A Chronic Sleep Fragmentation Model using Vibrating Orbital Rotor to Induce Cognitive Deficit and Anxiety-Like Behavior in Young Wild-Type Mice
Summary
Presented here is a protocol for chronic sleep fragmentation (CSF) model achieved by an electrically controlled orbital rotor, which could induce confirmed cognitive deficit and anxiety-like behavior in young wild-type mice. This model can be applied to explore the pathogenesis of chronic sleep disturbance and related disorders.
Abstract
Sleep disturbance is generally common in populations as a chronic disease or a complained event. Chronic sleep disturbance is proposed to be closely linked to the pathogenesis of diseases, especially neurodegenerative diseases. We recently found that 2 months of sleep fragmentation initiated Alzheimer’s disease (AD)-like behavioral and pathological changes in young wild-type mice. Herein, we present a standardized protocol to achieve chronic sleep fragmentation (CSF). Briefly, CSF was induced by an orbital rotor vibrating at 110 rpm and operating with a repetitive cycle of 10 s-on, 110 s-off, during light-ON phase (8:00 AM–8:00 PM) continuously for up to 2 months. Impairments of spatial learning and memory, anxiety-like but not depression-like behavior in mice as consequences of CSF modeling, were evaluated with Morris water maze (MWM), Novel object recognition (NOR), Open field test (OFT) and Forced swimming test (FST). In comparison with other sleep manipulations, this protocol minimizes the handling labors and maximizes the modeling efficiency. It produces stable phenotypes in young wild-type mice and can be potentially generated for a variety of research purposes.
Introduction
Sleep disturbance is increasingly common both in patients with sleep-disturbing conditions and healthy people with sleep-disturbing events. It has been observed that patients with neurodegenerative diseases, chronic pain, emotional stress, respiratory system diseases, urinary system diseases, etc., usually complain about unpleasant sleep experiences1,2,3,4,5. Obstructive sleep apnea (OSA), periodic limb movements in sleep (PLMS), sleep maintenance insomnia among other sleep disorders are the most common causes, which induce sleep fragmentation6,7. In developed countries, OSA has over 5% to 9% prevalence in adult population and 2% in child population8,9,10. Meanwhile, there is an increasing proportion of the healthy population experiencing sleep disturbance due to the overuse of smart phones, irregular sleep habits, annoying noises, and work duties, such as night shifts for caregivers. Sleep is acknowledged to be important for brain waste clearance11,12, memory consolidation13,14, metabolic balance15,16, among many other physiological processes. Yet, it still remains largely unknown whether long-term sleep disturbance gives rise to irreversible pathogenesis alterations in healthy human beings, and whether it is the etiology or a contributing factor of developing central nervous system diseases, such as neurodegenerative diseases in a couple of years down the road. Our goal is to report an experimental model that generates stable and evident cognitive deficit and anxiety-like behavior in young wild-type mice after a 2-month sleep fragmentation treatment. This model would be applied for answering the scientific questions listed above.
Sleep disturbance is listed as a potential risk factor for developing Alzheimer’s disease (AD) or dementia. Kang et al. first found and described the exacerbation of AD pathology by 6 h acute sleep deprivation17. Thereafter, many other studies reported that sleep deprivation or fragmentation could aggravate pathogenesis in transgenic AD mice models18,19,20. However, very few researchers have studied the consequence of sleep disturbance in young wild type mice; that is, whether sleep disturbance gives rise to AD-like behavior or pathological changes in young wild-type mice. In our recent publication, we reported that 2 months of sleep fragmentation induced evident spatial memory deficit and anxiety-like behavior, as well as increased intracellular Amyloid-β (Aβ) accumulation both in cortex and hippocampus in 2–3 month-old wild-type mice21. We also observed altered expression levels of endosome-autophagosome-lysosome pathway markers and microglia activation, which was similar to the pathological changes reported in APP/PS1 mice21,22.
This presented sleep fragmentation (SF) protocol was validated by Sinton et al.23 and modified by Li et al.24. In brief, an orbital rotor vibrating at 110 rpm interrupts sleep for 10 s every 2 min during light-ON phase (8:00 AM–8:00 PM). Sleep structure alteration in this model was previously characterized with electrophysiological sleep recordings and reported by Li et al.24, indicating a significant increase in the wake time and decrease in rapid eye movement (REM) sleep during the light-ON phase, with the total sleep and wake times (in 24 hour) unaffected after more than 4 weeks’ modeling24. Currently, total sleep or partial sleep deprivation are the most commonly used sleep manipulation models. Total sleep deprivation is usually performed by sustained gentle handling or exposing the animal to novel objects, alternatively by continuously rotating a bar or a running treadmill25,26,27,28,29. Due to ethical reasons, total sleep deprivation is usually shorter than 24 h. The most commonly applied partial sleep deprivation model is the water platform method, which primarily ablating REM sleep30,31,32. Other approaches using either a treadmill or a bar that sweeps along the bottom of the cage, could induce sleep fragmentation when set on at fixed intervals33,34,35,36,37,38. It is noteworthy that SF interrupts sleep and intermittently causes arousals across all sleep stages24. One of the prominent advantages of this CSF model applying orbital rotor is that it can be performed continuously for months automatically controlled by machines, which avoids frequent processing labor daily except for regular monitoring. Furthermore, the apparatus would allow to simultaneously model multiple cages of mice under uniformed interventions. During entire modeling sessions, mice are housed in their home cages with usual bedding and nesting materials, while some other methods require exposure to diversified environments and inevitable stress.
Sleep fragmentation was previously characterized by the sleep manipulation method, which mimics frequent arousals during the sleep phase and substantial sleep rebound during the wake phase. In some literatures, CSF was regarded as the animal model for OSA39,40. In this study, the rationale of the chosen frequency of arousal to be 30 times per hour is based on the observation of arousal indices in patients with moderate-to-severe sleep apnea. It was observed that 4 weeks’ sleep fragmentation significantly increased hypercapnic arousal latency and the tactile arousal threshold, which could at least last 2 weeks after recovery24. This phenotype was explained by revealing c-fos activation reduction in noradrenergic, orexinergic, histaminergic, and cholinergic wake-active neurons in response to hypercapnia, as well as reduced catecholaminergic and orexinergic projections into the cingulate cortex24. However, it is necessary to note that the most important feature in OSA is hypoxia caused by airway obstruction, which results in sleep disruption41,42. Sleep disturbance and repetitive hypoxia reciprocally interact with each other in OSA pathogenesis. Therefore, sleep fragmentation alone might not be able to fully demonstrate all key features of OSA in mice.
Herein, we present a standardized protocol to model chronic sleep fragmentation in young wild-type mice. Cognitive deficit and anxiety-like as well as depression-like behaviors after CSF treatment were evaluated by Morris water maze, Novel object recognition, Open field test, and Forced swimming test. It is important to note that this model should be taken as a whole that generates phenotypes of dysregulated sleep pattern, cognitive deficit, and anxiety-like behavior. The current model could potentially be applied, but not be limited, to the following purposes: 1) Further investigating the functional or molecular pathogenesis mechanisms induced by chronic sleep disturbance in young mice without genetic predisposition, 2) Identifying the direct pathway leading to neurodegeneration initiated by sleep disturbance, 3) Exploring the therapeutics for improving phenotypes induced by chronic sleep disturbance, 4) Studying the intrinsic protective/compensatory mechanisms in wild-type mice upon chronic sleep disturbance, 5) To be applied for studying sleep-wake regulation and state-transition mechanisms.
Protocol
This protocol was approved by the Institutional Animal Care and Use Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
1. Mice screening and preparation for the experiment
- Select wild-type adult (8–10 weeks old) male mice with weight of 20–28 g for the whole experiment.
NOTE: Wild-type C57BL/6 mice are obtained from the Hubei Research Center for Laboratory Animals, Hubei, China. - Randomly assign all mice to the CSF and the control group. House 3–5 mice in each cage to avoid social isolation stress. The number of mice housed in the control cages is matched with that housed in the paired CSF cages.
NOTE: Mice in the same group cages are pooled to perform follow-up behavioral experiments. - Locate the control cages in the same room with the CSF cages, to keep the surrounding environment and labor effects identical.
- Number and mark the mice in each group on their ears using an ear tag for monitoring purposes.
- Maintain ambient temperature and humidity between 21–23 °C and 35%–60 %.
- Maintain the ambient environment in 12-hour light-dark cycle (8:00 AM–8:00 PM light-ON, 8:00 PM–8:00 AM light-OFF), to avoid biased effect on normal sleep rhythm in mice.
- Minimize the noise and interference while the researcher is present in the modeling room.
- Provide mice with sufficient food and water. Use long nozzles with ball valve tips on water bottles, to prevent water leakage upon the platform movements. Fasten the water bottle on top of the cage with a spring to avoid the dislocation of the bottle during rotor running.
2. Preparation and setting of the orbital rotor
- Prepare an electrically controlled orbital rotor with enlarged platform (67 cm x 110 cm), on which 10 cages can be placed at most.
- Set the orbital rotor on during light-ON phase (8:00 AM–8:00 PM) controlled by a program timer, which is the time when mice exhibit the majority of their daily sleep.
- Set the orbital rotor with a speed of 110 rpm and a repetitive cycle of 10 s-on, 110 s-off controlled with a solid-state timer.
NOTE: The load capacity of the platform is 50 kg. The fixed amplitude of the rotor horizon vibrating is 2.5 cm. - Fasten the CSF cages on top of the rotor platform by thick springs to prevent dislocation of cages upon platform rotations.
3. Chronic sleep fragmentation modeling and monitoring
- Place cages of the CSF and the control mice into the modeling room for one week prior to experiments, to let mice adapt to the ambient environment.
- At the beginning of the modeling, ensure that all mice have free access to food and water during orbital rotations.
- At the beginning of the modeling, observe at least for 1 h to ensure the orbital rotor operating in gear.
- During the period of modeling, check that the orbital rotor is operating properly and mice conditions every 2 days to ensure mice have enough food and water. Change beddings of cages weekly.
- During the period of modeling, weigh the mice weekly at 8:00 AM when changing the bedding. Remove the mice with significant weight loss from the modeling, and also from the experimental groups.
NOTE: Significant weight loss is defined as weighing less than 20 g lasting for 2 weeks. - During the entire modeling sessions, remove the aggressor, if any, from the cage and, also from the experimental groups.
- After the termination of modeling, continue to maintain and feed the mice in the original room.
4. Morris water maze (MWM) test
- Preparation for the test
- Prepare the apparatus of a circular tank filled with warm water (20–23 °C).
- Suspend four signs with different shapes and colors on the curtain surrounding the tank in four quadrant directions as the distant vision reference. Make the water to appear opaque by the addition of powdered milk.
- Locate a platform in the middle of the southwest quadrant.
- The training test
- Subject mice to four consecutive trials between 8:00 AM and 12:00 AM each day over a 5-day training period.
- Release each mouse into the water facing the sidewall at one of four quadrants in four trials. In each trial, allow the mouse to swim for 60 s to find the platform. If the mouse is unable to arrive at the platform within 60 s, guide it to the platform and to remain there for 15 s.
- Use a video tracking system to automatically record the escape latency of mice to find the hidden platform.
- The probe test
- Conduct the probe test on the sixth day after 5 training days.
- Remove the platform. Release each mouse from the northeast quadrant and allow it to swim for 60 s
- Use a video tracking system to automatically record the track data of mice.
5. Novel object recognition (NOR) test
- The familiar phase
- Place mice in a tank (length 30 cm, width 28 cm, height 35 cm) in sequence, which contains two copies of objects (A1 and A2). Allow the mice to explore freely (10 min per trial).
- Use a video tracking system to automatically record the track data of mice.
- The test phase
- Conduct the test trial after a 1 h delay of the familiar phase. Replace one of the original objects by a novel object (“novel”) in the tank keeping the other one unchanged. Return the mice to the tank and allow it to explore for 5 min per trial.
- Use a video tracking system to automatically record the time spent in exploration of each object by each mouse.
NOTE: The exploration of the object is determined by licking, sniffing, chewing, or moving vibrissae while orienting the nose toward and less than 1 cm from the object. The Discrimination Index (DI) is calculated with the equation (TN − TF)/(TN + TF), where TN = time spent exploring the “novel” object and TF = time spent exploring the “familiar” object.
6. Open field test (OFT)
- Prepare the apparatus of a tank (30 cm x 28 cm x 35 cm).
- During the test, place each mouse into the center of the tank and allow it to explore freely for 5 min. Clean the tank with 75% ethanol after each trial to avoid the leftover effects of the previous mouse.
- Use a video tracking system to automatically record the track data of mice.
7. Forced swimming test (FST)
- Prepare the apparatus of an open cylindrical vessel, which contains water (20–23 °C) that is 15 cm deep.
- During the test, place each mouse into the cylinder and allow it to remain there for 6 min.
- Use a video track system to automatically record the immobility time during the last 4 min of the test by each mouse.
NOTE: The mouse is determined to be immobile when it stops struggling and floats in the water, making only movements which are necessary to keep its head above water.
8. Data Analysis
- Analyze data using statistical analysis software (e.g., GraphPad Prism 6.0).
- Express all data as the mean ± SEM.
- Compare the escape latency in MWM test between two groups using two-way ANOVA with repeated measures followed by Bonferroni posttests. Other comparisons between the CSF and the control groups are determined by unpaired t tests.
- Consider differences significant if P < 0.05 in all tests.
Representative Results
All the representative results and figures were reproduced from our recent publication21. The reuse of the figures was permitted by the original journal.
The entire experimental design is illustrated in the order of time, which indicates the timing of CSF modeling, behavioral tests of MWM, NOR, OFT, and FST (Figure 1A). We obtained weights of mice every week from the CSF and the control groups, to monitor their general conditions during the modeling sessions. No evident difference was found in the weight increase in mice between two groups during the modeling (Figure 1B).
To evaluate the effects of CSF on spatial learning and memory performance, we conducted MWM behavioral trial43,44. The CSF group displayed poorer escape capacities to find the platform throughout 5 training days in comparison with the control group (Figure 2A). In the probe test, the CSF mice spent significantly less time proportion in the targeted quadrant and crossed the previous platform location by fewer times (Figure 2B,C), without swimming speed difference (Figure 2D). These above results indicated that the spatial learning and memory retrieval capabilities of mice were impaired after CSF.
We also conducted NOR test to assess object recognition and short-term working memory after CSF45. In the familiar phase, there was no significant difference in the total exploration time between the CSF and the control group (Figure 3A). Correspondingly, no differences were found in the exploration time between objects A1 and A2, respectively in two groups (Figure 3B). The above results guaranteed that there were no differences in the mice’s abilities for exploration and preferences for location. In the test phase, the Discrimination Index (DI) of the CSF mice was significantly reduced versus controls (Figure 3C), which evidently indicated deficits in object recognition and short-term working memory after CSF.
We further performed OFT and FST, respectively to examine anxiety-like and depression-like behaviors of mice46,47. Interestingly, in the OFT, it was found that the CSF group spent less time in the central zone than the control group (Figure 4A), which illustrated that sleep fragmentation could induce anxiety-like behavior to a certain extent. Additionally, CSF mice exhibited longer total distance moved in the tank (Figure 4B), suggesting increased spontaneous activity after modeling. Nevertheless, this CSF modeling could not induce depression-like behavior, verified by non-significant difference in the immobility time between two groups subjected to the FST (Figure 4C).
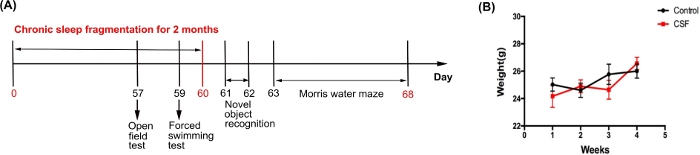
Figure 1: The flowchart of experimental design procedure. (A) The experimental design procedure indicating the timing of CSF modeling and behavioral tests (i.e., MWM, NOR, OFT, and FST). (B) Body weight curves of the CSF and the control mice during the first month after the CSF model was established. This figure has been modified from Xie et al.21 Please click here to view a larger version of this figure.
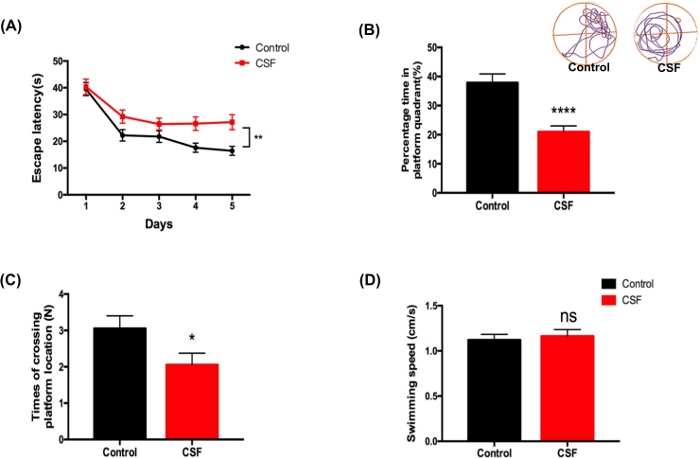
Figure 2: CSF impaired spatial learning and memory abilities evaluated by MWM test. (A) The CSF mice performed longer escape latency compared to the control mice during the 5-day training test. **p < 0.01. (B) In the probe test, the CSF mice exhibited less percentage time spent in the platform quadrant in contrast with the control mice. Upper panel shows representative tracings of two groups. ****p < 0.0001. (C) In the probe test, the CSF group performed less times of crossing the platform location when compared to the control group. *p < 0.05. (D) The swimming speed of two groups in the probe test. n.s. indicates changes between different groups were not significant. Data were all presented as mean ± SEM. n = 10 per group. This figure has been modified from Xie et al.21 Please click here to view a larger version of this figure.
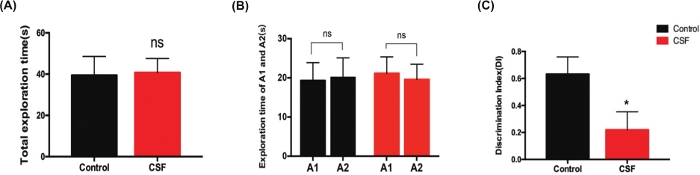
Figure 3: CSF impaired object recognition and short time working memory evaluated by NOR test. (A) The total exploration time between the CSF and the control mice in the familiar phase, n.s. indicates no significant changes between different groups. (B) The exploration time for objects A1 and A2 respectively between two groups in the familiar phase. n.s. indicates no significant changes between different groups. (C) In the test phase, the Discrimination Index (DI) of the CSF group was significantly decreased compared to that of the control group. *p < 0.05. Data were all presented as mean ± SEM. n = 10 per group. This figure has been modified from Xie et al.21 Please click here to view a larger version of this figure.

Figure 4: CSF exacerbated anxiety-like but not depression-like behavior evaluated by OFT and FST. (A) The CSF mice spent less time in the central zone during the observed 5 min compared with the control mice in OFT. *p < 0.05. (B) The CSF group displayed longer total distance moved in the tank versus the control group in OFT. *p < 0.05. (C) The immobility time between the CSF and the control groups in FST. n.s. indicates no significant changes between different groups. Data were all presented as mean ± SEM. n = 10 per group. This figure has been modified from Xie et al.21 Please click here to view a larger version of this figure.
Discussion
Critical steps in the current protocol include setting up sleep fragmentation machines with the optimized parameters according to the study purpose and maintaining the mice in comfortable and quiet living environment throughout the entire modeling sessions. It is also crucial to decide the proper timing to interrupt or stop sleep fragmentation and arrange behavioral tests for those mice. Like other sleep manipulation models, it is important to perform the protocol in a dedicated room with controlled light cycles and void of all possible unnecessary interferences. Efforts should be taken to avoid inducing noises and minimize the operating time conducted by the researchers for checking, refilling food, and water supply, changing the beddings, etc. In rare occasions, there are aggressors attacking the littermates, especially at the initiation of uncomfortable sleep disruption sessions. The aggressor when present should be removed out of the home cages as well as the experimental groups. Most of the experimental animals except for a few to our experience, would adapt to the treatment and manage to access the water and food as needed. Mice with intrinsic problems, such as deformed teeth, underweight and skin wounds might cause weight loss or weakness. They also need to be avoided being used for the modeling. As this protocol could potentially induce chronic stress and metabolic dysregulation, it is essential to use mice screened with uniformed criteria, such as body weight, for modeling and experiments.
In the described protocol, the orbital rotor would be automatically turned on during 8:00 AM–8:00 PM (light-ON) daily, which is the time when mice exhibit most of their daily life. The rotor was set running on a repetitive cycle of 10 s-on, 110 s-off during light-ON phase to induce frequent arousals. Various modeling durations would give rise to different phenotypes. Acute sleep fragmentation could result in absolute reduction in sleep duration, increased sympathetic nervous system activities, such as elevated cortisone levels and impaired insulin sensitivity23,24. However, chronic sleep fragmentation showed unaffected cortisone levels, and balanced total sleep time24. Any modifications based of the current protocol, such as light cycles, matched vibrating settings (speed, amplitude, repetitive cycle, etc.) and modeling durations, could potentially alter the phenotypes. It is required to conduct sleep recording and sleep structure analysis under different modeling settings to identify the sleep phenotypes. It might also result in distinctive behavioral and pathological changes. As we explored the cognitive deficit after long-term rather than one-night sleep fragmentation and tended to avoid the biased effects of intermittent sleep fragmentation on mice behaviors in MWM and NOR, we performed these two behavioral tests after terminating the CSF protocol on day 60. However, inevitably, the effect of recovery sleep in mice might have confounded the results for MWM and NOR shown.
Although this model is entitled with sleep fragmentation model, it is actually composed of fragmented sleep patterns during the light-ON phase, dysregulation of circadian rhythm, and compensatory sleep rebound during the light-OFF phase. This protocol could induce not only sleep pattern alterations, but also substantial neuroinflammation, metabolic imbalance, immune system disturbance, etc21,23,24. All these pathological processes may interact with each other and mediate phenotypes like an orchestra. This model should be taken as a whole to generate the mice with phenotypes of dysregulated sleep pattern, cognitive deficit, and anxiety-like behavior in young wild-type mice. As mentioned in the previous section, this model is not exactly mirroring OSA due to lack of repetitive hypoxia. Another limitation is that it is difficult to generate accurate pathological changes and sleep phenotypes in the same mice. The widely applied EEG/EMG electrode implantation for sleep recording unavoidably induced severe gliosis in the cortex48. In recent years, video monitoring and image analysis techniques based on artificial intelligence were applied in sleep studies, which would collect precise sleep information without invasive electrode implantation49,50,51.
The significances of this CSF method in comparison with existing methods include: 1) Different from sleep deprivation protocols that usually are performed for hours or days, the current protocol better mimics long-term sleep disturbance in healthy human beings. The compensatory sleep rebound in sleep fragmented mice perfectly mirrors the daytime somnolence and retardant working performance in people with poor sleep quality during the night52,53. 2) It is so far the only chronic sleep fragmentation model in young wild-type mice with confirmed cognitive deficit and anxiety-like but not depression-like behavior phenotypes, as well as evident molecular pathological changes in brain tissue. 3) This treatment causes milder irritations to mice so that the modeling could last for months, even with the possibility to be performed in longer periods of time. 4) With proper settings, this model can generate stable phenotypes of sleep disturbance, cognitive deficit, and anxiety-like behavior, which can be used either as disease models or interventions for different study designs. 5) Some sleep deprivation models require full session interference by researchers to apply gentle handling or novel objects. Except for regular monitoring, this method minimizes handling labors, which also eliminates the artificial bias.
This CSF protocol provides the opportunity to answer a number of key scientific questions, such as, is chronic sleep disturbance the cause or consequence of neurodegenerative diseases? Is chronic sleep disturbance induced pathogenesis during young age reversible? Do the compensatory mechanisms upon chronic sleep disturbance vary between the young and elder people, healthy people, and patients? This protocol can also be applied to explore therapeutics by assessing the severity and improvement of the behavioral and molecular phenotypes. It would also be applied to model the mice with chronic craniectomy, optic fiber implantation preparations for functional recordings. Moreover, it can possibly be used as interventional strategy to induce or aggravate phenotypes on top of pre-existing conditions. Finally, it can be used for studying sleep-wake state transitional mechanisms. Interestingly, the current CSF model could induce anxiety-like rather than depression-like behavior in mice, which is in line with the clinical observation that the sleep disturbance in patients would likely be associated much more with anxiety than with depression54,55. It provides a practical model to study emotional disorders in rodents.
In summary, we present the protocol of modeling chronic sleep fragmentation by use of a vibrating orbital rotor, which could produce stable phenotypes in young wild-type mice and minimize the modeling labors with high efficiency. It can be potentially generated for a variety of research purposes.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (61327902-6 to W. Wang and 81801318 to F.F. Ding). We acknowledge Dr. Sigrid Veasy for establishing the SF experimental system and kindly providing technical details. We acknowledge Dr. Maiken Nedergaard for instructive comments for related experiments.
Materials
| Any-maze behavior tracking system | Stoelting,Inc,USA | – | A video-tracking system which was used to record the behavior track of mice. |
| C57BL/6J mice | Hubei Research Center for Laboratory Animals, Hubei, China. | – | healthy male C57BL/6J mice aged 10-12 weeks were purchased from Hubei Research Center for Laboratory Animals |
| Graphpad Prism 6.0 Software | Graphpad Software,Inc.USA | – | Graphpad Prism 6.0 software was used to draw statistical graphs. |
| Morris water maze system | Shanghai XinRuan Information Technology Co.,Ltd,China | XR-XM101 | The system was used to perform Morris water maze test |
| Orbial rotor | Shanghai ShiPing Laboratory Equipment Co.,Ltd,China | SPH-331 | The orbital rotor was used to establish the chronic sleep fragmentation model |
| Solid state timer | OMRON Corporation, Kyoto, Japan | H3CR-F8-300 | The solid state time was used to control the frequency and time of the rotor running |
| Wooden Lusterless Tank | – | – | length 30 cm, width 28 cm, height 35 cm The tank was used to perform open field test and novel object recognition test |
Referencias
- Peter-Derex, L., Yammine, P., Bastuji, H., Croisile, B. Sleep and Alzheimer’s disease. Sleep Medicine Reviews. 19, 29-38 (2015).
- Mathias, J. L., Cant, M. L., Burke, A. L. J. Sleep disturbances and sleep disorders in adults living with chronic pain: a meta-analysis. Sleep Medicine. 52, 198-210 (2018).
- Murphy, M. J., Peterson, M. J. Sleep Disturbances in Depression. Sleep Medicine Clinics. 10 (1), 17-23 (2015).
- Walter, L. M., et al. Sleep disturbance in pre-school children with obstructive sleep apnoea syndrome. Sleep Medicine. 12 (9), 880-886 (2011).
- Helfand, B. T., et al. The relationship between lower urinary tract symptom severity and sleep disturbance in the CAMUS trial. Journal of Urology. 185 (6), 2223-2228 (2011).
- Kimoff, R. J. Sleep fragmentation in obstructive sleep apnea. Sleep. 19 (9), 61-66 (1996).
- Dhondt, K., et al. Sleep fragmentation and periodic limb movements in children with monosymptomatic nocturnal enuresis and polyuria. Pediatric Nephrology. 30 (7), 1157-1162 (2015).
- Young, T., Peppard, P. E., Gottlieb, D. J. Epidemiology of obstructive sleep apnea: a population health perspective. American Journal of Respiratory and Critical Care Medicine. 165 (9), 1217-1239 (2002).
- Peppard, P. E., et al. Increased prevalence of sleep-disordered breathing in adults. American Journal of Epidemiology. 177 (9), 1006-1014 (2013).
- Marcus, C. L., et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 130 (3), 714-755 (2012).
- Xie, L., et al. Sleep drives metabolite clearance from the adult brain. Science. 342 (6156), 373-377 (2013).
- Benveniste, H., et al. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology. 65 (2), 106-119 (2019).
- Stickgold, R., Walker, M. P. Memory consolidation and reconsolidation: what is the role of sleep. Trends in Neurosciences. 28 (8), 408-415 (2005).
- Stickgold, R. Sleep-dependent memory consolidation. Nature. 437 (7063), 1272-1278 (2005).
- Aalling, N. N., Nedergaard, M., DiNuzzo, M. Cerebral Metabolic Changes During Sleep. Current Neurology and Neuroscience Reports. 18 (9), 57 (2018).
- Rempe, M. J., Wisor, J. P. Cerebral lactate dynamics across sleep/wake cycles. Frontiers in Computational Neuroscience. 8, 174 (2014).
- Kang, J. E., et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 326 (5955), 1005-1007 (2009).
- Minakawa, E. N., et al. Chronic sleep fragmentation exacerbates amyloid beta deposition in Alzheimer’s disease model mice. Neuroscience Letters. 653, 362-369 (2017).
- Qiu, H., et al. Chronic Sleep Deprivation Exacerbates Learning-Memory Disability and Alzheimer’s Disease-Like Pathologies in AβPP(swe)/PS1(ΔE9) Mice. Journal of Alzheimer’s Disease : JAD. 50 (3), 669-685 (2016).
- Holth, J. K., et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 363 (6429), 880-884 (2019).
- Xie, Y., et al. Chronic sleep fragmentation shares similar pathogenesis with neurodegenerative diseases: Endosome-autophagosome-lysosome pathway dysfunction and microglia-mediated neuroinflammation. CNS Neuroscience & Therapeutics. 26 (2), 215-227 (2020).
- Ba, L., et al. Distinct Rab7-related Endosomal-Autophagic-Lysosomal Dysregulation Observed in Cortex and Hippocampus in APPswe/PSEN1dE9 Mouse Model of Alzheimer’s Disease. Chinese Medical Journal (England). 130 (24), 2941-2950 (2017).
- Sinton, C. M., Kovakkattu, D., Friese, R. S. Validation of a novel method to interrupt sleep in the mouse. Journal of Neuroscience Methods. 184 (1), 71-78 (2009).
- Li, Y., et al. Effects of chronic sleep fragmentation on wake-active neurons and the hypercapnic arousal response. Sleep. 37 (1), 51-64 (2014).
- Misrani, A., et al. Differential effects of citalopram on sleep-deprivation-induced depressive-like behavior and memory impairments in mice. Progress Neuro-psychopharmacology & Biological Psychiatry. 88, 102-111 (2019).
- Xu, A., et al. Roles of hypothalamic subgroup histamine and orexin neurons on behavioral responses to sleep deprivation induced by the treadmill method in adolescent rats. Journal of Pharmacological Sciences. 114 (4), 444-453 (2010).
- Saito, L. P., et al. Acute total sleep deprivation potentiates amphetamine-induced locomotor-stimulant effects and behavioral sensitization in mice. Pharmacology, Biochemistry, and Behavior. 117, 7-16 (2014).
- Spano, G. M., et al. Sleep Deprivation by Exposure to Novel Objects Increases Synapse Density and Axon-Spine Interface in the Hippocampal CA1 Region of Adolescent Mice. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 39 (34), 6613-6625 (2019).
- Morrow, J. D., Opp, M. R. Sleep-wake behavior and responses of interleukin-6-deficient mice to sleep deprivation. Brain, Behavior, and Immunity. 19 (1), 28-39 (2005).
- Arthaud, S., et al. Paradoxical (REM) sleep deprivation in mice using the small-platforms-over-water method: polysomnographic analyses and melanin-concentrating hormone and hypocretin/orexin neuronal activation before, during and after deprivation. Journal of Sleep Research. 24 (3), 309-319 (2015).
- Aleisa, A. M., Alzoubi, K. H., Alkadhi, K. A. Post-learning REM sleep deprivation impairs long-term memory: reversal by acute nicotine treatment. Neuroscience Letters. 499 (1), 28-31 (2011).
- Zagaar, M., Dao, A., Alhaider, I., Alkadhi, K. Regular treadmill exercise prevents sleep deprivation-induced disruption of synaptic plasticity and associated signaling cascade in the dentate gyrus. Molecular and Cellular Neurosciences. 56, 375-383 (2013).
- McKenna, J. T., et al. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neurociencias. 146 (4), 1462-1473 (2007).
- Tartar, J. L., et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. The European Journal of Neuroscience. 23 (10), 2739-2748 (2006).
- Guzman-Marin, R., Bashir, T., Suntsova, N., Szymusiak, R., McGinty, D. Hippocampal neurogenesis is reduced by sleep fragmentation in the adult rat. Neurociencias. 148 (1), 325-333 (2007).
- Nair, D., et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. American Journal of Respiratory and Critical Care Medicine. 184 (11), 1305-1312 (2011).
- McCoy, J. G., et al. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 30 (1), 52-60 (2007).
- Dumaine, J. E., Ashley, N. T. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 308 (12), 1062-1069 (2015).
- Carreras, A., et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep. 37 (11), 1817-1824 (2014).
- Khalyfa, A., et al. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget. 7 (34), 54676-54690 (2016).
- Ferreira, C. B., Cravo, S. L., Stocker, S. D. Airway obstruction produces widespread sympathoexcitation: role of hypoxia, carotid chemoreceptors, and NTS neurotransmission. Physiological Reports. 6 (3), (2018).
- Tripathi, A., et al. Intermittent Hypoxia and Hypercapnia, a Hallmark of Obstructive Sleep Apnea, Alters the Gut Microbiome and Metabolome. mSystems. 3 (3), (2018).
- D’Hooge, R., De Deyn, P. P. Applications of the Morris water maze in the study of learning and memory. Brain Research. Brain Research Reviews. 36 (1), 60-90 (2001).
- Vorhees, C. V., Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 1 (2), 848-858 (2006).
- Vogel-Ciernia, A., Wood, M. A. Examining object location and object recognition memory in mice. Current Protocols in Neuroscience. 69, 1-17 (2014).
- Kraeuter, A. K., Guest, P. C., Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods in Molecular Biology. 1916, 99-103 (2019).
- Porsolt, R. D., Bertin, A., Blavet, N., Deniel, M., Jalfre, M. Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. European Journal of Pharmacology. 57 (2-3), 201-210 (1979).
- Hauglund, N. L., Kusk, P., Kornum, B. R., Nedergaard, M. Meningeal Lymphangiogenesis and Enhanced Glymphatic Activity in Mice with Chronically Implanted EEG Electrodes. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 40 (11), 2371-2380 (2020).
- Nguyen-Michel, V. H., et al. Rapid eye movement sleep behavior disorder or epileptic seizure during sleep? A video analysis of motor events. Seizure. 58, 1-5 (2018).
- Zimmerman, J. E., Raizen, D. M., Maycock, M. H., Maislin, G., Pack, A. I. A video method to study Drosophila sleep. Sleep. 31 (11), 1587-1598 (2008).
- Abad, J., et al. Automatic Video Analysis for Obstructive Sleep Apnea Diagnosis. Sleep. 39 (8), 1507-1515 (2016).
- Sandlund, C., Hetta, J., Nilsson, G. H., Ekstedt, M., Westman, J. Impact of group treatment for insomnia on daytime symptomatology: Analyses from a randomized controlled trial in primary care. International Journal of Nursing Studies. 85, 126-135 (2018).
- Shekleton, J. A., Rogers, N. L., Rajaratnam, S. M. Searching for the daytime impairments of primary insomnia. Sleep Medicine Reviews. 14 (1), 47-60 (2010).
- Dixon, L. J., Lee, A. A., Gratz, K. L., Tull, M. T. Anxiety sensitivity and sleep disturbance: Investigating associations among patients with co-occurring anxiety and substance use disorders. Journal of Anxiety Disorders. 53, 9-15 (2018).
- Press, Y., Punchik, B., Freud, T. The association between subjectively impaired sleep and symptoms of depression and anxiety in a frail elderly population. Aging Clinical and Experimental Research. 30 (7), 755-765 (2018).

