Assembly and Characterization of Polyelectrolyte Complex Micelles
Summary
We provide protocols and representative data for designing, assembling, and characterizing polyelectrolyte complex micelles, core-shell nanoparticles formed by polyelectrolytes and hydrophilic charged-uncharged block copolymers.
Abstract
Polyelectrolyte complex micelles (PCMs), core-shell nanoparticles formed by self-assembly of charged polymers in aqueous solution, provide a powerful platform for exploring the physics of polyelectrolyte interactions and also offer a promising solution to the pressing problem of delivering therapeutic oligonucleotides in vivo. Developing predictive structure-property relationships for PCMs has proven difficult, in part due to the presence of strong kinetic traps during nanoparticle self-assembly. This article discusses criteria for choosing polymers for PCM construction and provides protocols based on salt annealing that enable assembly of repeatable, low-polydispersity nanoparticles. We also discuss PCM characterization using light scattering, small-angle X-ray scattering, and electron microscopy.
Introduction
When oppositely charged polyelectrolytes are mixed in aqueous solution, entropy gain from release of their counterions causes demixing of the solution into a polymer-rich condensed phase and a polymer-depleted supernatant1,2,3,4,5, a phenomenon known as polyelectrolyte complexation. If a neutral hydrophilic block is conjugated to one or both of the polyelectrolytes, nanoscale phase separation occurs instead (Figure 1A). The resulting self-assembled core-shell nanoparticles are variously referred to as polyelectrolyte complex micelles (PCMs), polyion complex micelles, block ionomer complexes, or coacervate-core micelles by analogy to surfactant micellization, even though all components of the system are hydrophilic6,7. A PCM's ability to encapsulate hydrophilic molecules such as proteins and nucleic acids, as well as the extensive tunability offered by the block copolymer carrier architecture makes them attractive candidates for delivering therapeutic molecules in vivo8,9,10,11,12,13.
Delivering therapeutic nucleic acids to cellular targets is a particularly important challenge, and one for which PCMs offer several advantages. Therapeutic nucleic acids (genetic DNA, mRNA, and oligonucleotides such as siRNA) have immense potential for improving human health, but must overcome numerous biological and physical barriers to realize that potential14,15,16. Bare nucleic acids are degraded by serum and cellular nucleases, are quickly cleared from circulation, and their strong negative charge makes it difficult for them to penetrate cell membranes without assistance. Current approaches for overcoming these barriers include costly chemical modifications to prevent damage from nucleases and/or encapsulation into various lipid nanoparticles assembled via hydrophobic interactions15,17,18. While these methods have proven effective for local injections and liver targeting, systemic use presents significant limitations of toxicity, immunogenicity, and limited biodistribution16. By contrast, PCMs use the negative charge of nucleic acids to condense them within the phase-separated core, while the neutral corona provides a steric barrier against degradation as well as a platform for incorporating ligands to enhance targeting or internalization11,19. In vitro and animal studies have shown that PCMs can effectively deliver various nucleic acid payloads20,21,22,23,24, but weaknesses in our ability to predict PCM properties such as size, shape, and stability from the properties of the constituent polymers have hindered their wider adoption.
Recent work by our group and others in the field has begun to address this problem by developing structure-property, and in some cases structure-property-function relationships for PCMs formed from nucleic acids and various cationic-neutral polymers7,25,26,27. Two consistent themes that have emerged from these studies are the importance of developing well-controlled, repeatable protocols for PCM assembly and the benefit of using multiple techniques to characterize the resulting nanoparticles. Polyelectrolytes, particularly those with high charge density like nucleic acids, interact with each other very strongly, and appear to readily become kinetically trapped upon mixing, resulting in PCM preparations that are highly sensitive to small variations in procedure and display high polydispersity and poor repeatability from batch to batch. PCMs have also been shown to adopt a wide range of shapes and sizes depending on the atomic-level configurations of their components, and capturing this diversity with any individual characterization technique is very difficult, particularly since some common techniques such as dynamic light scattering (DLS) require assumptions about particle shape for their interpretation.
In this article, we discuss material design and selection for PCMs, with a focus on oligonucleotides and cationic-neutral diblock copolymers. We then describe a salt annealing protocol that uses high salt concentrations followed by slow dialysis to avoid kinetic trapping during PCM assembly. The polyelectrolytes are mixed in high salt conditions where electrostatic attractions are screened, then the salt concentration is slowly lowered to allow the polyelectrolytes to settle into their most energetically favorable configurations, analogous to the slow cooling process of thermal annealing. Using this protocol, we are regularly able to achieve exceptionally low polydispersity and high repeatability for oligonucleotide PCMs7,26. Finally, we describe how four separate measurement techniques can be used to characterize PCMs over a very wide range of length scales, from external morphology to internal structure: DLS, multi-angle light scattering (MALS), small angle X-ray scattering (SAXS), and transmission electron microscopy (TEM). We hope that these protocols will enable more researchers to effectively explore the capabilities of these interesting nanoparticles.
Polymer Selection and Preparation
PCM properties are strongly influenced by the physical and chemical characteristics of the constituent polymers, making polymer selection a critical step in the design process. The most well-characterized block copolymers for nucleic acid PCMs are linear diblocks such as poly(lysine)-poly(ethylene glycol) (pLys-PEG), but PCMs can be formed between polyelectrolytes and a variety of hydrophilic neutral-charged polymers, which can be generated in a high throughput manner28. The choice of charged group strongly affects the stability of ion pairing and shape of micelles26, and PCM size has been shown to increase with the length of the charged block5,7,26 (Figure 2), thus allowing PCM properties to be tuned for the requirements of a desired application. For linear diblocks, we have found that the charged block should have at least 10 charges and be strongly charged at the desired pH. Longer charged blocks may promote PCM formation with oligonucleotides such as siRNA, which are difficult to complex with shorter blocks21. We have successfully observed PCM formation with block lengths up to 200, and the literature describes longer polymers. More flexibility is available in the choice of neutral blocks24, but experience has shown that very short neutral blocks lead to aggregation rather than nanoparticle formation, and that the minimum neutral length increases with charged block length. For pLys-PEG, a PEG MW of at least 3,000–5,000 is required for pLys lengths below ~50, and longer lengths are required as the charged block is increased further. Increased neutral block length results in increased PCM size, particularly shell thickness, due to steric crowding of the neutral polymers.
This manuscript presents a protocol for preparing PCMs from lyophilized high-purity pLys-PEG and oligonucleotides of known quantity, but should be readily adaptable to other systems as well. We have tested it successfully with several charged polypeptides, including polyarginine and polyglutamic acid, as well as several synthetic polyelectrolytes, such as polyacrylic acid and poly(vinylbenzyl trimethylammonium). We also describe preparing PCMs with a stoichiometric ratio of polyelectrolyte charges, but this is easily modified. We find it easiest to work in charge concentration units (c.c.), which also naturally accommodates polymers that are not fully charged. If either polymer is not well-characterized, care should be taken to accurately determine the polymer lengths/masses and ensure that excess salt is not present beyond that needed for charge neutralization by dialysis, for example. The presence of any retained water should also be accounted for when concentrations are calculated. Nucleic acid concentration can be conveniently quantified by absorbance at 260 nm, and the presence or absence of terminal phosphates should be considered when calculating the c.c.
When using oligonucleotides as polyanions, the hybridization state and chemical structure help determine the propensity for self-assembly and the characteristics of the resulting PCM5,7,26. Optimizing these, within the requirements for biological efficacy if using PCMs for delivery, will increase the likelihood of forming the desired structures. Helpful tools for analyzing hybridization include MATLAB functions for nucleic acids, NUPACK29, and IDT OligoAnalyzer. We recommend analyzing a candidate sequence to understand the strength of binding to 1) itself in a hairpin formation; 2) another copy of the same sequence (self-dimer); and 3) to other oligonucleotides present in the system. DNA and RNA melting temperatures for a specific sequence can also be calculated using the nearest-neighbor method30,31. Thermal annealing of nucleic acids (step 2.3) denatures any residual secondary structure in the individual nucleotides and promotes equilibrium folding.
PCM Characterization and Analysis
A wide range of techniques are available for characterizing nanoparticles, including static and dynamic light scattering, small angle scattering of electrons or neutrons, and electron microscopy. In this article, we provide protocols for two light scattering techniques, small angle X-ray scattering, and two electron microscopy techniques.
DLS measures the autocorrelation of temporal fluctuations in scattering intensity at one angle from Brownian motion of the sample. Fitting this data can provide hydrodynamic radius and polydispersity for spherical micelles (Figure 3). Multiple angle light scattering (MALS) measures the static scattering intensity at many angles. This angular dependence describes the shape of the nanoparticle but is limited to length scales longer than ~50 nm for visible light, which limits its effectiveness for smaller nanoparticles. Both techniques are based on refractive index mismatch and primarily describe the outside dimensions of the nanoparticle.
Small angle X-ray scattering (SAXS) uses X-rays as the scattering probe, and their shorter wavelength allows measurements over a range of ~0.1–100 nm. Fitting the observed scattering intensity vs. angle (conventionally expressed as momentum transfer q) provides information on PCM morphology (i.e., size and shape) and also internal structure. If an absolute intensity calibration is available, and if the scattering intensity can be extrapolated to zero angle, PCM mass and aggregation number can also be estimated32, making SAXS an extremely versatile and valuable method. Small angle neutron scattering (SANS) is sensitive over a similar range of length scales but is only available at specialized facilities and will not be explicitly discussed in this article33,34,35.
Recent years have seen the advent of benchtop SAXS instruments, but we find that synchrotron sources are better suited for PCM characterization, as their higher intensity allows data to be collected much faster for these low-contrast samples. We provide a brief protocol for acquiring PCM SAXS data at Beamline 12-ID-B at the Advanced Photon Source (Argonne National Laboratory, USA) from a user perspective. This protocol should be applicable to most synchrotron sources, but consulting with local staff before proposing an experiment is highly recommended. We also provide a data reduction and analysis protocol using Irena36, a free set of macros written for Igor Pro. Irena includes a versatile set of form factors for modeling SAXS data and allows for construction of multicomponent models that are capable of describing the complex scattering profile of PCMs (see Representative Results, Figure 4). Irena also has comprehensive documentation and tutorials available online. Before attempting the procedures below, we recommend familiarization with these, particularly the tutorial "Modeling of SAXS data with two main scatterer populations".
Radiation damage is a concern for X-ray scattering, but several measures can be employed to minimize it. In particular, we recommend using a flow cell setup with a syringe pump and PCM sample flowing during data acquisition, rather than a sealed capillary. This also greatly simplifies background subtraction. We also suggest taking multiple exposures of the flowing sample rather than one longer one in order to limit the flux that any single volume of sample sees and to allow for comparison of the exposure data to identify any damage.
In contrast to the scattering techniques, which generally require fitting to interpret, transmission electron microscopy (TEM) provides a real space visual image of the nanoparticles by passing an electron beam through the sample and projecting an image on a scintillation screen (Figure 5). We present protocols for two TEM techniques in this article. Cryo TEM freezes micelle samples into a thin layer of vitreous ice, preserving structural conformation with minimal foreign substances, optimal for micelles ≤~10-100 nm in radius. Negative stain TEM uses a heavy metal salt (e.g., uranium) to surround the sample after it has been dried on the surface of a grid. The dense stain will scatter more electrons than the sample, adding contrast and producing a negative image of the sample. Cryo TEM is recommended for high-quality images. However, it is more costly, time consuming, and may not provide sufficient contrast. When this is a concern, negative stained samples should be used. Examples of each are shown in Figure 5.
Each of these techniques reports on slightly different aspects of the nanoparticles, with different strengths and limitations. Light scattering is readily available, and is often the fastest approach, but has substantial limitations in size and shape resolution. SAXS can provide information over a large range of length scales at reasonably high throughput, but requires specialized equipment to acquire the data, as well as modeling to interpret it. TEM images are straightforward to interpret but can be limited in contrast and are inherently low throughput. Our experience has shown that using multiple techniques for characterization greatly increases the information that can be obtained about PCM properties and simplifies interpretation of data sets obtained from each one alone. For example, SAXS and TEM primarily examine a PCM's dense core, while light scattering reports on the overall dimensions of the nanoparticle. Thus, combining them allows measurement of both core and corona size. TEM's ability to acquire real space images can provide ground truth data to enable selection of appropriate form factors for modeling SAXS data that might otherwise be ambiguous. This article describes protocols for all four techniques, and an example process for using them to characterize an unknown sample is given in the Discussion section.
Protocol
1. Preparation of Materials
- Weigh out lyophilized diblock polymer and add water up to nearly the volume required for a stock solution of 10 mg/mL final concentration. Vortex at maximum speed for 2 min.
- Sonicate for 5 min. Very long diblocks may require additional sonication. The stock solution should appear completely transparent and homogeneous.
- Adjust pH to 7.4 using NaOH or HCl as needed. Add water to the final volume. pLys-PEG solutions are fairly stable but should be refrigerated for longer-term storage and the pH must be checked before use. Lyophilization is preferable to freezing.
- Resuspend lyophilized oligonucleotide(s) at desired stock concentration, typically 2–5 mM molecular concentration for lengths of 50 nt or below. Vortex thoroughly to ensure full dissolution.
- Calculate molar concentrations using molecular weight or length as described in the Introduction.
- Calculate molar charge concentrations (c.c.), where
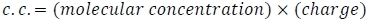
The polyelectrolyte charge is the number of charged monomers, while the nucleic acid charge is the number of bases minus 1, assuming no phosphorylation. Keep in mind that double-stranded DNA will have twice as many charges per molecule compared to single-stranded DNA.
- Create diluted stock at 20 mM c.c. for each polymer.
2. Nucleic Acid Polyelectrolyte Micelle Preparation
- In a 1.5 mL microcentrifuge tube, mix the following to a total volume of 280 μL:
- 200 μL of nuclease-free water (ultrapure water for PCMs not containing nucleic acids).
- 40 μL of 10x phosphate-buffered saline (PBS, 137 mM sodium chloride, 10 mM phosphate, pH 7.4 when diluted to 1x) or other suitable buffer37.
- 40 μL of 20 mM c.c. oligonucleotide. For double-stranded oligonucleotides, add 20 μL of each strand at 20 mM c.c.
- Incubate the oligonucleotide solution for 5 min at 70 °C. If the calculated melting temperature for the oligonucleotides is higher, the temperature should be increased accordingly. Note that RNA will degrade at elevated temperatures, so this step should not be longer if this or other sensitive components are present.
- Cool for 15 min. Add 40 μL of 20 mM c.c. diblock, then vortex immediately for 20 s at maximum speed. Incubate 5 min at room temperature (RT).
- Perform the salt anneal.
- Add 80 μL of 5 M sodium chloride for a final concentration of 1 M NaCl and final volume of 400 μL. Vortex for 10 s at maximum speed.
- Incubate for 10 min at room temperature, then load into dialysis cartridges. Note that the listed molecular weight cutoffs are determined for globular proteins and will not be accurate for linear polymers. We find that a 2k MWCO cartridge avoids sample loss and also provides for gradual changes in ionic strength.
- Prepare dialysis baths.
- Calculate the volume of dialysis bath needed:
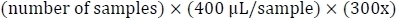
- Mix 10x PBS (or other desired buffer), 5 M NaCl, and ultrapure water for a final solution of 1x PBS and 0.5M NaCl, as well as two solutions of 1x PBS.
- Calculate the volume of dialysis bath needed:
- Load dialysis cartridges.
- Label cartridges with permanent marker. Soak cartridges in buffer for at least 2 min to hydrate membranes.
- Remove cap by twisting counterclockwise. Load sample using gel loading pipette tip. Remove excess air by gently squeezing membranes. Replace cap.
- Put cartridges in the 1x PBS, 0.5 M sodium chloride dialysis bath. Cartridges should float, with both membranes exposed to the bath. Foam floats can be used if needed.
- Dialysis
- Incubate for 24 h stirring slowly with a magnetic stir bar.
- Move the cartridges to a new bath of 1x PBS or other desired working buffer. Incubate for 24 h, stirring slowly with a magnetic stir bar. Repeat this step.
- Recover the sample.
- Remove the cartridges from the bath. Remove cap and recover sample using a gel loading pipette tip. Note that the recovered volume may be higher than the initial 400 μL due to membrane swelling. Record recovered volume if slight dilution is a concern.
- Place the sample into a clean 1.5 mL microcentrifuge tube. PCMs prepared in this way should be stable for several months when refrigerated, provided that nuclease contamination has been avoided.
3. Dynamic light scattering
- To ensure dust-free conditions, buffers should be carefully filtered (filtered 3x through a 0.22 μm syringe or vacuum filter) and glassware thoroughly cleaned between samples. It is also important to ensure that the sample has reached thermal equilibrium before conducting the measurement.
- Dilute the PCM sample to 0.2 mM c.c. (10x if using the protocol described above) with the desired working buffer and load into a suitable cuvette. We use a small-volume cuvette, which requires ~200 μL of sample after dilution.
- Set DLS detector position to 90°.
- Adjust the laser power and/or attenuator so the count rate is 100,000–200,000 counts per second (cps), if possible. Count rates as low as 10 kcps can be used, but measurement times may need to be extended to obtain good statistics (step 4.1). Higher count rates should be avoided, as multiple scattering will confound the measurement.
- Acquire data for 1 min. The count rate should be constant over the entire acquisition time; if not, this indicates that some component of the sample or instrument has not yet equilibrated.
- Examine the autocorrelation data. The long-time baseline should be very flat, and the autocorrelation curve should be smooth, with minimal scatter, as shown in Figure 3A. Baseline drift indicates lack of equilibrium, and noise in the data can be improved by acquiring more data.
- Fit autocorrelation function.
- Use REPES38,39 to perform regularized inverse Laplace transformation to deliver a distribution of relaxation times and a diffusion coefficient, D. This method then calculates hydrodynamic radius, Rh, using the Stokes-Einstein equation:
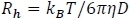
Figure 1B shows a representation of Rh and Figure 3B shows the result of REPES. - Alternatively, use other methods, including CONTIN40,41 (an alternative regularization algorithm), or non-negative least-squares fitting (NNLS). Consistent results from multiple fitting methods is a signature of high-quality data. Note that cumulant analysis (standard on many instruments) gives nonphysical values for multimodal size/length distributions.
- Use REPES38,39 to perform regularized inverse Laplace transformation to deliver a distribution of relaxation times and a diffusion coefficient, D. This method then calculates hydrodynamic radius, Rh, using the Stokes-Einstein equation:
4. Multi-angle light scattering
NOTE: Light scattering intensity vs. angle can be measured on a variety of instruments. We have obtained good results using both goniometer-based instruments and multiple-detector instruments, run in batch mode.
- Adjust the PCM concentration and illumination intensity to provide sufficient signal/noise vs. a buffer-only sample at all angles without saturating any detector. The latter can be tested by preparing samples at various dilution factors and checking for linearity of intensity vs. concentration (assuming minimal interaction between the PCMs).
- Record the light scattering rate from 15° to 135° for 1 min per angle. If the sample and instrument are properly equilibrated, the scattering rate will be constant over the measurement time.
- Plot the normalized scattering rate, Isin(θ), vs. q, where I is the scattering rate. q is the scattering vector (photon momentum transfer) as defined by
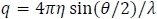
where η = the solvent refractive index, θ = the measurement angle, and λ = the wavelength of the light source. Figure 4 shows a plot of MALS scattering intensity.
5. Small angle X-ray scattering
- Data acquisition
- Prepare PCM samples as described above at 2 mM charge concentration for oligonucleotide PCMs for ample scattering above background. For PCMs lacking heavy atoms (e.g., phosphorus in nucleic acids), higher concentrations may be required. Scattering length densities can be estimated using calculators such as SASSIE42.
- In order to minimize radiation damage by scavenging free radicals, add glycerol from a concentrated (e.g., 50%) stock solution so that the micelle solution contains 1% (v/v) glycerol. Note that neat glycerol is highly viscous and difficult to accurately measure. Diluting with water or buffer is highly recommended.
- Prepare a large volume of working buffer with 1% glycerol for use as a background monitor.
- Prepare the beamline specific flow cell apparatus and calibrate detector. The protocol uses a 3 mm diameter quartz capillary connected to a computer-controlled syringe pump with a small diameter, and minimal length polyethylene tubing. A minimum volume of ~140 μL per sample is needed with this setup.
- Determine sample exposure parameters. The optimum exposure will vary based on beam intensity, detector sensitivity, and the concentration, scattering strength, and damage susceptibility of the sample, but the goal is to expose the sample to the minimal flux required to obtain sufficient scattering intensity over the q range of interest.
- For oligonucleotide/pLys-PEG PCMs, 30x 0.2 s exposures at a 1 Hz repetition rate produce good data quality with little perceptible damage. For new samples, the following procedure may be helpful:
- Prepare PCM samples over a range of concentrations (e.g., 10–10,000 μM c.c.).
- Starting at an intermediate concentration, alternate PCM and buffer-only samples with varying exposure times. See below for data acquisition and reduction procedure. Sample signal should have good statistics (small statistical error, or smooth variation across q). If the statistics are poor, the exposure time may be increased.
- Sample signal should also be clearly distinguishable from background over the q range of interest. Compute and plot the (signal–background)/background ratio vs. q to determine signal/background ratio. If the signal/background ratio is low, sample concentration should be increased.
- Verify that the scattering intensity (normalized to concentration) is independent of sample concentration by acquiring data at higher and lower concentrations, scaling exposure time if needed. Interparticle interactions (the most likely cause of concentration dependence) will be most pronounced in the low q range.
- Acquire data for PCM samples and background (i.e., buffer with glycerol).
- Trigger the syringe pump to move the sample through the capillary. Either bidirectional or once-through motion is acceptable, but care should be taken to isolate each sample (e.g., by inserting an air bubble between samples). Samples may be recovered and can be reused if no radiation damage is seen.
- Once flow has started, trigger the X-ray exposure and data acquisition program described above. Care should be taken that the beam exposure ends before fluid flow does.
- After each sample, perform azimuthal averaging and plot the 1D profiles (i.e., intensity vs. q) for each exposure together. They should be identical within statistical error. Changing signal over time can indicate radiation damage.
- Isolated anomalous profiles may indicate the presence of microbubbles. If bubbles are observed frequently, decreasing the flow rate may help.
- Average the 1D scattering profiles.
- Acquire data for buffer-only samples frequently (once per every 4–5 PCM samples) and compare these over time. Increased signal from buffer-only samples indicates that the capillary may be contaminated with radiation-damaged sample.
- When contamination is noticed, wash the capillary with bleach, and consider reducing exposure time if possible.
- Data reduction and analysis using Irena
- Import micelle and background ASCII data sets (SAS/Data import & export/Import ASCII SAS data).
- Subtract background scattering from sample data. Typically, the highest q values (e.g. q > 0.5) show incoherent scattering from the solvent dominating the signal. Scaling the background data to match the sample data over this q range removes any variation due to beam intensity variation and sample concentration.
- Plot the sample and background together on a log-log scale. Verify flat intensity at high q (SAS/Data Manipulation/Data Manipulation I). Compute the sample/background ratio (Data1/Data 2), plot on a linear-log scale, and verify the high-q asymptote.
- Compute the average (sample/background) ratio over this q range (use a custom macro or copy/paste into a spreadsheet from the data browser).
- Using the Data Manipulation macro, scale the background (e.g., Modify Data 2) using the ratio computed above and plot the ratio of background-subtracted signal to background vs. q ([Data1-Data2]/Data2), verifying that it now asymptotes to zero at high q. Record this ratio; it should be <1–2% away from 1.0 for each sample.
- Plot the background-subtracted signal (Data1-Data2) vs. q and save the data with a new name. Do not overwrite the original data.
- If high-q data is not available, use a scaling factor of 1 for background subtraction, but be aware that inaccuracies may be introduced in q ranges where the signal/background ratio is small.
- Open the Modeling macro, (SAS/Modeling), then load and plot the background-subtracted data (Data cntrls/Add data). Do not scale in this macro.
- First, find an approximate model for the external surface of the PCM (micelle size/shape):
- In Data cntrls, select low to moderate q range (e.g., ~0.003Å-1 < q < ~0.1Å-1). If oscillations are visible, include them.
- Choose a form factor appropriate for the data. The slope at low q is indicative of nanoparticle shape, which can also be verified through TEM and/or MALS. Use Schulz-Zimm spheroid (q0), cylinder (q-1), or flexible cylinder (q-2) models. Irena provides tools for fitting power laws (SAS/Support Tools for plots and tables).
- In Model cntrls, select the first scattering population (1P) and make sure it is the only one in use (Select the Use? checkbox).
- Select Size dist. for Model and choose the desired Distribution type and Form Factor. Set initial parameters for the search by entering values in the Scale, Mean Size, and Ancho fields and click Calculate Model to draw the resulting form factor.
NOTE: The Flexible Cylinder form factor can be added as a User Form Factor and downloaded from https://usaxs.xray.aps.anl.gov/software/irena. Parameters 1 and 2 correspond to the length of the cylinder and the Kuhn length, respectively. - Once reasonable parameters have been found, click Fit Model to perform a nonlinear least-squares fit to the data. The Size distribution model gives mean radius and width.
To calculate polydispersity (PDI) use
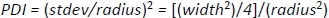
As with any nonlinear fitting procedure, it may be necessary to adjust the data range (q region) and starting parameters in order to obtain a stable, physically reasonable fit. - Once a reasonable fit is obtained, save it (Store in Notebook/Store in Folder).
- Next, model the scattering of the individual polymers within the PCM core. This can be captured by a power law model (e.g., q-2 for ideal chains, q-5/3 for swollen chains, etc.). Irena implements this through a Beaucage model43:
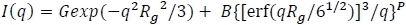
where P is the power law and G and B are prefactors.- Adjust the data controls to cover the entire q range and replot the model (Calculate Model). Typically, excess scattering will be observed in the moderate to high q range (e.g., q > ~0.1Å-1).
- Use the data controls to select the q range where excess scattering (>10x the form factor model) is observed.
- Add a second scattering population (2P) and make sure it is the only one in use (deselect Use? for 1P).
- Select Unified level for the model. B and P are the relevant parameters. Use the plotting support tools or the Fit P/B between csrs macro to obtain an initial guess for these parameters, and adjust the Guinier factors G and Rg to ensure that the model does not predict excessive scattering at low q.
- As for the form factor, perform a nonlinear fit and record the parameters and model.
- Next, If a diffraction peak is present, as in Figure 4, add a third model for a diffraction peak in the q range of interest (q = ~0.22 Å-1 in this case).
- Once approximate fit values are obtained for the individual scattering populations, turn on all three together (select Use? for each) and optimize the combined fit.
- Check that each value remains physically reasonable. The result of this procedure should be a composite model that describes the SAXS data well over a large range of size scales, as illustrated in Figure 4. Save the fit using the Store in Folder button to store within Igor.
6. Transmission Electron Microscopy (TEM)
- Cryo TEM
- Select the grid. We recommend holey carbon support film on a standard TEM grid or lacey carbon as an alternative. In either case, the holes between the carbon will provide an imaging area of pure vitreous ice and sample and no film.
- Place the grid carbon side up in a glow discharging apparatus on a clean glass slide. Wrapping the slide in laboratory film can help with grid handling. Avoid touching the center of the grid with tweezers and always pinch near the edge of the grid.
- Expose the grid for 30 s.
- Prepare a vitrification robot for sample deposition.
- Set to 100% humidity and RT and add blotting paper. Prepare liquid ethane and liquid nitrogen baths at the base of the robot. See online tutorials and videos for additional help with vitrification robot preparation and use.
- Dilute sample 5x.
- Using the negative action tweezers provided with the vitrification robot, pick up a grid, then attach the tweezers to the robot and move the tweezers into the chamber.
- While in the robot, add 4 μL of the sample to the carbon side of the grid using a pipette through the hole in the side of the machine.
- Incubate for 4 min.
- Using the robot, blot 3–5 s with filter paper.
- The vitrification robot will plunge the grid into liquid ethane.
- Remove the tweezers and move the grid to liquid nitrogen and into a storage container, which should also be under liquid nitrogen. This process fixes the sample into a thin layer of vitreous ice. Minimize the time the grid spends out of the liquid ethane or liquid nitrogen during this step.
- Cool the cryo sample holder using liquid nitrogen. Keep a Dewar and reservoir full.
- When ready to image, load the grid onto the cryo sample holder. Keep the sample under liquid nitrogen or briefly in the extremely cold nitrogen steam just above the liquid surface.
- Image the grid at 120 kV between 75kx and 150kx in thin and thick ice, because different sized micelles may prefer a certain ice thickness.
- Limit beam exposure to avoid melting ice and damaging the sample. Do not focus directly where planning to image; focus nearby. Only expose the area of interest while capturing an image.
- Be sure to differentiate liquid ethane drops from the sample when viewing images (see Figure 5).
- Conventional TEM using negative staining
- Prepare the stain.
- Boil ~10 mL of ultrapure water. Weigh out 0.1 g uranyl formate (UFo) into a 15 mL conical tube.
- Add 5 mL of the hot water to the UFo powder for a 2% solution. Close tightly and wrap in aluminum foil to block light. A 1% uranyl acetate stain is also commonly used.
- Vortex or shake vigorously for 5 min. Fastening the tube to the vortexer will help. Filter through a 0.2 μm syringe filter into a clean conical tube.
- Let cool 10 min to RT. Add 25 μL of 5M NaOH and vortex immediately for 2 min.
- Alternatively, freeze 200 μL aliquots of 2% UFo. When ready for use, thaw an aliquot, add 1 μL of 5 M NaOH, and vortex for 2 min.
- Keep stain wrapped in foil or away from light.
- Dilute sample 10x in 1x PBS (or desired buffer).
- Select grid. We recommend carbon support film on copper grids. The darker, shinier side of the grid is the carbon-coated side where the sample will be deposited and stained.
- Place the grid carbon side up in a glow discharging apparatus on a clean glass slide. See step 6.1.2.
- Expose the grid for 30 s.
- Pick up the grid with the carbon side still facing up and hold with negative action tweezers by the edge of the grid to prevent tearing the imaging area in the center. Set down the tweezers with the grid still held carbon side up.
- Apply a 4 μL droplet of sample to the top (carbon side) of the grid with a pipette.
- Incubate 4 min.
- With ~1 min left, pipette a 10 μL and a 20 μL drop of UFo solution onto a piece of clean laboratory film.
- Use filter paper to wick the sample from the edge of the grid (perpendicular contact) to avoid any contact with the imaging surface.
- Using the tweezers (still holding the grid), immediately place the sample side of the grid down on the 10 μL UFo droplet, then immediately wick off the liquid (wash step). It is important not to let the grid dry, so do not stop in between steps.
- In a similar manner, apply the 20 μL UFo droplet to the grid. Hold the grid on the UFo for 40 s. Wick the liquid off and let the grid dry.
- Image the dry grid at 120 kV between 20,000x and 100,000x.
- Be sure to properly dispose of all UFo-contaminated materials using the institution's safety service for radioactive waste.
- When necessary, brightness/contrast enhancement and a median filter can be applied to TEM images in ImageJ to reduce background noise. Post processing should be done uniformly, only for images that are not utilized for quantitative measurements such as intensity, and should always be reported.
- Prepare the stain.
Representative Results
In order to illustrate the characterization methods described above, we show typical results for PCMs assembled from oligonucleotides and block copolymers of various lengths and chemistries (Figure 1). Figure 2 provides an example of how PCM core size (as determined from SAXS and TEM, Figure 4 and Figure 5) varied with charged block length. Figure 3 shows DLS data and fitting results for spherical PCMs formed from relatively long block copolymers and short single-stranded oligonucleotides. Figure 4 illustrates how complex SAXS intensity spectra could be accurately fit by combining models for the multiple spatial correlations that were present (external surface, intra-core scattering, inter-helix ordering), and how MALS could be used to extend scattering measurements to longer length scales. Finally, Figure 5 shows representative electron microscopy data for PCMs of varying morphology.
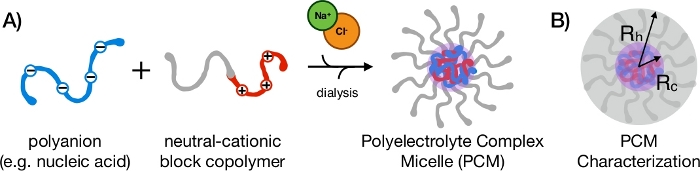
Figure 1: Assembly and characterization of nucleic acid PCMs. (A) Anionic polymers, such as oligonucleotides, formed phase-separated complexes with cationic regions of diblock copolymers. The presence of a hydrophilic neutral block (gray) resulted in formation of stable PCM nanoparticles. (B) PCMs were core-shell nanoparticles with multiple parameters to characterize. The overall size (hydrodynamic radius, Rh) could be determined using DLS, the core radius (Rc) could be found using SAXS and TEM, corona size could be calculated as Rh-Rc, and morphology could be determined over multiple length scales by combining SAXS, MALS, and TEM. Please click here to view a larger version of this figure.
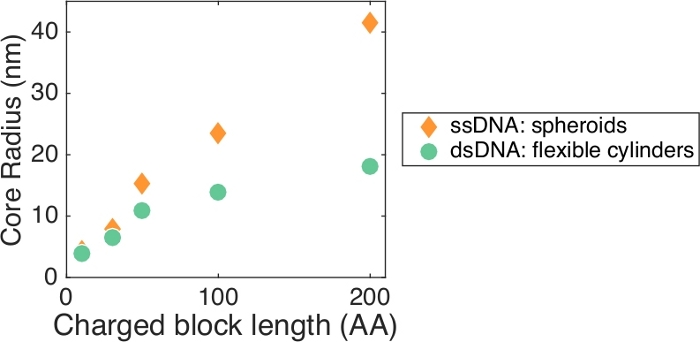
Figure 2: Micelle size dependence. Micelle core size was primarily determined by the length of the charged block of the block copolymer, and largely independent of the length of the homopolymer7,26. This allows for control of PCM size by choice of block polymer. The data shown here are for pLys-PEG with 88 nt/bp DNA and have been previously reported26. Please click here to view a larger version of this figure.
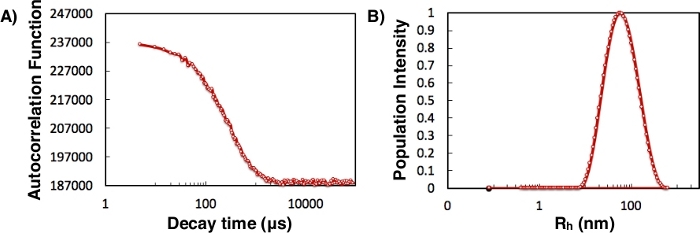
Figure 3: Dynamic light scattering. (A) Autocorrelation function (arbitrary units) for 10 nt single-stranded DNA + pLys(100)-PEG(10k) PCM. (B) Hydrodynamic radius distribution (histogram) from REPES fit. The autocorrelation function decayed to a flat value with a single time scale, resulting in a single size peak in the REPES size distribution. Please click here to view a larger version of this figure.
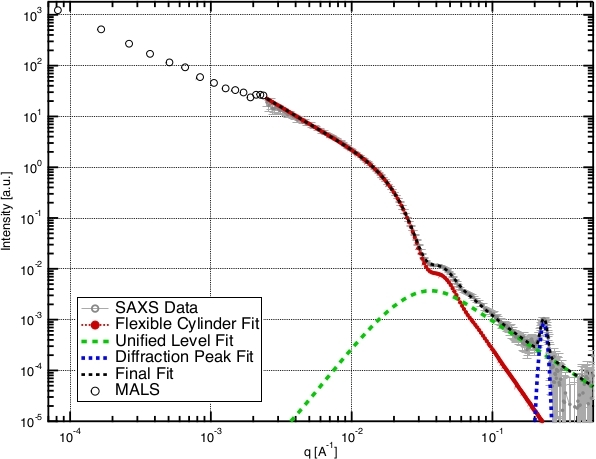
Figure 4: Representative SAXS and MALS data and fit for a cylindrical micelle. SAXS data (gray circles) are shown for PCMs assembled from pLys(50)-PEG(5k) and 88 bp double-stranded DNA. At low q (< 10-2 Å-1), the intensity showed an approximately q-2 dependence on momentum transfer, implying a flexible cylinder shape (worm-like micelle). MALS data (open black circles) show the same dependence, indicating that the micelles were at least several micrometers in length (corroborated by TEM imaging, Figure 5C,D). Spheroidal micelles would show a flat dependence (q0) of intensity on q in this range. The colored lines illustrate the multicomponent fitting procedure for PCM SAXS data described in section 5. Scattering at low q (large distance scales) was dominated by the external surface of the PCM, and fit well by a flexible cylinder model (red). At higher q values (smaller length scale), scattering was dominated by the individual polymers inside the PCM core, fit here by a power law (green) with low q cutoff. We also observed parallel packing of double-stranded DNA helices within the PCM core, resulting in a quasi-Bragg diffraction peak (blue). The black dashed line shows that combining these models accurately described the SAXS data, and the addition of light scattering data (open circles) extended the size range over nearly four orders of magnitude. Fitting results gave a PCM population with mean radius = 11.0 nm and PDI = 0.03, power law at high q = 1.81 and the diffraction peak represents inter-helix spacing of 2.71 nm. SAXS data have been previously reported26 and are publicly available44. Please click here to view a larger version of this figure.
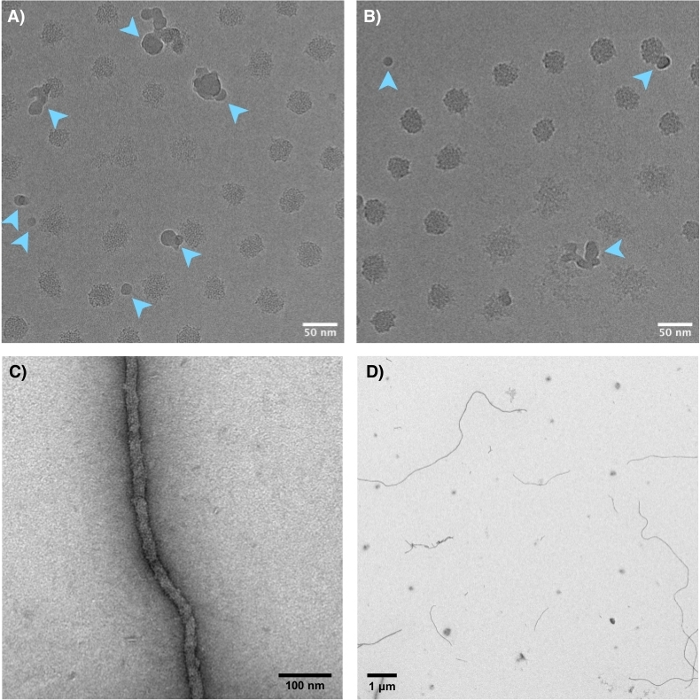
Figure 5: TEM images of nucleic acid PCMs. (A–B) Cryo TEM of 22 nt single-stranded DNA + pLys(50)-PEG(5k) PCMs, showing predominantly spherical morphology. Blue arrows indicate liquid ethane droplets, not to be confused with the PCMs (textured spheroidal objects). (A) is slightly under-focused, adding slight contrast while preserving resolution. (B) is substantially under-focused, adding more contrast but sacrificing clarity. Brightness and contrast adjustments and a two pixel median filter were applied to both images. (C–D) Negative stained TEM of 88 bp double-stranded DNA + pLys(50)-PEG(5k) PCMs, which are long flexible cylinders. In both cases, core radii from TEM were consistent with the values obtained from fitting SAXS data. Please click here to view a larger version of this figure.
Discussion
As mentioned above, the protocols presented here are written with a focus on oligonucleotides as the polyanion component and pLys-PEG as the cationic-neutral block copolymer, but we have tested them with a variety of polymers, such as poly(acrylic acid), polyglutamate, and PEG-poly(vinylbenzyl trimethylammonium), and believe they will be generally applicable for most polyelectrolyte pairs. One parameter that may need to be optimized is the salt concentration used for annealing, because it should be high enough that PCMs do not form at the beginning of the anneal. This can be checked experimentally by DLS, or by comparison to observation of phase separation with the polyelectrolytes alone (no neutral block). Thermal annealing can be used if salt annealing is undesirable, though the resulting polydispersities are larger7. The concentrations used for characterization also may need to be optimized, because larger nanoparticles scatter more light than small ones, and nucleic acids are more efficient at scattering X-rays than many other polymers due to the presence of electron-dense phosphorus atoms in the backbone. It may also be necessary to more closely control the pH of the buffer if either polyelectrolyte has a pKa close to the working condition.
In this article we present protocols for two light scattering techniques (i.e., multi-angle/static light scattering and dynamic light scattering), as well as small angle X-ray scattering, and both cryo and conventional negative stain transmission electron microscopy, with representative data for each. Not all techniques are necessary for all scenarios, and others are available as well, raising the question of which should be employed when. Ample review literature exists on this subject45,46, but we suggest the following when characterizing a new PCM or similar nanoparticle. Begin by checking for aggregation, both by visual inspection for turbidity and by optical microscopy. If no aggregation is observed, the next step is to determine whether any nanoparticles exist. DLS is a quick way to determine this because PCMs scatter light vigorously, and weak or nonexistent light scattering is a strong indicator of poor nanoparticle formation. While DLS can confirm the presence of nanoparticles, it is difficult to determine their size and shape without reference to other data, as most analysis methods rely on the Stokes-Einstein relation, which assumes spherical particles. MALS can confirm spherical shapes (flat normalized intensity vs. angle) but may not be able to determine the shape of nonspherical particles unless the size distribution is both narrow and happens to fall in the correct range for resolution. As a result, we recommend performing TEM, SAXS, or both on any PCM sample in order to fully characterize its properties.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We thank Phil Griffin and Tera Lavoie of the Soft Matter Characterization Facility and Advanced Electron Microscopy Facility, respectively, at The University of Chicago. We also thank Xiaobing Zuo and Soenke Seifert of the Advanced Photon Source at Argonne National Laboratory and NIST Center for Hierarchical Materials Design (CHiMaD) for support. We thank Jeff Ting and Michael Lueckheide for their contributions to this work.
Materials
| 70 mm circle filter paper | Whatman | 1001-070 | Filter paper for wicking during grid prep |
| Carbon Film TEM grid | Electron Microscopy Sciences | CF200-Cu | TEM grid |
| DAWN | Wyatt Technology | DAWN | MALS instrument |
| DNA oligonucleotide | Integrated DNA Nanotechnologies Inc | Custom oligonucleotide | |
| Lacey Carbon TEM grid | Electron Microscopy Sciences | LC200-Cu | TEM grid |
| Methoxy-poly(ethylene glycol)-block-poly(l-lysine hydrochloride) PEG5k – PLKC50 | Alamanda Polymers Inc | mPEG5K-b-PLKC50 | Example block copolymer |
| Milli-Q | Millipore Sigma | Ultrapure water | |
| NanoDrop | Thermo Scientific | For measuring nucleic acid concentration | |
| negative-action tweezers | Dumont | N7 | Tweezers for grid preparation |
| Parafilm "M" | Bemis Company Inc | PM996 | Laboratory film |
| Quantifoil Holey Carbon TEM grid | Electron Microscopy Sciences | Q210CR1.3 | TEM grid |
| Research Goniometer and Laser Light Scattering System | Brookhaven Instruments | BI-200SM | DLS/MALS instrument |
| Slide-A-Lyzer G2 2K 0.5 mL | Thermo Scientific Pierce Protein Biology | 87723 | Dialysis cartridge |
| small volume cuvette | Brookhaven Instruments | BI-SVC | Cuvette for DLS/MALS |
| Solarus 950 Advanced Plasma System | Gatan | Solarus 950 | Plasma system for TEM grids |
| Talos TEM | FEI | Talos | TEM used for cryo samples |
| Tecnai Spirit TEM | FEI | Spirit | TEM used for dry samples |
| Uranyl Formate | SPI-Chem | 16984-59-1 | For negative staining samples for TEM |
| Vitrobot | FEI | Vitrobot | Vitrification robot for cryo grid preparation |
Referencias
- Spruijt, E., Westphal, A. H., Borst, J. W., Cohen Stuart, M. A., van der Gucht, J. Binodal compositions of polyelectrolyte complexes. Macromolecules. 43 (15), 6476-6484 (2010).
- van der Gucht, J., Spruijt, E., Lemmers, M., Cohen Stuart, M. A. Polyelectrolyte complexes: bulk phases and colloidal systems. Journal of Colloid and Interface Science. 361 (2), 407-422 (2011).
- Priftis, D., Laugel, N., Tirrell, M. Thermodynamic characterization of polypeptide complex coacervation. Langmuir. 28 (45), 15947-15957 (2012).
- Fu, J., Schlenoff, J. B. Driving Forces for Oppositely Charged Polyion Association in Aqueous Solutions: Enthalpic, Entropic, but Not Electrostatic. Journal of the American Chemical Society. 138 (3), 980-990 (2016).
- Vieregg, J. R., et al. Oligonucleotide-Peptide Complexes: Phase Control by Hybridization. Journal of the American Chemical Society. 140 (5), 1632-1638 (2018).
- Voets, I. K., de Keizer, A., Cohen Stuart, M. A. Complex coacervate core micelles. Advances in Colloid and Interface Science. 147-148, 300-318 (2009).
- Lueckheide, M., Vieregg, J. R., Bologna, A. J., Leon, L., Tirrell, M. V. Structure-Property Relationships of Oligonucleotide Polyelectrolyte Complex Micelles. Nano Letters. 18 (11), 7111-7117 (2018).
- De Kruif, C. G., Weinbreck, F., de Vries, R. Complex coacervation of proteins and anionic polysaccharides. Current Opinion in Colloid & Interface Science. 9 (5), 340-349 (2004).
- Vieregg, J. R., Tang, T. Y. D. Polynucleotides in cellular mimics: Coacervates and lipid vesicles. Current Opinion in Colloid & Interface Science. 26, 50-57 (2016).
- Marciel, A. B., Chung, E. J., Brettmann, B. K., Leon, L. Bulk and nanoscale polypeptide based polyelectrolyte complexes. Advances in Colloid and Interface Science. 239, 187-198 (2017).
- Cabral, H., Miyata, K., Osada, K., Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chemical Reviews. 118 (14), 6844-6892 (2018).
- Tan, Z., et al. Block Polymer Micelles Enable CRISPR/Cas9 Ribonucleoprotein Delivery: Physicochemical Properties Affect Packaging Mechanisms and Gene Editing Efficiency. Macromolecules. 52 (21), 8197-8206 (2019).
- Horn, J. M., Kapelner, R. A., Obermeyer, A. C. Macro- and Microphase Separated Protein-Polyelectrolyte Complexes: Design Parameters and Current Progress. Polymers. 11 (4), 578 (2019).
- Juliano, R. L. The delivery of therapeutic oligonucleotides. Nucleic Acids Research. 44 (14), 6518-6548 (2016).
- Kanasty, R., Dorkin, J. R., Vegas, A., Anderson, D. Delivery materials for siRNA therapeutics. Nature Materials. 12 (11), 967-977 (2013).
- Lorenzer, C., Dirin, M., Winkler, A. M., Baumann, V., Winkler, J. Going beyond the liver: progress and challenges of targeted delivery of siRNA therapeutics. Journal of Controlled Release. 203, 1-15 (2015).
- Allen, T. M., Cullis, P. R. Liposomal drug delivery systems: from concept to clinical applications. Advanced Drug Delivery Reviews. 65 (1), 36-48 (2013).
- Li, W., Szoka, F. C. Lipid-based nanoparticles for nucleic acid delivery. Pharmaceutical Research. 24 (3), 438-449 (2007).
- Miyata, K., Nishiyama, N., Kataoka, K. Rational design of smart supramolecular assemblies for gene delivery: chemical challenges in the creation of artificial viruses. Chemical Society Reviews. 41 (7), 2562-2574 (2012).
- Oishi, M., Nagasaki, Y., Itaka, K., Nishiyama, N., Kataoka, K. Lactosylated poly(ethylene glycol)-siRNA conjugate through acid-labile ss-thiopropionate linkage to construct pH-sensitive polyion complex micelles achieving enhanced gene silencing in hepatoma cells. Journal of the American Chemical Society. 127 (6), 1624-1625 (2005).
- Christie, R. J., et al. Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano. 6 (6), 5174-5189 (2012).
- Kuo, C. H., et al. Inhibition of atherosclerosis-promoting microRNAs via targeted polyelectrolyte complex micelles. Journal of Materials Chemistry B. 2 (46), 8142-8153 (2014).
- Ge, Z., et al. Targeted gene delivery by polyplex micelles with crowded PEG palisade and cRGD moiety for systemic treatment of pancreatic tumors. Biomaterials. 35 (10), 3416-3426 (2014).
- Van Bruggen, C., Hexum, J. K., Tan, Z., Dalai, R. J., Reineke, T. M. Nonviral Gene Delivery with Cationic Glycopolymers. Accounts of Chemical Research. 52 (5), 1347-1358 (2019).
- Hayashi, K., et al. Influence of RNA Strand Rigidity on Polyion Complex Formation with Block Catiomers. Macromolecular Rapid Communications. 37 (6), 486-493 (2016).
- Marras, A. E., Vieregg, J. R., Ting, J. M., Rubien, J. D., Tirrell, M. V. Polyelectrolyte Complexation of Oligonucleotides by Charged Hydrophobic-Neutral Hydrophilic Block Copolymers. Polymers. 11 (1), 83 (2019).
- Phillips, H. R., et al. Glycopolycation-DNA Polyplex Formulation N/P Ratio Affects Stability, Hemocompatibility, and in Vivo Biodistribution. Biomacromolecules. 20 (4), 1530-1544 (2019).
- Ting, J. M., Wu, H., Herzog-Arbeitman, A., Srivastava, S., Tirrell, M. V. Synthesis and Assembly of Designer Styrenic Diblock Polyelectrolytes. ACS Macro Letters. 7 (6), 726-733 (2018).
- Zadeh, J. N., et al. NUPACK: Analysis and design of nucleic acid systems. Journal of Computational Chemistry. 32 (1), 170-173 (2011).
- Santa Lucia, J. A unified view of polymer, dumbbell, and oligonucleotide DNA nearest-neighbor thermodynamics. Proceedings of the National Academy of Sciences of the United States of America. 95 (4), 1460-1465 (1998).
- Xia, T., et al. Thermodynamic parameters for an expanded nearest-neighbor model for formation of RNA duplexes with Watson-Crick base pairs. Bioquímica. 37 (42), 14719-14735 (1998).
- Orthaber, D., Bergmann, A., Glatter, O. SAXS experiments on absolute scale with Kratky systems using water as a secondary standard. Journal of Applied Crystallography. 33 (2), 218-225 (2000).
- Srivastava, S., et al. Gel phase formation in dilute triblock copolyelectrolyte complexes. Nature Communications. 8, 14131 (2017).
- Lindhoud, S., et al. Salt-induced disintegration of lysozyme-containing polyelectrolyte complex micelles. Langmuir. 25 (19), 11425-11430 (2009).
- Lindhoud, S., de Vries, R., Schweins, R., Stuart, M. A. C., Norde, W. Salt-induced release of lipase from polyelectrolyte complex micelles. Soft Matter. 5 (1), 242-250 (2009).
- Ilavsky, J., Jemian, P. R. Irena: tool suite for modeling and analysis of small-angle scattering. Journal of Applied Crystallography. 42 (2), 347-353 (2009).
- Sambrook, J., Fritsch, E. F., Maniatis, T. . Molecular Cloning: a Laboratory Manual. , (1989).
- Jakeš, J. Regularized positive exponential sum (REPES) program-A way of inverting laplace transform data obtained by dynamic light scattering. Collection of Czechoslovak Chemical Communications. 60 (11), 1781-1797 (1995).
- Schillen, K., Brown, W., Johnsen, R. M. Micellar Sphere-to-Rod Transition in an Aqueous Triblock Copolymer System – a Dynamic Light-Scattering Study of Translational and Rotational Diffusion. Macromolecules. 27 (17), 4825-4832 (1994).
- Provencher, S. W. Contin – a General-Purpose Constrained Regularization Program for Inverting Noisy Linear Algebraic and Integral-Equations. Computer Physics Communications. 27 (3), 229-242 (1982).
- Provencher, S. W. A Constrained Regularization Method for Inverting Data Represented by Linear Algebraic or Integral-Equations. Computer Physics Communications. 27 (3), 213-227 (1982).
- Sarachan, K. L., Curtis, J. E., Krueger, S. Small-angle scattering contrast calculator for protein and nucleic acid complexes in solution. Journal of Applied Crystallography. 46 (6), 1889-1893 (2013).
- Beaucage, G. Approximations leading to a unified exponential power-law approach to small-angle scattering. Journal of Applied Crystallography. 28 (6), 717-728 (1995).
- Marras, A. E., Vieregg, J. R., Ting, J. M., Rubien, J. D., Tirrell, M. V. . Materials Data Facility. , (2018).
- Modena, M. M., Rühle, B., Burg, T. P., Wuttke, S. Nanoparticle Characterization: What to Measure. Advanced Materials. , (2019).
- Mourdikoudis, S., Pallares, R. M., Thanh, N. T. K. Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale. 10 (27), 12871-12934 (2018).

