Effectiveness of the Air Stripping in Two Salmonid Fish, Rainbow Trout (Oncorhynchus Mykiss) and Brown Trout (Salmo Trutta Morpha fario)
Summary
The principal aim of this study is to standardize and test the pneumatic method (air stripping) of collecting eggs in rainbow trout and brown trout. This method allows effective and simple collection of the eggs without the necessity of fish abdomen massage.
Abstract
Egg collection is one of the most crucial procedures during fish reproduction in salmonid hatcheries. Classic methods involve the use of hand massage on fish abdomens to expel the eggs. An alternative method uses the pressure of gas injected into the body cavity, which causes the subsequent release of the eggs. This method is believed to have less negative effects on both the welfare and egg quality of the broodstocks. Herein, we compare the results of air and hand stripping methods with respect to one-year survival and egg quantity and quality in two salmonid fish, rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta morpha fario). Our results indicate that air stripping yielded a better quality of eggs and higher one-year survival rate in rainbow trout. In addition, air stripping resulted in lower mortality rate than the group subjected to hand stripping (25% vs. 35%). The pH and hatching rate of the hand stripped group was lower than those of the air stripped group. In the case of brown trout, the quality of eggs obtained by both hand and air-stripping methods was similar; however, the one-year losses in fish were higher in air stripped group (15% compared to 0% in hand stripped fish). Although the advantages of air stripping method over hand stripping in terms of egg quality might not be observed in all salmonid species, the air-stripping procedure might be a promising option to be adopted in hatcheries as it ensures a high level of reproducibility and efficiency.
Introduction
The rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta morpha fario) belong to the Salmonidae family of Salmoniformes1. The production of these two species in global aquaculture has gained rapid growth, owing to their commercial and recreational importance. In Poland, production of salmonid fish stands at around 20 000 tonnes, with rainbow trout being the dominant species. On the other hand, the brown trout represents a major source of freshwater fish resources in Europe because of its commercial value in aquaculture and importance for angling. In many aquatic ecosystems, the existence of brown trout populations is threatened. As a result, artificial reproduction has been applied to restock local populations of this species2.
Rainbow trout and brown trout attain sexual maturity usually at the age of three, with males maturing a year earlier than females1. Under artificial conditions, eggs and sperm of rainbow trout and brown trout are usually collected by gentle abdominal massage. In practice, on a small scale, a hand-stripping method of collecting eggs is cost-effective. However, on a large scale, the hand-stripping method may be labor-intensive and exhausting. As a result, this technique can result in broken eggs due to the weariness of the hatchery workers. Breakage of eggs often leads to a decrease in the pH of ovarian fluid and the release of egg yolk, both of which negatively affect the fertilization of eggs3. Furthermore, this traditional method of egg collection (repeatedly pressing of the abdomen) may also result in broodstock mortality caused by skin infection associated with the deterioration of the protective barrier of mucus.
Pneumatic method of egg collection was first used in Australia in 19574. The method is based on the injection of gases (air, nitrogen or oxygen) into the body cavity to expel eggs using gas pressure. This fast and easy technique has been successfully applied in salmonid fish without any negative side effects on the fish4. Recently, this method was used in wild species (Northern pike, Esox lucius) to collect mature eggs in artificial conditions5. It was shown that air stripped eggs had higher fertilization and hatching rates in comparison with hand stripped eggs, regardless of the solution used for fertilization (Woynarovich solution, Billard buffer or hatchery water)5.
The principal aim of this study was to test the pneumatic method (air stripping) of collecting eggs in rainbow trout and brown trout in comparison with traditional hand stripping method. Efficiencies of both methods (volume of obtained eggs and time of collection, quality of gametes (ovarian fluid pH), as well as hatching rate and post-spawning mortality) were compared.
Protocol
Procedures were carried out in accordance with the Local Committee on the Ethics of Animal Experiments in Olsztyn, Poland.
1.Equipment Preparation
- Use a syringe and needle for injection of the gas into the fish body cavity.
- Set the gas pressure at 0.5 bar, and maintain this pressure to be below 1 bar throughout.
- Control the air flow. For the salmonid fish, 1.5 L/min is the most efficient speed of gas exhaustion. Maintain the optimum speed because higher speed might not obtain all the eggs while a slower speed might unnecessarily prolong the procedure.
- Connect the syringe with a screwed needle (0.8-1.2 mm diameter) to avoid needle detachment caused by the gas pressure.
2. Air Spawning
- Anesthetize the fish with 0.2% Propiscin. Place the fish into a water bath with the 0.2% Propiscin and wait until the fish becomes unresponsive.
- Carefully dry out before spawning by wiping with a dry cotton cloth.
- Place the fish on the slant bed about 35-40°, on her side tail side down. With a gentle push of fish abdomen, check the egg flow from the genital tract. If the eggs are not present, the fish should not be spawned.
- Place a container covered with mesh below the fish papilla to collect the eggs.
- Quickly insert the needle below the abdominal fins.
- Freely remove the eggs by the gas until only singular eggs are removed from the fish body.
- Gently massage the abdomen to remove excess gas from the fish body.
- After spawning, gently transfer the fish to a tank with water flow. Fish released to tank after air spawning might initially swim on the surface of the water. After several minutes the fish releases the remaining gas while swimming and starts swimming normally.
NOTE: The obtained eggs are very clean and free from blood or feces contamination.
Representative Results
Mature males and females of rainbow trout (age 2+, 1700 ± 328 g) and brown trout (age 3+, 1900 ± 435 g) were obtained from the Salmonid Fish Hatchery (Rutki-Żukowo, Inland Fishery Institute in Olsztyn, Poland). The males and females were placed in separate tanks with a volume of about 5 m3. The water temperature was 12±1 °C. Before manipulation, fish were anesthetized with 0.2% Propiscin. The fish were divided into two groups. Part of them underwent a traditional method to obtain eggs, i.e., abdominal pressure (n = 20). The remaining groups underwent the pneumatic procedure with the use of air (n = 20). Eggs from each female were collected separately and placed in dry plastic bowls.
During hand stripping of the eggs, the fish was first wiped with a dry cloth while holding the fish on her side, tail down. The duration of spawning was measured starting from the point where the operator holds the fish and either, start pressing abdomen or inject the needle. The end point was set at the time when eggs stop flowing to the bowl. For hand stripping, one skilled worker was chosen to conduct the whole experiment, and the air stripping procedure was carried out by another skilled worker.
During the procedure, the volume of the eggs obtained was measured using a measuring cylinder and the ovarian fluid was collected separately from each female specimen. Thereafter, its pH level was analyzed using a pH electrode. Broodstock fish used in our experiments were marked with Passive Integrated Transponder (PIT) tags. Tagging allowed us to observe the mortality rate in each group of fish.
Immediately after collection, each sample was subjected to fertilization. Sperm for fertilization was collected about 10 minutes prior to spawning and stored diluted in Morisawa solution6 (0.1 M NaCl, 0.04 M KCl, 0.003 M CaCl2, 0.0015 M MgCl2 x 6 H2O, 0.05 M Tris, pH 8.5), in the ratio 1:9 at 4 °C. The mixture of sperm obtained from four rainbow trout or brown trout males was used respectively.
Since hatching rate is the most appropriate measure of egg quality, rainbow trout and brown trout eggs obtained by hand and pneumatic stripping methods were subjected to fertilization. Eggs were fertilized with about 100,000 sperm per egg, using about 100 eggs from each spawned female. The sperm concentrations were measured in the Bürker chamber after sperm solutions were diluted 1: 2000 with 0.7% NaCl. Eggs were fertilized with the sperm in 5 mL of the Billard solution (0.125 M NaCl, 0.002 M Tris buffer, 0.003 M glycine, and 0.001 mM CaCl2, pH 9.0) for each variant7. Two replications were performed for each sample of eggs. Three minutes after fertilization, the egg samples were carefully washed twice in hatchery water. Then, eggs were placed in a special grid, placing each experimental variant separately. Fertilized eggs were placed in incubators at 8 °C and the number of eyed-eggs was determined about four weeks later. The percentage of hatched eggs was also determined according to the formula: (eggs which were fertilized x 100%) / (all eggs in the concrete variant).
The differences between experimental treatments were determined with paired t-tests at a level of significance P < 0.05. The data expressed as a percentage was transformed using arc-sin transformation prior to statistical analysis. Statistical analyses were conducted with GraphPad Prism software.
The duration of egg collection by hand stripping method was shorter (approximately 10 s.) in comparison to air stripping method in both species (Figure 1; P< 0.05). The methods (traditional or pneumatic) of stripping did not have an influence on the volume of eggs obtained from both rainbow trout and brown trout females (Figure 2). Ovarian fluid pH in rainbow trout after hand stripping was 7.6, whereas, in the air stripping method, the pH was increased to 8.0 (Figure 3; P < 0.05). However, irrespective of the methods of egg collection, the ovarian fluid pH in brown trout was unaffected (Figure 2). In rainbow trout, the hatching rate was significantly higher (Figure 4; P < 0.05) in air-stripped eggs than in hand-stripped eggs. The percentage mortality was higher after hand stripping in comparison to air stripping in rainbow trout. However, in brown trout, percentage mortality was higher (15%) in air-stripped eggs than in hand-stripped eggs (Table 1).
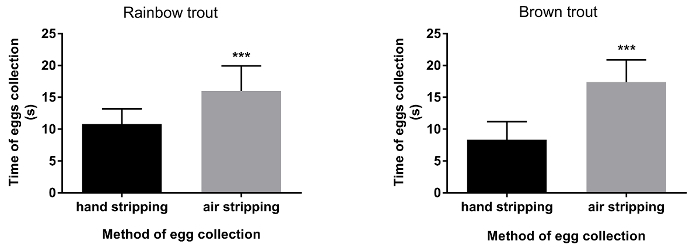
Figure 1. Time of stripping after traditional (hand stripping) and pneumatic (air stripping) method of eggs collection in rainbow trout and brown trout. Statistical significance is indicated by asterisks representing P-value (*** P < 0.001), error bars indicate SD (n=20). Please click here to view a larger version of this figure.
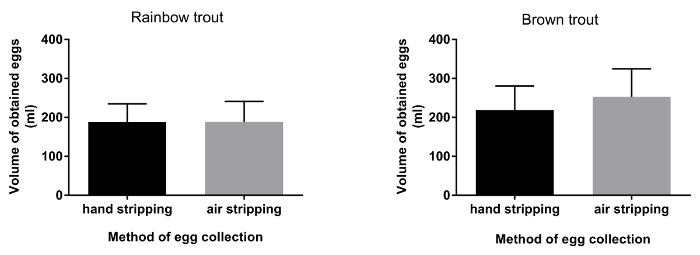
Figure 2. The volume of eggs obtained after traditional (hand stripping) and pneumatic (air stripping) methods of egg collection in rainbow trout and brown trout. Error bars indicate SD (n=20). Please click here to view a larger version of this figure.
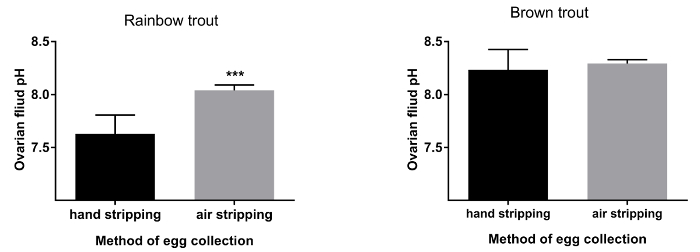
Figure 3. Ovarian fluid pH after traditional (hand stripping) and pneumatic (air stripping) methods of egg collection in rainbow trout and brown trout. Statistical significance is indicated by asterisks representing P-value (*** P < 0.001), error bars indicate SD (n=20). Please click here to view a larger version of this figure.
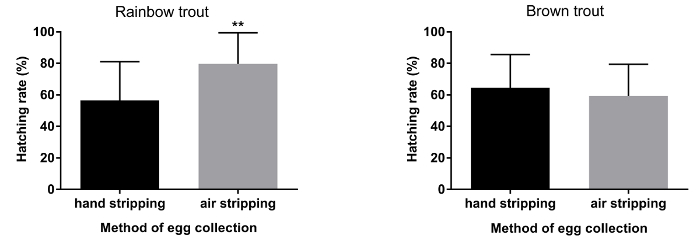
Figure 4. Percentage of hatching rate after traditional (hand stripping) and pneumatic (air stripping) methods of egg collection in rainbow trout and brown trout. Statistical significance is indicated by asterisks representing P-value (** P < 0.01)., error bars indicate SD (n=20). Please click here to view a larger version of this figure.
| Species | Method of egg collection | |
| Hand stripping | Air stripping | |
| Rainbow trout | 35 | 25 |
| Brown trout | 0 | 15 |
Table 1. Percentage of post-spawning mortality after traditional (hand stripping) and pneumatic (air stripping) methods of egg collection in rainbow trout and brown trout as measured over one year.
Discussion
The pneumatic method of egg collection from fish, although more time-consuming than the traditional method (hand stripping), can secure a high quality of matured oocytes (this work). This is related to the low risk of mechanical disruption of the eggs while applying this procedure. High pH of the ovary fluid, as well as an increase in the fertilization rate, attest to the usefulness of the pneumatic method. Overall, the air stripping method of egg collection in fish reproduction could be a promising option in terms of standardization of the hatchery protocols, as their successful application is not related to the manual excellence of the operators.
Although the air spawning seems to be an automatic option, we found that it might take more time than manual spawning. This difference in duration can be obvious especially when well-experienced hatchery workers apply hand-stripping procedures. However, it should be pointed out that the duration of spawning with the use of air pressure is still satisfactory; about 20 seconds per fish. Moreover, the effectiveness of this technique is not dependent on workers’ experience, as is the case with hand stripping method.
Although we did not observe any differences between volumes of obtained eggs, the differences in the pH of ovarian fluid were evident in the case of rainbow trout. This effect was most probably related to the broken eggs, as the pH is a good indicator of this phenomenon3. Unfertilized eggs are more susceptible to mechanical injury and their resistance increase after egg activation during the hardening time8. Unfertilized broken eggs are responsible for lower fertilization rates in salmonids as reported elsewhere. Therefore, maximum care must be taken during egg collection. Our data suggest that unfertilized eggs of rainbow trout are weaker than eggs of brown trout. Rainbow trout eggs collected by hand stripping had lower pH and hatching rate compared to those obtained by air stripping. This was not seen in the case of brown trout. Our experiments suggest that for salmonid species with softer eggs, the air-stripping procedure might be beneficial in terms of post-spawning quality.
Individual mortality of the broodstock after spawning is always visible in salmonid fish hatcheries. However, their occurrence is related to both individual condition and health status of the fish. Air stripping seems to be a mild method of egg collection as there was no massive loss of the skin mucus, an important barrier against pathogens from the water. In spite of the fact that we did not observe any post-spawning mortality (within one month from spawning), whole year observation showed some loses of fish. In the case of rainbow trout, we observed about 10% higher losses in hand stripped group, whereas, in brown trout, air stripping resulted in 15% mortality over the year. We can assume that air stripping could be more beneficial for rainbow trout than brown trout in terms of egg quality and overall mortality of the broodstock.
Although air stripping is beneficial to rainbow trout eggs but not to brown trout, we suggest that this procedure might be more standardized compared to hand stripping procedure. In air stripping, the pressure applied to the abdomen of fish is the only factor that affects the quality and quantity of the matured oocytes obtained. The use of this method in hatcheries alleviates salmon egg losses, which mainly occur as a result of broken eggs11. Since no special skills are required to carry out air-stripping procedure, we expect that similar quantity and quality of eggs can be obtained by both skilled and non-skilled hatchery workers. We recommend air-stripping procedure as a promising option for new hatcheries seeking for egg collection procedure characterized by high level of reproducibility and efficiency, as well as little or no experience by the operators.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
The presented study was supported by Project “Pneumatic method of fish stripping – possible application, influence on the gametes’ quality and quantity and the welfare of fish” (acronym: PNEUFISH) financed under “Operational Program Development of the Fisheries Sector and Coastal Areas 2007-2013” (OR-61724-OR1400001/10), funds appropriated to Institute of Animal Reproduction and Food Research Polish Academy of Sciences, Olsztyn, Poland, and support by COST Office (Food and Agriculture COST Action FA1205: AQUAGAMETE). We would like to thank, Stanley Ifeanyi Ugwu for great support in English editing. We want to acknowledge also the animation producers “Studio Filmowe Ruchome Obrazki”.
Materials
| Glycine pure P.A. | AVANTOR | 527560117 | sperm activating buffer |
| Trizma base | Sigma Aldrich | T1503 | sperm activating buffer |
| Sodium chloride Bioreagent | Sigma Aldrich | S5886 | sperm activating buffer |
| Calcium chloride anhydrous | Sigma Aldrich | C4901 | sperm activating buffer |
| Propiscin (0.2% etomidate solution) | IRS Olsztyn, Poland | not indicated | |
| compressor | Thomas Sheboigan WI USA | DT/SR 070800001882 | |
| reduction valve | Camozzi | cRJUS U7J | |
| air hose | ZEC T.P.U. | SH.98 | |
| syrgine | EFD | 7012118 | |
| Air spawning stage | Biopasz, Poland | PNEU001 | |
| Orion Ross Ultra electrode | Thermo Scientific, Waltham, MA, USA | 8102BNUWP |
Referencias
- Nelson, J. S. . Fishes of the World. , (2006).
- García-Marín, J. L., Sanz, N., Pla, C. Proportions of native and introduced brown trout in adjacent fished and unfished Spanish rivers. Conservation Biology. 12, 313-319 (1998).
- Dietrich, G. J., Wojtczak, M., Slowinska, M., Dobosz, S., Kuzminski, H., Ciereszko, A. Broken eggs decrease pH of rainbow trout (Oncorhynchus mykiss) ovarian fluid. Aquaculture. 273, 748-751 (2007).
- Zurbuch, P. E. A structure for easy fish recovery during drainage of an impoundment. Progressive FishCulturist. 27, 237-238 (1965).
- Cejko, B. I., et al. Effects of different stripping methods of female and activation medium on fertilization success in northern pike (Esox lucius). Czech Journal of Animal Sciences. 10, 481-486 (2016).
- Morisawa, S., Morisawa, M. Induction of potential for sperm motility by bicarbonate and pH in rainbow trout and chum salmon. Journal of Experimental Biology. 136, 13-22 (1988).
- Billard, R., Cosson, J., Perchec, G., Linhart, O. Biology of sperm and artificial reproduction in carp. Aquaculture. 129, 95-112 (1995).
- Iuchi, I., Ha, C. R., Sugiyama, H., Nomura, K. Analysis of chorion hardening of eggs of rainbow trout, Oncorhynchus mykiss. Development Growth and Differentiation. 38, 299-306 (1996).
- Wojtczak, M. A., Kowalski, R. K., Dobosz, S., Goryczko, K., Kuźminski, H., Glogowski, J., Ciereszko, A. Assessment of water turbidity for evaluation of rainbow trout (Oncorhynchus mykiss) egg quality. Aquaculture. 242, 617-624 (2004).
- Tabrizi, E. N., Khara, H., Nezami, S. A., Lorestani, R., Shamspour, S. Broken Eggs Influence on Fertilization Capacity and Viability of Eggs, Turbidity and pH of Ovarian Fluid and Fertilization Water in the Endangered Caspian Brown Trout, Salmo Trutta Caspius. International Journal of Biology. 3 (1), 161-166 (2011).
- Carl, G. C. Beware of the broken egg! A possible cause of heavy losses of salmon eggs. Progresive Fish-Culturist. 53, 30-31 (1941).

