Expanding the Comprehension of the Tumor Microenvironment using Mass Spectrometry Imaging of Formalin-Fixed and Paraffin-Embedded Tissue Samples
Summary
In the era of cancer immunotherapy, interest in elucidating tumor microenvironment dynamics has increased strikingly. This protocol details a mass spectrometry imaging technique with respect to its staining and imaging steps, which allow for highly multiplexed spatial analysis.
Abstract
Advances in immune-based therapies have revolutionized cancer treatment and research. This has triggered growing demand for the characterization of the tumor immune landscape. Although standard immunohistochemistry is suitable for studying tissue architecture, it is limited to the analysis of a small number of markers. Conversely, techniques such as flow cytometry can evaluate multiple markers simultaneously, although information about tissue morphology is lost. In recent years, multiplexed strategies that integrate phenotypic and spatial analysis have emerged as comprehensive approaches to the characterization of the tumor immune landscape. Herein, we discuss an innovative technology combining metal-labeled antibodies and secondary ion mass spectrometry focusing on the technical steps in assay development and optimization, tissue preparation, and image acquisition and processing. Before staining, a metal-labeled antibody panel must be developed and optimized. This hi-plex image system supports up to 40 metal-tagged antibodies in a single tissue section. Of note, the risk of signal interference increases with the number of markers included in the panel. After panel design, particular attention should be given to the metal isotope assignment to the antibody to minimize this interference. Preliminary panel testing is performed using a small subset of antibodies and subsequent testing of the entire panel in control tissues. Formalin-fixed, paraffin-embedded tissue sections are obtained and mounted on gold-coated slides and further stained. The staining takes 2 days and closely resembles standard immunohistochemical staining. Once samples are stained, they are placed in the image acquisition instrument. Fields of view are selected, and images are acquired, uploaded, and stored. The final stage is image preparation for the filtering and removal of interference using the system's image processing software. A disadvantage of this platform is the lack of analytical software. However, the images generated are supported by different computational pathology software.
Introduction
The importance of the numerous cell types surrounding clonal tumor populations is a crucial element in the categorization of carcinogenesis. Interest in elucidating this tumor microenvironment (TME) composition and interactions has risen continuously following the establishment of immune-based therapy as part of the cancer treatment arsenal. Therefore, treatment strategies have shifted from a tumor-centric approach to a TME-centric one1.
Efforts to elucidate the roles of immune cells in tumor surveillance and cancer development have increased strikingly in recent years2,3. In medical research, a plethora of methods, including cytometry-based methods and singleplex and multiplex imaging technologies, arose as part of this attempt to decipher the unique interactions of multiple elements of TMEs.
Pioneering methods such as flow cytometry (invented in the 1960s), fluorescence-activated cell sorting, and mass cytometry are focused mainly on identifying and quantifying TME components4. Even though cytometry-based quantitative techniques allow for immune landscape phenotyping, determining the cellular spatial distribution is impossible. Conversely, methods such as standard singleplex immunohistochemistry preserve the tissue architecture and enable researchers to analyze cellular distribution, though a reduced number of targets in a single tissue section is a limitation of these methods5,6. Over the past several years, multiplexed imaging technologies for single-cell resolution such as multiplex immunofluorescence, barcoding fluorescence imaging, and imaging mass spectrometry have emerged as comprehensive strategies for acquiring information on simultaneous marker staining using the same tissue section7.
Here we present a technology that couples metal tagged antibodies and secondary ion mass spectrometry and enables single-cell resolution quantification, marker co-expression (phenotyping), and spatial analysis using formalin-fixed, paraffin-embedded (FFPE), and fresh frozen (FF) tissue samples8,9. FFPE samples are the most widely used materials for tissue archiving samples and represent a more readily available resource for multiplexed imaging technologies than fresh frozen samples10. Additionally, this technology offers the possibility of reacquiring images months after. Herein, we discuss our staining and image processing protocols using FFPE tissue samples.
Protocol
Tissue samples were obtained for research purposes in accordance with the Institutional Review Board of The University of Texas MD Anderson Cancer Center, and samples were further de-identified.
1. Antibody selection
- Define the research questions to choose the best available antibody clones. Use the Human Protein Atlas, published research, and antibody manufacturer websites as research resources.
NOTE: The selection of adequate human tissue controls and the same human tissue controls to be stained in the different steps is critical to make sure that the markers are stained correctly, in the right place, and in the correct cellular compartment. A small tissue microarray (TMA) including normal tissue and neoplastic tissues is always recommended for staining validation. - Use only carrier-free antibodies to design the panel.
NOTE: The use of carrier-free antibody formulations is required if a commercial carrier-free antibody is not available; purification kits can be used to adjust the antibody to the correct formulation. Protein additives such as bovine serum albumin are known to diminish conjugation capacity. - Test and titrate each antibody using standard chromogenic immunohistochemistry to determine the subcellular pattern of a marker's expression; membrane, cytoplasmic, or nuclear staining; and expression in specific cells in control tissues.
NOTE: However, each antibody must be tested in pH 6 citrate and pH 9 ethylenediaminetetraacetic acid (EDTA), and whether the panel will be stained with pH 6 citrate or pH 9 EDTA must be determined. The use of pH 9 EDTA is recommended to obtain good signals, especially when working with this methodology. - Choose the optimal staining concentration according to the pattern of expression in positive controls.
- Assign each antibody to one of the available metal isotopes according to the immunohistochemical marker abundance, subcellular localization, and cellular co-expression with the other targets.
- Stain the antibody with the corresponding assigned metal and compare with the expression in the same control tissue used previously.
NOTE: Antibody metal conjugation starts with antibody preparation, reduction, and subsequent conjugation (Supplementary File 1). Each metal-loaded polymer tube is sufficient for labeling 100 µg of an antibody.
2. Antibody panel design
- Create small batches of antibody staining. Test up to five markers in a cocktail.
NOTE: Testing antibodies already conjugated and analyzed using immunohistochemistry in batches facilitates the early identification of channel interferences and provides the opportunity to reconjugate the antibodies with another metal. It also provides an opportunity to evaluate adequate marker co-expression. - Stain each small batch with four different antibody concentrations (0.05 µg/mL, 0.2 µg/mL, 1 µg/mL, and 4.0 µg/mL).
NOTE: Comparing different titers, identify the antibody concentration that results in the correct location and highest expression with minimum background staining (best signal-to-noise ratio).
3. FFPE tissue sectioning
- Select gold-coated slides (specific for this purpose) and keep them close to the microtome. Prepare the microtome by inserting a new blade into the holder. Set the section thickness to 4-5 μm.
- Put FFPE blocks on ice before sectioning. Prepare a tissue floatation bath by heating distilled water or deionized water (diH2O) to 40-45 °C.
NOTE: Using diH2O or distilled water reduces the possibility of contamination. - Start sectioning. Place the tissue section in the water using tweezers and let it flatten. Use the slides to remove tissue sections from the water.
- Place the slides in a slide rack and let them dry at room temperature overnight.
4. FFPE antibody staining
NOTE: The staining process takes place on two separate days.
CAUTION: The solutions employed in this protocol are potentially corrosive and represent hazards to the skin and eyes. Wearing gloves, a lab coat, and protective eye and face equipment is advised and part of our institution's biosafety policy.
- Reagent preparation (day 1)
- Dilute 50 mL of 20x Tris-buffered saline with Tween (TBS-T) into 950 mL of diH2O to make 1 L of TBS-T.
- Dilute 8 mL of 10x Tris-ethylenediaminetetraacetic acid (EDTA) into 72 mL of diH2O to make a 1x heat-induced epitope retrieval (HIER) solution.
- Dilute donkey serum in 1x TBS-T to a final concentration of 5% to make blocking buffer. Store the solution at 4 °C until ready to use.
- HIER preparation: Pretreatment (PT) module
CAUTION: Wear chemical protective gloves when handling any reagents or parts immersed in any reagents used in the PT module.- Dilute 140 mL of 10x phosphate-buffered saline (PBS) into 1,260 mL of diH2O to make 1.4 L of 1x PBS. Alternatively, dilute seven PBS tablets into 1.4 L of diH2O.
- Fill a tank with 1.4 L of PBS and place the prepared slide chamber container with 100 mL of 1x HIER solution into the tank.
- Preheat the tank in a PT module to 75 °C.
- Excess paraffin removal
- Bake slides at 70 °C in an oven for at least 20 min.
NOTE: Some tissues or sections may need longer baking times. The recommended baking time for brain tissue or a TMA is 1 h. This can be extended to 16 h (overnight) or according to lab experience. - Place baked slides in a slide holder and perform the following washing steps on a shaker under a fume hood. Wash the slides in the following solution: Xylene 2x for 30 s (metal-free), 100% alcohol 2x for 30 s (metal-free), 95% alcohol 2x for 30 s (metal-free), 80% alcohol for 30 s (metal-free), 70% alcohol for 30 s (metal-free), and diH2O 2x for 30 s (metal-free).
- Keep the slides in a fresh diH2O container until ready for antigen retrieval.
NOTE: The slides should not be dried until the end of the procedure.
- Bake slides at 70 °C in an oven for at least 20 min.
- Antigen retrieval
- Place the slides in a preheated slide chamber containing 1x HIER buffer inside the PT module. Place the cover on the tank. Close and latch the lid.
- Press the Menu button on the PT module to open the main menu and press the Setup cycle (time and temperature) button to create a custom program.
- Run the PT module at 97 °C for 40 min. Afterward, the PT module will automatically cool to 65 °C.
- Remove the slides from the PT module once the 65 °C temperature is reached. Keep the slides at room temperature for 30 min.
NOTE: The PT module will not open until the temperature cools to 65 °C. Do not touch the golden surface of the slides during this process, and always use gloves.
- Antibody blocking
- Set a shaker to 70 rpm.
- Wash the slides by dipping them in 1x TBS-T for 5 min 2x.
- Using a hydrophobic barrier pen, draw a border around the tissue section at least 1 mm away from the slide edges and let it dry for 15-30 s.
NOTE: Take care to prevent the pen fluid from touching the tissue section to avoid any tissue interference with the staining. Do not press the pen down too hard while drawing the barrier to avoid potential leaks. For optimal staining and image acquisition results, the tissue sections are placed in the middle of the gold-coated slides in a frame no larger than 15 mm x 30 mm to allow enough space to draw the barrier without touching the tissue or the guide dots placed on the outer edge of the slide. - To remove any pen residue, dip the slides in TBS-T.
- In a moisture chamber at room temperature, incubate the tissue section with 100-200 μL of blocking buffer for 20 min. Leave the tissue incubating in the blocking buffer until an antibody master mix is ready.
- Antibody panel preparation
NOTE: The final volume of the antibody panel cocktail varies according to the estimated tissue section surface area. To cover an area of 20 mm x 20 mm, prepare 100-200 μL of the cocktail.
Do the following to prepare an antibody panel cocktail of metal isotope-conjugated antibodies:- Confirm the final volume of the cocktail that equals the antibody cocktail volume added to the blocking buffer volume.
- Confirm the specifications for all antibodies, dilutions, and/or antibody concentrations in a panel spreadsheet for the project.
- Spin down all the antibody-containing tubes at 10,000 x g for 5 min. Add individual antibodies to the blocking buffer and mix very well. Do not disturb the bottom of the antibody tube when pipetting it.
- Filter the antibody panel by prewetting a 0.1 μm centrifugal filter device with 100 μL of blocking buffer. Spin the filter at 10,000 x g for 2 min and remove the blocking buffer flow-through with a pipette.
- Transfer the antibody panel into a spin column and spin the filter at 10,000 x g for 2 min. Discard the spin column and use the flow-through as the filtered antibody panel.
NOTE: Perform the staining immediately after making the cocktail to prevent metal exchange. If storage of the antibody cocktail for an extended time is needed, it can be subjected to lyophilization.
- Antibody staining
- Carefully remove the blocking buffer from the slides by tipping each slide on its side and gently tapping the edges against a task wiper.
- Place the slides in a moisture chamber and add 100-200 μL of antibody master mix to the tissue (avoid contact with tissue and the creation of air bubbles).
- Add positive and negative control slides to the staining batch.
- Incubate the slides at 4 °C overnight (14-16 h).
- Reagent preparation (day 2)
- Dilute 100 mL of 10x low-barium PBS, pH 7.4, in 900 mL of diH2O to make 1x PBS.
- Prepare 350 mL of a working stock solution of 2% glutaraldehyde in low-barium PBS (340 mL of low-barium PBS + 10 mL of 70% glutaraldehyde).
NOTE: Perform the dilution under a hood. Glutaraldehyde is a very viscous liquid. Use a 1 mL pipette and add 1 mL of low-barium PBS to the glutaraldehyde tube every time until it becomes liquid enough to pour out of the tube. - Dilute 10x Tris, pH 8.5, in 900 mL of diH20 to make 1x Tris.
- Fixation and dehydration
- Remove the antibody master mix from each slide by tipping the slide on its side and gently tapping the edge against a task wiper.
- Wash the slides with light to moderate agitation in the following buffers: 1x TBS-T, 3x times for 5 min each (metal-free); filtered 2% glutaraldehyde for 5 min; filtered 1x tris, pH 8.5, 3x for 30 s; filtered diH2O 2x for 30 s; 70% alcohol for 30 s (metal-free); 80% alcohol for 30 s (metal-free); 95% alcohol for 30 s (metal-free); and 100% alcohol for 30 s (metal-free).
- Gently tap the edge of each slide against a task wiper to remove excess alcohol and allow residual alcohol to evaporate at room temperature (~5-10 min).
- Dry slides in a desiccator for at least 1 h prior to image acquisition or keep the slides in a vacuum chamber for long-term storage.
5. Image acquisition
- Slide setup
NOTE: Before scanning using the instrument, the slides must be defined and set up using the web-based image management application following the steps described below.- On the slide page in the web-based image management application, click Accession New Slide and enter the desired details (name, slide identification, location, and description).
- Create scanning sections by clicking Add Section. Fill in the information for each section (name of the project, slide name, block, and position). To save the new slides/sections created, click Enviar.
- Under the resources tab, open the panels page and select the panel to be used. For new panels, click Create New Panel.
- After selecting the panel, click on Sections. Add the sections created in step 2. by clicking Edit Section Assignment. Save by clicking Enviar.
- Instrument setup
NOTE: This instrument (see Table of Materials) is operated using a specific control software program (see Table of Materials).- Log in to the control software. Click Wake Up if the instrument is in sleep mode.
NOTE: At least 1 h before the operation, warming up the instrument is recommended, which helps with beam focus stabilization. - To load a slide, click on Exchange Sample and then click Continuar to open the door. Put the slide in the loading slot with the sample facing up and the label on the right. Click Continuar to close the door.
NOTE: If the slide is not loaded correctly, a warning sound and notification to adjust the slide will appear. - Select the project and then select the slide name for the loaded sample.
- Visualize the panoramic image on the slide optical image pane.
- Log in to the control software. Click Wake Up if the instrument is in sleep mode.
- Field of view (FOV) selection and acquisition
- Click on the Mode menu and select SED (Secondary Electron Detector). Click on the imaging mode menu and pick QC −300 μm.
- Click on Jog Stage to control the FOV location and then click on the arrows to navigate and select the position of the FOV.
- Click Add FOV, set 400 μm x 400 μm as the field size, and select fine as the imaging mode and 1 ms of dwell time. Click Confirm.
NOTE: The set dwell time will depend on the desired resolution. The dwell time increases as the resolution increases. The acquisition time may vary depending on multiple factors, including detector break-in period and length of operation. Our mean acquisition time for one FOV is about 17 min. Use the instrument nonstop for up to 8 h, which allows for scanning of about 28 FOVs. The quality of the acquired images decreases after many consecutive hours of use. - After creating an FOV list, proceed to image focus and adjust the stigmation by moving the beam to a tissue region that will not be imaged. Adjust the parameters until a clear central image is obtained.
NOTE: To optimize image acquisition, keeping the tissue section centralized on the slide is ideal. While focusing, a clear image of only the central area can be obtained. If the tissue section is too large, the edges will be out of focus, and the image will be blurred. - Click on Start Run. Acquired images will be automatically uploaded and stored in the web-based image management application.
6. Image preparation
- Image isobaric correction
NOTE: The purpose of isobaric correction is to remove spillover signal between channels resulting from the presence of isotopes of equal or very similar mass whose differences in mass cannot be detected using the mass analyzer.- In the web-based image management application, click on the green Download icon to save the FOVs (TIFF images).
- Download the most updated version of the image processing software (see Table of Materials) available on the about tab in the web-based image management application and save it in a file. Open the .zip archive file and follow the installation steps. Open the software once the installation is complete.
- Select the folder containing the images to be corrected by clicking on the File icon on the input pane of the image processing software. An FOV list will be loaded.
- Click on the Filter icon on the input pane to apply default corrections. For each channel, visualize images obtained before and after correction on the right side of the screen.
- Click on the Floppy disk icon on the output pane to save the corrected image.
- Image filtering: Voronoi tessellation
NOTE: Voronoi tessellation diagrams illustrate a partition of space containing seed points into Voronoi cells. The aim is to estimate the cell density and define cell boundaries. Using the Voronoi tessellation calculation, a mask is generated and applied to the original image for filtering.- Click on the Filtering tab and select Voronoi Tesselation on the filtering parameters menu.
- On the input pane, select the MassCorrected.tiff image. Click on the Filter icon on the input pane.
- Click on the Floppy disk icon on the output pane to save the filtered image. The two resulting files will have the suffixes -Filtered.tiff and -Filtered.json.
NOTE: The image named MassCorrected-Filtered.tiff then can be uploaded to a third-party digital analysis software program or open-source software program.
Representative Results
Tonsil and lung adenocarcinoma TMA tissue sections (5 mm thick) were obtained and placed on the middle of gold-coated slides following the specifications regarding tissue size and secure margins of the slides. Free glass margins of 5 mm and 10 mm between the edge of the tissue and the lateral and inferior borders of the glass slides, respectively, are necessary for optimal staining. The tissue sections were baked overnight in an oven prior to staining to assure proper adherence of the section to the slide. The antibody panel was composed of 23 markers. In the same staining batch, a tonsil slide and a TMA slide containing multiple tissues were included to evaluate the expression of markers scantly expressed in lung adenocarcinoma samples (Figure 1). Positive controls need to be chosen according to the targets of the antibodies used in the panels; for example, to evaluate SOX10 expression, melanoma samples are needed, and to evaluate GAFP expression, glioblastoma samples are needed (Figure 2).
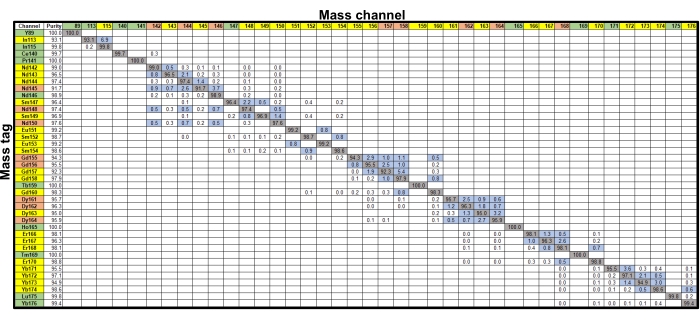
Figure 1: Isotopic purity and channel cross-talk. The matrix shows the percent cross-talk derived from probe purity and oxides (blue boxes, ≥0.5%; clear boxes, <0.5%). For the mass tags, probes that contributed at least 0.5% cross-talk into no channels (green), one or two channels (yellow), or more than two channels (orange) are shown. Mass channels receiving at least 0.5% cross-talk from no probes (green), one or two probes (yellow), or more than two probes (orange) are also shown. Please click here to view a larger version of this figure.
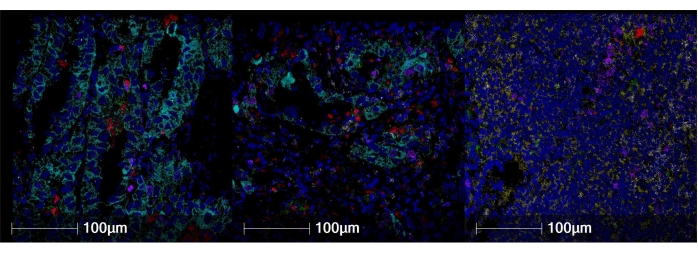
Figure 2: Representative staining images in different tissues. (A) Lung adenocarcinoma. (B) Nonneoplastic kidney. (C) Tonsil. CD20 is shown in yellow, Ki67 is shown in magenta, CD3 is shown in white, CD11c is shown in green, CD68 is shown in red, cytokeratin is shown in cyan, and double-stranded DNA (dsDNA) is shown in blue. Magnification, 200x. Please click here to view a larger version of this figure.
This staining procedure closely resembles standard immunohistochemical staining and occurred on two consecutive days. The five main steps of the staining protocol are 1) excess paraffin removal, 2) antigen retrieval, 3) antibody blocking, 4) antibody staining, and 5) fixation and dehydration (Figure 3). A fundamental step in the staining procedure is to take precautions to avoid metal contamination of the samples, including using metal-free reagents stored in plasticware and careful handling of the samples to limit mechanical damage. Preparing the antibody cocktail immediately before staining is recommended. This reduces the metal exchange. Once the staining was complete, we stored the slides and scanned them the next day. Before image acquisition, a necessary step is slide setup using a web-based image management application (Figure 4).
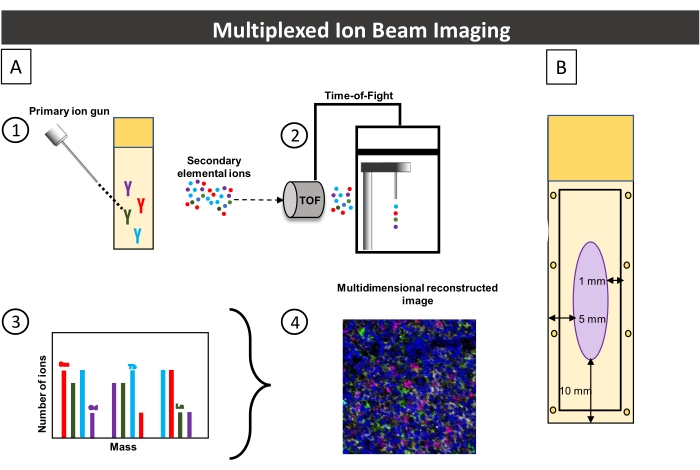
Figure 3: Image acquisition workflow and the associated slide. (A) Image acquisition workflow summary. 1) An FFPE tissue section stained with metal-conjugated antibodies is rasterized using a primary ion gun, releasing secondary ions. 2) A time-of-flight mass spectrometer separates, and measures ions based on their mass at the pixel level. 3) Pixel-based quantification of secondary ions. The y-axis corresponds to the number of ions detected (peak), and the x-axis corresponds to the mass of each metal. 4) Based on the peak of each mass spectrum, multidimensional images are reconstructed. (B) Schematic of the slide used in this procedure. For optimal staining tissue, the section must be positioned at least 5 mm from the slide's lateral edge and 10 mm from its inferior edge. When drawing the hydrophobic barrier around the tissue section, it must be kept inside the rectangle at least 1 mm from the slide's edge. Please click here to view a larger version of this figure.
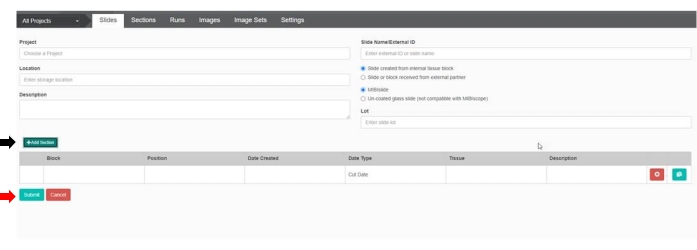
Figure 4: Slide setup in the web-based image management application. Before slide scanning, setting up each slide is necessary. Enter the desired details that can help with slide identification. Click on Add Section (black arrow) to include the block and position information. To save, click Enviar (red arrow). Please click here to view a larger version of this figure.
On the scanning day, turn on the acquisition instrument using the control software. Clicking on Exchange Slide opened the door of the instrument, allowing loading of a slide. We positioned the slide on the loading slot facing up with the label on the right side. Only one slide can be scanned at once. The next step was selecting the FOVs and adjusting the focus and stigmation following the steps described in the protocol. We acquired images by clicking on Start Run. The acquisition time varies depending on the FOV size and resolution desired. The FOV size ranges from 200 μm x 200 μm to 800 μm x 800 μm, which corresponds to a frame size range of 128 pixels x 128 pixels to 2048 pixels x 2048 pixels. Three standard resolutions are available: coarse, fine, and superfine. Scanning larger FOVs at superfine resolution is time-consuming, with the final acquisition time for one FOV ranging from 25 s to 4.7 h (Figure 5).
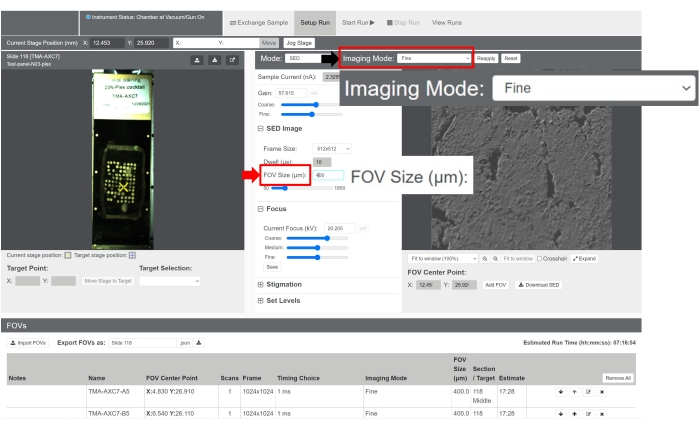
Figure 5: Image acquisition using the control software. The acquisition settings can be adjusted as desired. To modify the scanning resolution, click on the dropdown menu by Imaging Mode (black arrow). To modify the FOV size, enter a number in the FOV Size (μm) field (red arrow). Please click here to view a larger version of this figure.
After scanning was complete, an image set including all FOVs was automatically uploaded to the web-based image management application. Besides storage of images, this application enables the visualization of all channels together or separately, image adjustments, and downloading images for further preparation. The standard visualized image is saved as a TIFF file (Figure 6).
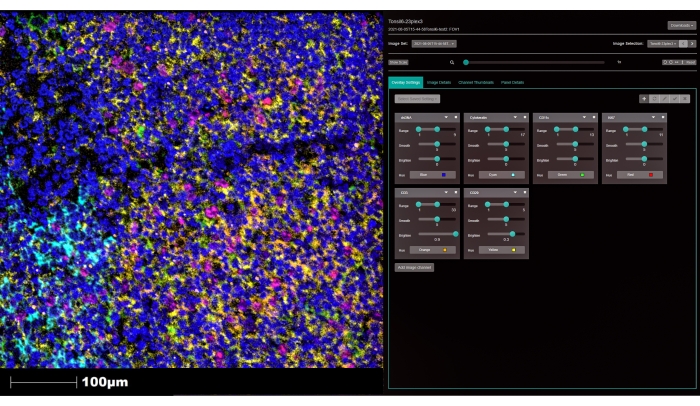
Figure 6: Image visualization using the web-based image management application. Acquired images are stored and visualized using the online platform. Adjusting the visualization parameters and changing the color of each marker is possible. Click on Add image channel (white arrow) to visualize other markers. Magnification, 200x. Please click here to view a larger version of this figure.
Metal interferences are inherent to the metal-labeling methods despite the use of strategies to minimize them. To remove the excess background signals from metal isotope conjugated to the antibodies and prepare images for analysis, we used the associated image processing software (see Table of Materials). The image preparation procedure has two steps: 1) isobaric correction, which removes signals between channels, and 2) filtering, which removes signals caused by aggregates on the image.
To start the isobaric correction, the file containing the saved TIFF image was selected by clicking on the File icon of the input pane. The software automatically loads all archived TIFF images in the file, allowing batch analysis. Next, we corrected the image by clicking on the Filter icon of the input pane. Two resulting files with the suffix -MassCorrected were automatically generated: one archived in TIFF format and the other archived in JSON format. By default, the resulting archives were saved in the same file from which the initial image was loaded (Figure 7).
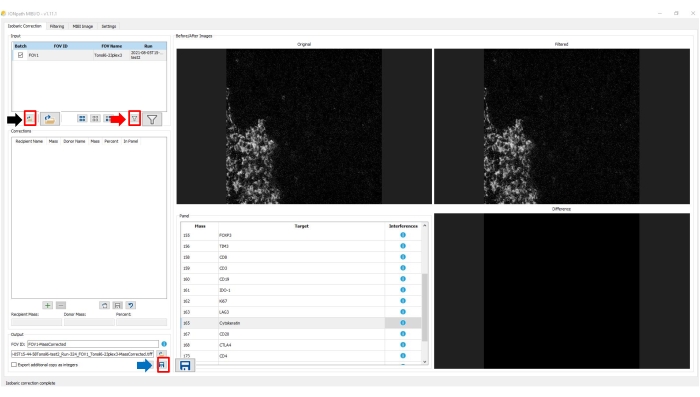
Figure 7: Image preparation: correction step. To load an image for preparation in the image processing software, click on the File icon in the input pane (black arrow) and select the image. To apply the default correction procedure, click on the Filter icon in the input pane (red arrow). To save the updated, corrected image, click on the Floppy disk icon on the output pane. Please click here to view a larger version of this figure.
The second step of image preparation is filtering, which can be selected on the upper tab in the software. We selected the corrected image on the input pane. In this step, automated Voronoi tessellation was used as the filtering parameter. Clicking on the filter icon on the input panel automatically applied the selected filter to all channels. In both image preparation steps (correction and filtering), the images of each channel before and after processing and signal differences are displayed on the right side of the screen.
Similar to the isobaric correction step, two new archives, one in TIFF and one in JSON format, were generated. In this step, the suffix following the file name was -Filtered. Therefore, the final image obtained was named MassCorrected-Filtered.tiff. By completing these steps, we prepared the image for the analysis using the preferred digital pathology software (Figure 8 and Figure 9). By using this technique, we were able to analyze all 23 markers in the antibody panel with minimal interferences between channels at the subcellular level in a single tissue section.
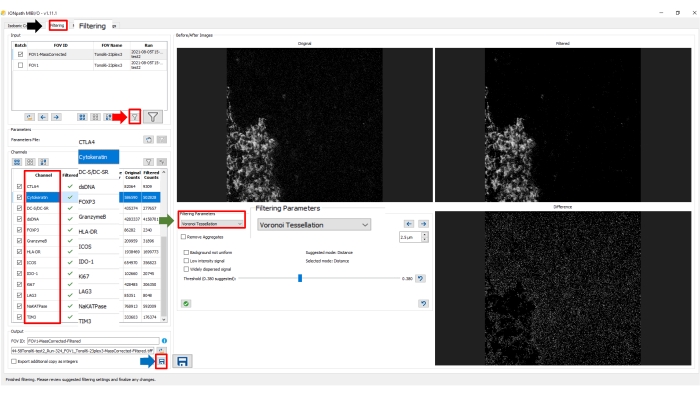
Figure 8: Image preparation: filtering step. Select Filtering in the upper tab (black arrow) in the image processing software. Under Filtering Parameters, select the desired method (green arrow). On the input pane, select MassCorrected image. To apply the selected procedure to the image, click on the Filter icon on the input pane (red arrow). To save the updated filtered image, click on the Floppy disk icon on the output pane. Please click here to view a larger version of this figure.
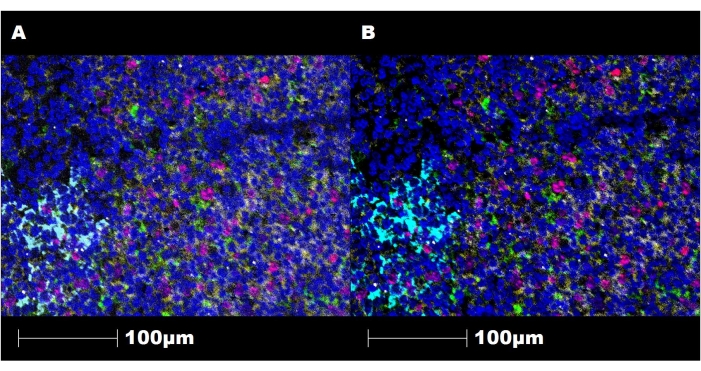
Figure 9: Representative images of the staining before and after image preparation. (A) Image of a tonsil tissue section stained using this technique and visualized using a third-party digital image analysis software program before filtering and correction. (B) Image of the same section under the same conditions after filtering and correction steps. CD20 is shown in yellow, Ki67 is shown in magenta, CD3 is shown in white, CD11c is shown in green, cytokeratin is shown in cyan, and double-stranded DNA (dsDNA) is shown in blue. Magnification, 200x. Please click here to view a larger version of this figure.
Supplementary File 1: Antibody panel list. Each antibody was conjugated to a specific metal isotope with a different mass as listed. Please click here to download this File.
Discussion
The comprehensive elucidation of the complex, intricate interactions among the multiple components of a TME remains a pivotal objective of cancer research. Manufacturers have introduced numerous multiplexed assays as part of this effort, especially over the past 5 years. Multiplexed spatial analysis is a versatile and powerful tool that facilitates the simultaneous categorization of several targets while preserving structural morphology in tumor samples. Spatial analysis techniques can be performed using fluorescence imaging or mass spectrometry, such as the technique described herein11,12,13.
Before staining, antibody optimization using a regular immunohistochemistry protocol followed by the staining protocol is required to standardize antibody staining reproducibility. A very well-known fact is that different regular antibody clones may have optimal expression under different conditions. However, to conjugate antibodies with metal tags, carrier-free antibodies are required, as is defining the same pH for antigen retrieval for all the antibodies integrated into panel, which is a drawback of this method. Failure to complete this step can result in low-quality images that cannot be successfully analyzed. Dedicating time to investigation of the medical literature and antibody manufacturers' websites to select the best well-validated, reliable antibodies from among those that are commercially available is imperative. When validating the antibodies and the panels, constructing smaller panels is useful. This helps in identifying possible channel interferences and evaluating correct expression of the markers.
One of the distinctive features of this technique is the ability to study up to 40 metal-labeled antibodies on a single tissue section. Despite being highly advantageous, metal-labeled mass spectrometry techniques are subject to challenging metal interferences, such as isobaric interferences. Isobaric interferences are characteristic of metal-labeling mass spectrometry methods and are the result of the existence of isotopes of equal or very similar mass whose difference in mass is smaller than the mass analyzer detection capacity. These interferences can be caused by isotopic contamination or polyatomic species. Isotopic contamination refers to an element's purity. In nature, most elements are present in a mixture of isotopes. Even when subjected to enrichment, 100% purity cannot be obtained. It results in more than one element with the same mass and produces cross-talk among channels. The median purity of the 40 available metal isotopes is 98%. The interferences due to polyatomic species originate from free metal binding to negatively charged species such as hydrides, carbides, nitrides, oxides, hydroxides, and cyanides, adding from +1 to +26 to the final mass of the metal tag. Developing strategies to minimize possible noise sources is crucial to achieving good staining and imaging. The first necessary step in panel construction is careful metal isotope-antibody assignment. Markers that are generally widespread in tissue sections, such as cytokeratin and CD45, should be conjugated to metal isotopes with a high mass (greater than that of 160Gd) to minimize the effects of polyatomic interferences, and scant markers should be conjugated to isotopes with masses ranging from 140Ce to 160Gd. Something to keep in mind is that the risk of metal interference increases as the number of markers increases. However, with careful placement of metal and markers, the impact of residual interferences can be reduced with further image processing using the image processing software.
Complementary procedures also can be employed to reduce metal contamination of the samples in tissue cutting and staining. As described in the protocol above, the use of distilled water or diH2O in the water bath prevents this contamination. Likewise, the reagents used in staining must be metal-free. A useful tip is to avoid storing reagents in glass containers, as glassware facilitates metal contamination. To obtain optimal overall results, in the staining step, we recommend baking slides overnight to secure good adherence of tissue sections to the slides.
Unlike other multiplexed methods, this method uses FFPE samples14. Combined formalin fixation and paraffin embedding remains the most widely used form of sample preservation and preparation. The use of archived blocks allows for the performance of more extensive studies than does the use of fresh frozen samples, including retrospective research, and has a lower cost. This method also allows for whole-slide imaging and slide rescanning. This captures a broader area of analysis than does selecting only parts of the slide and enables scanning at multiple magnifications.
This technique has limitations related to the instrument's price and the requirement of purchasing materials such as gold-coated slides directly from manufacturers. Attempts to use uncoated slides, slides coated with a material other than gold, or slides obtained from companies other than the manufacturers may result in no signal acquisition and extensive signal interference and may, ultimately, damage the instrument.
Another significant drawback of this method is the absence of specific software for analysis. This method generates images in TIFF format, which is widely used and can be readily uploaded to third-party digital analysis software. Computational pathology software tools enable comprehensive analysis, including tissue compartmentalization, cell segmentation, marker quantification in any cellular compartment, and nearest neighbor and proximity analysis. Acquiring a third-party digital analysis software for use, increases the cost of an already expensive technique15,16.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge Don Norwood from Editing Services, Research Medical Library at MD Anderson for editing this article and the Multiplex Immunofluorescence and Image Analysis Laboratory at the Department of Translational Molecular Pathology at MD Anderson. This publication resulted in part from research facilitated by the scientific and financial support for the Cancer Immune Monitoring and Analysis Centers-Cancer Immunologic Data Commons Network (CIMAC-CIDC) provided through the National Cancer Institute (NCI) Cooperative Agreement (U24CA224285) to The University of Texas MD Anderson Cancer Center Cancer Immune Monitoring and Analysis Center (CIMAC).
Materials
| 100% Reagent Alcohol | Sigma-Aldrich | R8382 | |
| 95% Reagent Alcohol | Sigma-Aldrich | R3404 | |
| 80% Reagent Alcohol | Sigma-Aldrich | R3279 | |
| 70% Reagent Alcohol | Sigma-Aldrich | R315 | |
| 20X TBS-T | Ionpath | 567005 | |
| 10X Low-Barium PBS pH 7.4 | Ionpath | 567004 | |
| 10X Tris pH 8.5 | Ionpath | 567003 | |
| 4°C Refrigerator | ThermoScientific | REVCO | |
| Aerosol Barrier Pipette Tips P10 | Olympus | 24-401 | |
| Aerosol Barrier Pipette Tips P20 | Olympus | 24-404 | |
| Aerosol Barrier Pipette Tips P200 | Olympus | 24-412 | |
| Aerosol Barrier Pipette Tips P1000 | Olympus | 24-430 | |
| Centrifugal Filter Ultrafree-MC | Fisher Scientific | UFC30VV00 | |
| Deionized H2O | Ionpath | 567002 | |
| Donkey serum | Sigma-Aldrich | D9663 | |
| EasyDip Slide Staining Jar, Green | Electron Microscopy Sciences | 71385-G | |
| EasyDip Slide Staining Jar, Yellow | Electron Microscopy Sciences | 71385-Y | |
| EasyDip Slide Staining Kit (Jar+Rack), White | Electron Microscopy Sciences | 71388-01 | |
| EasyDip Stainless Steel Holder | Electron Microscopy Sciences | 71388-50 | |
| Glutaraldehyde 70% EM Grade | Electron Microscopy Sciences | 16360 | |
| Heat Induced Epitope Retrieval (HIER) buffer: 10X Tris with EDTA, pH 9 | Dako | S2367 | |
| Heat resistant slide chamber | Electron Microscopy Sciences | 62705-01 | |
| Hydrophobic barrier pen | Fisher | 50-550-221 | |
| MIBI/O software | Ionpath | NA | |
| MIBIcontrol software | Ionpath | NA | |
| MIBIslide | Ionpath | 567001 | |
| MIBIscope | Ionpath | NA | |
| Microcentrifuge | Eppendorf | 5415D | |
| Microtome | Leica | RM2135 | |
| Moisture Chamber (Humid Chamber) | Simport | M922-1 | |
| Phosphate Buffered Saline (PBS) Tablets | Fisher Scientific | BP2944100 | |
| PT Module | Thermo Scientific | A80400012 | |
| Rapid-Flow Sterile Disposable Filter Units | Fisher Scientific | 097403A | |
| Shaker | BioRocker | S2025 | |
| Spin column (Ultrafree-MC Spin Filter, 0.5mL 0.1μm ) | MillQ | UFC30VV00 | |
| Slide oven | Fisher Scientific | 6901 | |
| Vaccum Cabinet Desiccator | VWR | 30621-076 | |
| Task-whipe | Kimberly Clark | 34155 | |
| Xylene | Sigma-Aldrich | 534056-4L |
Referencias
- Laplane, L., Duluc, D., Bikfalvi, A., Larmonier, N., Pradeu, T. Beyond the tumour microenvironment. International Journal of Cancer. 145 (10), 2611-2618 (2019).
- Galli, F., et al. Relevance of immune cell and tumor microenvironment imaging in the new era of immunotherapy. Journal of Experimental & Clinical Cancer Research. 39 (1), 89 (2020).
- Liu, C. C., Steen, C. B., Newman, A. M. Computational approaches for characterizing the tumor immune microenvironment. Immunology. 158 (2), 70-84 (2019).
- Eisenstein, M. Cell sorting: Divide and conquer. Nature. 441 (7097), 1179-1185 (2006).
- Scognamiglio, G., et al. Multiplex immunohistochemistry assay to evaluate the melanoma tumor microenvironment. Methods in Enzymology. 635, 21-31 (2020).
- Guo, N., et al. A 34-marker panel for imaging mass cytometric analysis of human snap-frozen tissue. Frontiers in Immunology. 11, 1466 (2020).
- Fu, T., et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. Journal of Hematology & Oncology. 14 (1), 98 (2021).
- Rost, S., et al. Multiplexed ion beam imaging analysis for quantitation of protein expresssion in cancer tissue sections. Laboratory Investigation. 97 (8), 992-1003 (2017).
- Keren, L., et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell. 174 (6), 1373-1387 (2018).
- Mathieson, W., Thomas, G. A. Why formalin-fixed, paraffin-embedded biospecimens must be used in genomic medicine: An evidence-based review and conclusion. The Journal of Histochemistry and Cytochemistry. 68 (8), 543-552 (2020).
- Baghban, R., et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Communication and Signaling. 18 (1), 59 (2020).
- Cai, S., Allam, M., Coskun, A. F. Multiplex spatial bioimaging for combination therapy design. Trends in Cancer. 6 (10), 813-818 (2020).
- Allam, M., Cai, S., Coskun, A. F. Multiplex bioimaging of single-cell spatial profiles for precision cancer diagnostics and therapeutics. NPJ Precision Oncology. 4, 11 (2020).
- Rad, H. S., et al. The Pandora’s box of novel technologies that may revolutionize lung cancer. Lung Cancer. 159, 34-41 (2021).
- Tan, W. C. C., et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commununications. 40 (4), 135-153 (2020).
- Ptacek, J., et al. Multiplexed ion beam imaging (MIBI) for characterization of the tumor microenvironment across tumor types. Laboratory Investigation. 100 (8), 1111-1123 (2020).

