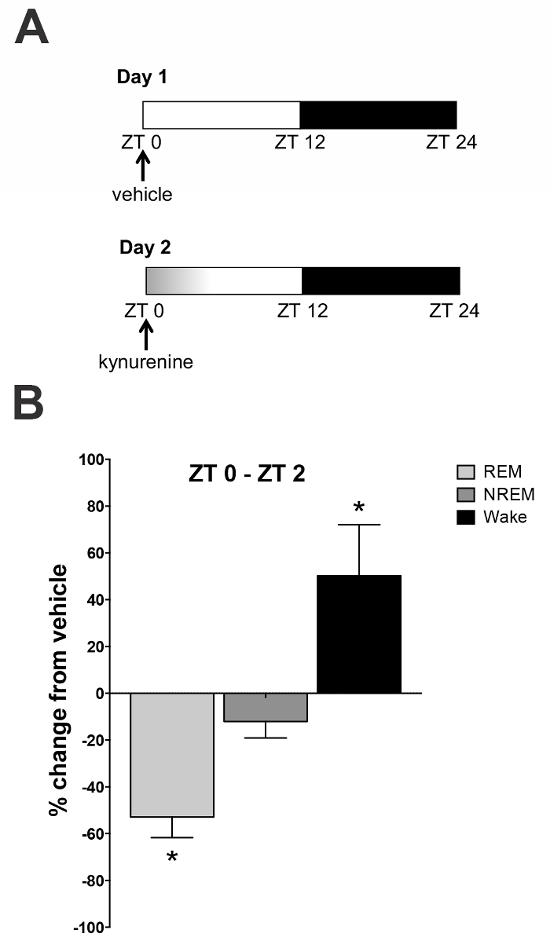
Figure 5: Effect of a kynurenine elevation on sleep-wake architecture. (A) Saline (day 1) and kynurenine (100 mg/kg; day 2) were administered by an intraperitoneal injection at zeitgeber time (ZT) 0. The sleep-wake behavior was recorded for the subsequent 24 h. (B) The rapid eye movement (REM) sleep duration was reduced and the wake duration was increased in the first 2 h following the kynurenine injection. * P <0.05 indicates the significance by a two-way analysis of variance (ANOVA) followed by a Bonferroni t-test correction. N = 6–8 per group. All data are mean ± SEM. This figure has been modified from Pocivavsek et al.11. Please click here to view a larger version of this figure.
| Mobile phase composition | 50 mM sodium acetate, pH 6.2 |
| 5% acetonitrile | |
| Mobile phase flow rate | 0.5 mL/min |
| Post-column derivatization reagent | 500 mM zinc acetate |
| Post-column derivatization flow rate | 0.1 ml/min |
| Column type | ReproSil-Pur C18 |
| Column dimensions | 4 x 150 mm2 |
| Detector temperature | 4.0 ºC |
| Kynurenine wavelength | Ex: 365, Em: 480 |
| Kynurenine retention time | ~6 min |
| KYNA wavelength | Ex: 344, Em: 398 |
| KYNA retention time | ~11 min |
Table 1: Parameters for an HPLC determination of kynurenine pathway metabolites.
Discussion
For a reliable assessment of KYNA in the brain after a peripheral kynurenine administration, it is critical to combine and interpret biochemical and functional experiments. Here, we present a detailed protocol that permits new users to establish effective methods for measuring the plasma kynurenine and brain KYNA of rats. The measurement of kynurenine in the plasma confirmed the accurate injection and the measurement of the metabolite KYNA confirms the de novo synthesis in the brain. There are several advantages of the described fluorometric HPLC method, adapted from Shibata18, to derivatize KYNA in the presence of Zn2+, including the ability to precisely detect endogenous amounts of KYNA in the brain in the nanomolar range. In addition, the method has been adapted to detect the bioprecursor kynurenine in the plasma in the micromolar range by adjusting the fluorometric wavelengths of excitation and emission, as described in the protocol.
Critical steps within the protocol, such as specific times between the treatment and the euthanasia, are described to accurately determine the time course of the KYNA formation in the brain and guide the design and analysis of the behavioral and sleep-wake monitoring experiments. Hippocampal-mediated learning was assessed using the passive avoidance paradigm and a sleep-wake analysis was conducted with telemetric recordings of EEG/EMG data. As we determined that the brain KYNA levels were highest 2 h post-injection, we timed the behavioral paradigm training session accordingly, so that the acquisition in the passive avoidance task occurred when the KYNA levels were highest, at ZT 2. In addition, we also focused the analysis of the sleep-wake behavior on the critical ZT 0 to ZT 2 time frame during which the KYNA levels were highest in the brain. Taken together, the carefully considered experimental design has allowed us to bridge together findings from biochemical and functional experiments, introducing KYNA as a modulator of both cognition and sleep-wake behavior11.
A number of limitations should be considered in the described protocol. To stimulate the endogenous production of KYNA, the direct bioprecursor kynurenine was injected peripherally into rats. Kynurenine readily crosses the blood-brain barrier and the stimulated KP metabolism occurs in a variety of brain regions2. We presently focus on the elevation in astrocyte-derived KYNA in the hippocampus; however, it is important to consider that systemic injections of kynurenine also elevate metabolites of the neurotoxic branch of the KP, including 3-hydroxykynurenine (3-HK) and quinolinic acid. To tease apart the effects caused by the elevations in 3-HK compared to those caused by KYNA elevations, the methodology should be adjusted. Additionally, the perfuse elevation in KP metabolism throughout the brain11 provides a limited insight into the specific brain regions that are mediating the alterations in behavior.
When designing the behavioral experiment described here, we optimized the passive avoidance paradigm with respect to existing methods, as described in detail, to engage hippocampal-mediated contextual memory. Memory is comprised of three major subprocesses: encoding, consolidation, and retrieval. Optimal conditions for memory consolidation processes occur during sleep when newly encoded memory is integrated into long-term storage19,20. As studies in rodents have focused on narrow time windows of memory consolidation and identified that memory appears most sensitive when sleep is delayed after the acquisition, we chose to elevate the kynurenine and KYNA formation in the brain during this sensitive time window, impacting the acquisition and the consolidation, to test the hypothesis of this article. We did not, however, presently test if the retrieval of the memory was impacted by kynurenine elevations, as previously shown21. It is critically important to also consider the brain regions engaged in rodents to perform a task. While for this study, we were mostly interested in the role of the hippocampus in modulating cognition, modifications to the protocol may be useful to test animals in alternative behavioral paradigms that have been shown to be impacted by an acute kynurenine challenge, such as fear conditioning and working memory tasks2,7,8,9,10.
Additionally, in lieu of systemic injections of kynurenine to elevate the brain KYNA, alternative strategies to consider include the direct infusion of KYNA into an area of interest or the targeted inhibition of the synthesizing enzyme KAT II by pharmacological tools or molecular knock-down approaches in specific brain regions, to test mechanistic hypotheses. While these approaches may also introduce potentially invasive procedures that independently impact the functional outcomes measured, it is critical to design experiments with optimal control conditions.
Lastly, the sleep recording EEG/EMG protocol described here has the advantage of being telemetric rather than tethered, eliminating electrical noise, movement artifacts, and the risk of injury in an animal that has pulled the tethered cables. The physical size and light weight of the telemetric device, and its placement subcutaneously rather than intraperitoneally in the rat to optimize the post-surgical recovery, enables wireless recordings that capture naturalistic sleep-wake behavior with a free range of movement. However, an important consideration when using the telemetric system is the limited ability to recording from multiples channels simultaneous. The current paradigm pairs one EEG channel and one EMG channel, and allows for the scoring of REM, NREM, and wake behavior.
The combination of methods described presently provides insight into behavioral and biochemical assays to elucidate the functional outcomes of a kynurenine elevation. These protocols demonstrate a relationship between the KP, cognition, and sleep, a previously unexplored dynamic. Utilizing the detailed experimental methods provided here will enhance the scientific understanding of the functional impacts of kynurenine and KYNA neurobiology in animals. Ultimately, continued work in this field may lead to new therapeutic approaches to alleviate outcomes in individuals combatting psychiatric disturbances and disruptions in sleep.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The present study was funded in part by the National Institutes of Health (R01 NS102209) and a donation from the Clare E. Forbes Trust.
Materials
| Wistar rats | Charles River Laboratories | adult male, 250-350 g | |
| L-kynurenine sulfate | Sai Advantium | ||
| ReproSil-Pur C18 column (4 x 150 mm) | Dr. Maisch GmbH | ||
| EZ Clips | Stoelting Co. | 59022 | |
| Mounting materials screws | PlasticsOne | 00-96 X 1/16 | |
| Nonabsorbable Sutures | MedRep Express | 699B | CP Medical Monomid Black Nylon Sutures, 4-0, P-3, 18", BOX of 12 |
| Absorbable Sutures | Ethicon | J310H | 4-0 Coated Vicryl Violet 1X27'' SH-1 |
| Dental Cement | Stoelting Co. | 51458 | |
| Drill Bit | Stoelting Co. | 514551 | 0.45 mm |
| Name | Company | Catalog Number | Comments |
| Alliance HPLC system | |||
| E2695 separation module | Waters | 176269503 | |
| 2475 fluorescence detector | Waters | 186247500 | |
| post-column reagent manager | Waters | 725000556 | |
| Lenovo computer | Waters | 668000249 | |
| Empower software | Waters | 176706100 | |
| Name | Company | Catalog Number | Comments |
| Passive avoidance box for rat | |||
| Extra tall MDF sound attenuating cubicle | MedAssociates | ENV-018MD | Interior: 22"W x 22"H x 16"D |
| Center channel modulator shuttle box chamber | MedAssociates | ENV-010MC | |
| Stainless steel grid floor for rat | MedAssociates | ENV-010MB-GF | |
| Auto guillotine door | MedAssociates | ENV-010B-S | |
| Quick disconnect shuttle grid floor harness for rat | MedAssociates | ENV-010MB-QD | |
| Stimulus light, 1" white lens, mounted on modular panel | MedAssociates | ENV-221M | |
| Sonalert module with volume control for rat chamber | MedAssociates | ENV-223AM | |
| SmartCtrl 8 input/16 output package | MedAssociates | DIG-716P2 | |
| 8 Channel IR control for shuttle boxes | MedAssociates | ENV-253C | |
| Infrared source and dectector array strips | MedAssociates | ENV-256 | |
| Tabletop interface cabinet, 120 V 60 Hz | MedAssociates | SG-6080C | |
| Dual range constant current aversive stimulation module | MedAssociates | ENV-410B | |
| Solid state grid floor scrambler module | MedAssociates | ENV-412 | |
| Dual A/B shock control module | MedAssociates | ENV-415 | |
| 2' 3-Pin mini-molex extension | MedAssociates | SG-216A-2 | |
| 10' Shock output cable, DB-9 M/F | MedAssociates | SG-219G-10 | |
| Shuttle shock control cable 15', 6 | MedAssociates | SG-219SA | |
| Small tabletop cabinet and power supply, 120 V 60 Hz | MedAssociates | SG-6080D | |
| PCI interface package | MedAssociates | DIG-700P2-R2 | |
| Shuttle box avoidance utility package | MedAssociates | SOF-700RA-7 | |
| Name | Company | Catalog Number | Comments |
| Sleep-Wake Monitoring Equipment | |||
| Ponehmah software | Data Sciences International (DSI) | PNP-P3P-610 | |
| MX2 8 Source Acquisition interface | Data Sciences International (DSI) | PNM-P3P-MX204 | |
| Dell computer, Optiplex 7020, Windows 7, 64 bit | Data Sciences International (DSI) | 271-0112-013 | |
| Dell 19" computer monitor | Data Sciences International (DSI) | 271-0113-001 | |
| Receivers for plastic cages, 8x | Data Sciences International (DSI) | 272-6001-001 | |
| Cisco RV130 VPN router | Data Sciences International (DSI) | RV130 | |
| Matrix 2.0 | Data Sciences International (DSI) | 271-0119-001 | |
| Network switch | Data Sciences International (DSI) | SG200-08P | |
| Neuroscore software | Data Sciences International (DSI) | 271-0171-CFG | |
| Two biopotential channels transmitter, model TL11M2-F40-EET | Data Sciences International (DSI) | 270-0134-001 |
Referencias
- Leklem, J. E. Quantitative aspects of tryptophan metabolism in humans and other species: a review. The American Journal of Clinical Nutrition. 24 (6), 659-672 (1971).
- Pocivavsek, A., Notarangelo, F. M., Wu, H. Q., Bruno, J. P., Schwarcz, R., Pletnikov, M. V., Waddington, J. L. Astrocytes as Pharmacological Targets in the Treatment of Schizophrenia: Focus on Kynurenic Acid. Modeling the Psychophathological Dimensions of Schizophrenia – From Molecules to Behavior. , 423-443 (2016).
- Hilmas, C., et al. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. Journal of Neuroscience. 21 (19), 7463-7473 (2001).
- Perkins, M. N., Stone, T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Research. 247 (1), 184-187 (1982).
- DiNatale, B. C., et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicological Sciences. 115 (1), 89-97 (2010).
- Wang, J., et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. The Journal of Biological Chemistry. 281 (31), 22021-22028 (2006).
- Alexander, K. S., Wu, H. Q., Schwarcz, R., Bruno, J. P. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berlin). 220 (3), 627-637 (2012).
- Chess, A. C., Landers, A. M., Bucci, D. J. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behavioural Brain Research. 201 (2), 325-331 (2009).
- Chess, A. C., Simoni, M. K., Alling, T. E., Bucci, D. J. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophrenia Bulletin. 33 (3), 797-804 (2007).
- Pocivavsek, A., et al. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 36 (11), 2357-2367 (2011).
- Pocivavsek, A., Baratta, A. M., Mong, J. A., Viechweg, S. S. Acute Kynurenine Challenge Disrupts Sleep-Wake Architecture and Impairs Contextual Memory in Adult Rats. Sleep. 40 (11), (2017).
- Halassa, M. M., et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 61 (2), 213-219 (2009).
- Erhardt, S., et al. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neuroscience Letters. 313 (1-2), 96-98 (2001).
- Linderholm, K. R., et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophrenia Bulletin. 38 (3), 426-432 (2012).
- Sathyasaikumar, K. V., et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophrenia Bulletin. 37 (6), 1147-1156 (2011).
- Schwarcz, R., et al. Increased cortical kynurenate content in schizophrenia. Biological Psychiatry. 50 (7), 521-530 (2001).
- Pocivavsek, A., Rowland, L. M. Basic Neuroscience Illuminates Causal Relationship Between Sleep and Memory: Translating to Schizophrenia. Schizophrenia Bulletin. 44 (1), 7-14 (2018).
- Shibata, K. Fluorimetric micro-determination of kynurenic acid, an endogenous blocker of neurotoxicity, by high-performance liquid chromatography. Journal of Chromatography. 430 (2), 376-380 (1988).
- Buzsaki, G. Memory consolidation during sleep: a neurophysiological perspective. Journal of Sleep Research. 7, 17-23 (1998).
- Graves, L. A., Heller, E. A., Pack, A. I., Abel, T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learning & Memory. 10 (3), 168-176 (2003).
- Yamashita, M., Yamamoto, T. Tryptophan circuit in fatigue: From blood to brain and cognition. Brain Research. 1675, 116-126 (2017).

