氯离子的动态电化学测试
Summary
Dynamic measurement of chloride ions is presented. Transition time of an Ag/AgCl electrode, during a chronopotentiometric technique, can give the concentration of chloride ions in electrolyte. This method does not require a stable conventional reference electrode.
Abstract
This protocol describes the dynamic measurement of chloride ions using the transition time of a silver silver chloride (Ag/AgCl) electrode. Silver silver chloride electrode is used extensively for potentiometric measurement of chloride ions concentration in electrolyte. In this measurement, long-term and continuous monitoring is limited due to the inherent drift and the requirement of a stable reference electrode. We utilized the chronopotentiometric approach to minimize drift and avoid the use of a conventional reference electrode. A galvanostatic pulse is applied to an Ag/AgCl electrode which initiates a faradic reaction depleting the Clˉ ions near the electrode surface. The transition time, which is the time to completely deplete the ions near the electrode surface, is a function of the ion concentration, given by the Nernst equation. The square root of the transition time is in linear relation to the chloride ion concentration. Drift of the response over two weeks is negligible (59 µM/day) when measuring 1 mM [Clˉ]using a current pulse of 10 Am-2. This is a dynamic measurement where the moment of transition time determines the response and thus is independent of the absolute potential. Any metal wire can be used as a pseudo-reference electrode, making this approach feasible for long-term measurement inside concrete structures.
Introduction
基于Ag / AgCl电极的过渡时间测定中氯离子传感器呈现。这样做的目的是在长期连续监测在电解液中的氯离子的过程中,避免固有漂移。计时电位测量,这是一种动态测量方法中,Ag / AgCl电极的用于此目的。这里的Ag / AgCl电极的电势的变化率的刺激(恒电流脉冲)期间进行测量。这种方法的优点是通过躲避液体结参考电极,而是使用任何金属线作为一个假参比电极,因此允许CL离子浓度为长期(岁)的检测和原位应用中,如证明混凝土结构内部的测量。
在混凝土结构中的氯离子是降解1,2的主要原因之一。它启动了钢筋点蚀第二结果在结构3的最终失败。因此,在具体测量CL离子不可避免地要预测的结构4,5的使用寿命和维护周期。不同感测原理已经报道为氯离子的测量在混凝土如电化学6,7,光学8,9和电磁10,11。然而,光学和电磁方法具有笨重的设置,是难以集成作为一个独立的系统,并与选择性12的问题。在电化学技术,一个Ag / AgCl电极的电位测量是本领域的方法6,7,13的状态。尽管有希望的结果,这种方法仅限于实验室规模的测量,因为在有缺陷的数据14,15在参考电势和扩散势降导致的漂移。基于动态电化学测量(DEM)的过渡时间的方法可以缓解这个问题由于潜在漂流16。
在DEM,一个系统的所施加的刺激反应测定17-19。这种系统的例子是计时。这里所施加的电流脉冲被用作刺激消耗电极表面附近的离子和相应的电势响应被测量。在一个Ag / AgCl电极的阳极电流启动一个法拉第反应(位于Ag + CL 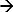 氯化银+ E),导致氯离子接近电极表面耗尽。的电势变化是所施加的电流的函数,并且在电解质12,20中(选择性)离子的浓度。这些离子完全消耗电极附近的时刻表面电位升高的变化率迅速,给人拐点21。潜在的时间响应曲线(测时电位)上的拐点表示的过渡时间,并且可以从被确定最大的潜在响应22的第一导数的。的过渡时间是离子浓度的特性。这种方法已被用来确定不同的离子浓度17和电解质23,24的pH。在一个Ag / AgCl电极作为工作电极的情况下(向其中电流被施加)的耗尽离子将是氯离子17。因此,测量其过渡时间将决定它的浓度。
氯化银+ E),导致氯离子接近电极表面耗尽。的电势变化是所施加的电流的函数,并且在电解质12,20中(选择性)离子的浓度。这些离子完全消耗电极附近的时刻表面电位升高的变化率迅速,给人拐点21。潜在的时间响应曲线(测时电位)上的拐点表示的过渡时间,并且可以从被确定最大的潜在响应22的第一导数的。的过渡时间是离子浓度的特性。这种方法已被用来确定不同的离子浓度17和电解质23,24的pH。在一个Ag / AgCl电极作为工作电极的情况下(向其中电流被施加)的耗尽离子将是氯离子17。因此,测量其过渡时间将决定它的浓度。
Protocol
Representative Results
Discussion
的过渡时间是拐点的时刻;它在理论上是独立基准电位即参考电极。因此,任何金属线可以用作过渡时间测量的伪参考电极。在对比中混凝土氯离子的现有电位测量这种方法能够长期和校准免费测量。此外,灵敏度和浓度的检测范围可以通过调节所施加的电流脉冲来调节。对于更高的CL浓度,这是在混凝土的情况下,较高的电流脉冲,应适用于保持6秒内的过渡时间。
虽?…
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work is a part of the STW project “Integral solution for sustainable construction (IS2C, Fleur van Rossem for her support during the chip fabrication, Justyna Wiedemair for the chip design and Allison Bidulock for her support during the manuscript preparation.
Materials
| Platinum wire (≥99.99% trace metals) | Sigma Aldrich, the Netherlands | EP1330-1EA | |
| Potassium chloride (BioXtra, ≥99.0%) | Sigma Aldrich, the Netherlands | P9333-500G | |
| Potassium hydroxide (90% pure reagent grade) | Sigma Aldrich, the Netherlands | 484016-1KG | |
| Ferric chloride | Sigma Aldrich, the Netherlands | 451649-1G | |
| potassium nitrate (> 99% reagent grade) | Sigma Aldrich, the Netherlands | P6083-500G | |
| Ag/AgCl liquid junction reference electrode | BASi, USA | model MF-2079 | |
| VSP potentiostat | Biologic Science Instruments, France | VSP 300 | |
| Steel wire | Microlab TU Delft | ||
| Silver wire | Sigma Aldrich, the Netherlands |
Referencias
- Page, C., Treadaway, K. Aspects of the electrochemistry of steel in concrete. Nature. 297, 109-115 (1982).
- Koleva, D. A., Hu, J., van Breugel, K., Boshkov, N., de Wit, H. Conventional and pulse cathodic protection of reinforced concrete: electrochemical approach and microstructural investigations. ECS Transactions. 1, 287-298 (2006).
- Montemor, M., Simoes, A., Ferreira, M. Chloride-induced corrosion on reinforcing steel: from the fundamentals to the monitoring techniques. Cement and Concrete Composites. 25, 491-502 (2003).
- Wegen, G., Polder, R. B., Breugel, K. V. Guideline for service life design of structural concrete: A performance based approach with regard to chloride induced corrosion. Heron. 57 (3), (2012).
- Yoon, I., Koenders, E. Theoretical time evolution of critical chloride content in concrete. Structural Durability & Health Monitoring. 5, 275-294 (2010).
- Du, R. G., Hu, R. G., Huang, R. S., Lin, C. J. In situ measurement of Cl-concentrations and pH at the reinforcing steel/concrete interface by combination sensors. Analytical Chemistry. 78, 3179-3185 (2006).
- Angst, U., Elsener, B., Larsen, C. K., Vennesland, &. #. 2. 1. 6. ;. Potentiometric determination of the chloride ion activity in cement based materials. Journal of Applied Electrochemistry. 40, 561-573 (2010).
- Laferrière, F., Inaudi, D., Kronenberg, P., Smith, I. F. A new system for early chloride detection in concrete. Smart Materials and Structures. 17, 045017 (2008).
- Tang, J. L., Wang, J. N. Measurement of chloride-ion concentration with long-period grating technology. Smart Materials and Structures. 16, 665 (2007).
- Kohri, M., Ueda, T., Mizuguchi, H. Application of a near-infrared spectroscopic technique to estimate the chloride ion content in mortar deteriorated by chloride attack and carbonation. Journal of Advanced Concrete Technology. 8, 15-25 (2010).
- Tripathi, S. R., Inoue, H., Hasegawa, T., Kawase, K. Non-destructive Inspection of Chloride Ion in Concrete Structures Using Attenuated Total Reflection of Millimeter Waves. Journal of Infrared, Millimeter, and Terahertz Waves. 34, 181-186 (2013).
- Abbas, Y., Olthuis, W., van den Berg, A. A chronopotentiometric approach for measuring chloride ion concentration. Sensors and Actuators B: Chemical. 188, 433-439 (2013).
- Climent-Llorca, M. A., Viqueira-Pérez, E., Lòpez-Atalaya, M. M. Embeddable Ag/AgCl sensors for in-situ monitoring chloride contents in concrete. Cement and Concrete Research. 26, 1157-1161 (1996).
- Myrdal, R. . The electrochemistry and characteristics of embeddable reference electrodes for concrete. , (2014).
- Angst, U., Vennesland, &. #. 2. 1. 6. ;., Myrdal, R. Diffusion potentials as source of error in electrochemical measurements in concrete. Materials and Structures. 42, 365-375 (2009).
- Abbas, Y., de Graaf, D. B., Olthuis, W., van den Berg, A. No more conventional reference electrode: Transition time for determining chloride ion concentration. Analytica Chimica Acta. 821, 81-88 (2014).
- Meyer, R. E., Posey, F. A., Lantz, P. M. Chronopotentiometry of the Ag− AgCl system and analysis for the chloride ion. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 19, 99-109 (1968).
- Olthuis, W., Langereis, G., Bergveld, P. The metrits of differential measuring in time and space. Biocybernetics and Biomedical Engineering. 21, 5-26 (2001).
- Bakker, E., Bhakthavatsalam, V., Gemene, K. L. Beyond potentiometry: robust electrochemical ion sensor concepts in view of remote chemical sensing. Talanta. 75, 629-635 (2008).
- Olthuis, W., Bomer, J., Bergveld, P., Bos, M., Van der Linden, W. Iridium oxide as actuator material for the ISFET-based sensor-actuator system. Sensors and Actuators B: Chemical. 5, 47-52 (1991).
- Bergveld, P., Eijkel, J., Olthuis, W. Detection of protein concentrations with chronopotentiometry. Biosensors and Bioelectronics. 12, 905-916 (1997).
- Iwamoto, R. Derivative chronopotentiometry. Analytical Chemistry. 31, 1062-1065 (1959).
- Olthuis, W., Bergveld, P. Simplified design of the coulometric sensor-actuator system by the application of a time-dependent actuator current. Sensors and Actuators B: Chemical. 7, 479-483 (1992).
- Olthuis, W., Bergveld, P. Integrated coulometric sensor-actuator devices. Microchimica Acta. 121, 191-223 (1995).
- Bard, A. J., Faulkner, L. R. . Electrochemical methods: fundamentals and applications. , (2001).
- Bakker, E., Bühlmann, P., Pretsch, E. Polymer Membrane Ion-Selective Electrodes-What are the Limits? . Electroanalysis. 11, 915-933 (1999).

