Subclavian Vein Blood Sampling in Conscious Rats
Summary
Here we present a combination of effective rat restriction and subclavian vein puncture methods that enable rapid, safe, and repeated blood collection in rats without anesthesia.
Abstract
There are several established methods for obtaining repeated blood samples from rats, with the most commonly employed methods being lateral tail vein sampling without anesthesia and jugular vein sampling with anesthesia. However, most of these methods require assistance and anesthetic equipment and sometimes pose difficulties in terms of blood collection or the poor quality of blood samples. In addition, these methods of blood collection consume significant time and human resources when repeated blood sampling is required for a large number of rats. This study presents a technique for repetitive blood sampling in non-anesthetized rats by a single proficient individual. Highly satisfactory blood samples can be obtained by puncturing the subclavian vein. The method demonstrated an impressive overall success rate of 95%, with a median time of merely 2 min from rat restraint to the completion of blood collection. Furthermore, performing consecutive blood collections within the designated range does not inflict any harm on the rats. This method is worth promoting for blood collection, especially in large-scale pharmacokinetic studies.
Introduction
Rats are one of the most common experimental animals, and there are many ways to obtain blood samples. For experiments involving a single blood collection at the concluding stage, a sufficient amount of blood can be obtained through cardiac puncture or abdominal aorta blood collection1. However, some studies require repeated blood collection from rats for routine blood or biochemical analysis, especially in pharmacokinetics and toxicology studies, where repeated blood collection is required to determine the absorption, distribution, and metabolism of drugs2.
Currently, although tail vein blood collection is the most common method for blood sampling from rats, despite not requiring anesthesia, this method can be challenging for repeated collections, and the volume of blood collected is relatively small3,4. Additionally, although blood can be collected from the saphenous and penile veins, the amount of blood obtained is limited, and anesthesia is required1,5. Furthermore, blood samples collected from the submandibular venous plexus, as well as sublingual, jugular, and subclavian veins provide higher-quality samples but typically require anesthesia or the assistance of multiple individuals1,6,7,8,9. Finally, retro-orbital sinus/canal blood collection not only necessitates anesthesia but may also potentially cause injury and stress to the rats9.
The quality of blood samples typically obtained from major veins is generally of the highest standard1. At present, some studies have found that continuous microsampling through the jugular vein is a very suitable method for toxicological research in rats although this method usually requires jugular vein catheterization10,11,12. Therefore, it is worth exploring how to obtain high-quality blood samples in accordance with the 3R principle of animal research without surgical intervention. The objective of this study was to present a method for efficiently extracting blood from the subclavian vein in rats. This technique enables the swift collection of satisfactory samples through a single-person procedure without the need for anesthesia.
Protocol
This study adhered to the guidelines outlined in the 8th edition of the Guide for the Care and Use of Laboratory Animals13. The research received approval from the Ethics Committee of Lanzhou University Second Hospital and was documented in adherence to The ARRIVE guidelines 2.014. Twelve healthy Wistar rats (six males weighing 290-330 g and six females weighing 250-280 g) aged 12-16 weeks were accommodated in the GLP Animal Laboratory of Lanzhou University for 3 days before the actual experiment. The rat cages utilized were of the R5 type, measuring 545 mm x 395 mm x 200 mm, and were equipped with autoclaved bedding material. All rats were provided unrestricted access to both food and water. The laboratory maintained an average humidity of 25%, an average temperature of 24 °C, and a light cycle that alternated between day and night (7:00 AM/7:00 PM). At the conclusion of the study, all animals were humanely euthanized using an overdose of isoflurane. For comprehensive information regarding the materials and instruments employed in this study, please refer to the Table of Materials.
1. Sample size calculation and animal selection
- Choose the resource equation method15 to estimate the animal sample size using equation (1).
E = Total number of animals − Total number of groups (1)
Where E is the degree of freedom of analysis of variance (ANOVA) and ranges from 10 to 20.
NOTE: In this study, 12 animals were divided into two groups A and B (three males and three females per group). - Define the primary outcome of this study as the success rate and time consumption of repeated blood sampling by a single individual.
- Define the secondary outcome measures as changes in rat body weight, food and water intake, as well as the incidence of adverse events (such as clavicle fractures, subcutaneous hematomas, pneumothorax, and mortality).
- Define successful blood sampling as meeting the following criteria: i) fewer than three punctures for a single blood collection; ii) a total time (from rat restraint to completion of blood collection) not exceeding 5 min; and iii) achieving the targeted blood volume while obtaining clear plasma. Consider any deviation from these criteria a sampling failure.
2. Animal restraint and blood collection
NOTE: Blood samples from group A and B rats were collected by two experienced researchers, both of whom had drawn at least 100 blood samples. Blood samples were gathered from both groups of rats for a total of 96 times over the course of 4 days. This blood collection method does not require anesthesia or additional restraint devices for the rats. However, it necessitates adept handling techniques.
- At 8:00 AM on the day before blood sampling (day 1), assign each rat to its individual cage while its food and water are weighed. Then, have another researcher, blinded to the measurements, record the rats' weight, food consumption, and water intake every day at 8:00 AM from day 1 onwards.
- To follow this protocol, first draw blood at 10:00 AM and then at 10:00 PM each day, collecting 0.15 mL of blood alternately from the subclavian veins on both sides.
NOTE: The amount of blood to be collected was determined by the maximum volume that the lowest-weight rat could tolerate within a week. - Flush a syringe with sodium heparin (25 U/mL) and disinfect the injection site with alcohol.
- Gently stroke the rat's back skin and pinch its neck repeatedly to help the rat relax (Video 1).
- Using the non-dominant hand's thumb and index finger, grip and lift the rat's neck skin firmly (Figure 1A and Video 1).
- With coordination from the dominant hand, use the remaining three fingers and palm of the non-dominant hand to secure the rat's back skin and immobilize its front limbs (Figure 1B,C and Video 2).
NOTE: If the rat resists or struggles, this procedure can be repeated several times to help the rat become accustomed to the handling. The following steps are key to successful blood collection. - Using the non-dominant hand's index finger, gently push down on the rat's head skin while the other fingers, along with the palm, assist in outwardly rotating the shoulder joint. During this process, use the dominant hand to fully extend the rat's shoulder joint (Figure 1D-F and Video 2).
- Grip the rat firmly with the non-dominant hand to align the rat's head and body in a straight line (Figure 1G,H). Then, use the dominant hand to locate the position of the clavicle and confirm the puncture site (Figure 1I, Video 2, and Video 3).
NOTE: Shaving the rat is not necessary. In Figure 1, shaving was done only to show the clavicle and puncture position to a greater extent. When restraining rats, especially rats >350 g, allowing the rat to rest its feet on a solid surface will help support their body weight. Additionally, the restrainer should monitor the respiratory rate of each rat while collecting blood to ensure the restraint is not too tight, which may cause respiratory distress. - Holding the syringe parallel to the rat's body in the dominant hand, with the needle tip facing upwards and the syringe scale toward the experimenter, maintain an approximately 15° angle with the midline of the rat's body. Insert the needle 0.5 cm below the clavicle notch (at the junction of the proximal third of the clavicle and the sternum), ensuring that the needle remains parallel to the rat's body (Figure 1J and Video 3).
NOTE: Particular attention should be paid to the angle and depth of the needle insertion to avoid piercing the blood vessel or causing inadvertent damage to adjacent vessels. - Gently withdraw the syringe slightly to create a negative pressure, often accompanied by a palpable sensation of breakthrough upon entering the blood vessel (particularly pronounced during the initial blood collection). Maintain this position and collect 0.1-1.0 mL of blood at a constant speed as needed (following the IACUC guidelines of approximately 4-5.3 mL/kg of blood per week1) (Figure 1K and Video 3).
- If there is no blood upon puncture, try gently adjusting the angle and depth of the needle or gently rotate the syringe (Video 3). If three consecutive attempts on the same side are unsuccessful, stop all bleeding and then switch to the opposite side for the puncture.
NOTE: Swift puncture through the skin is advisable to prevent the rat from struggling due to discomfort. - Apply a cotton swab for hemostasis and return the rat to its cage (Video 4).
- Process the blood samples according to the experimental requirements.
3. Blood sample processing
- Dispose of the syringe needle into a sharps container. Transfer the collected blood into a 1.5 mL microcentrifuge tube previously rinsed with heparin. Place the tube into a centrifuge, setting it at 4 °C and 1,200 x g, and centrifuge for 10 min to separate plasma. Transfer the serum using a 1.0 mL Pasteur pipette into a clean microcentrifuge tube and store it at -80 °C.
NOTE: To prevent hemolysis due to pressure, remove the needle tip when necessary. During plasma aspiration, avoid drawing blood cells from the bottom of the tube. Occasionally, the surface of the syringe might collect rat fur; take care not to allow any fur to enter the tube, as it can lead to clotting.
4. Statistical analysis
- Present all data as mean ± standard deviation and test them for homogeneity of variance.
- Use Fisher's exact test to compare the success rates between groups.
- Use a two-sample independent t-test to compare the overall means between the two groups.
- Use analysis of variance (ANOVA) for continuous measurements such as blood sampling time, body weight, food intake, and water consumption. Consider P < 0.05 statistically significant.
Representative Results
High-quality plasma specimens exhibit a pale-yellow hue, clarity, and transparency, devoid of any red tinge or clotting, as depicted in Figure 2A. Figure 2B shows hemolysis (left side) or coagulation (right side) as a result of improper procedures, respectively. Over the course of 96 blood collection sessions within 4 days, the average single blood collection times for groups A and B were 119.87 ± 33.62 s and 123.28 ± 30.96 s, respectively. There was no significant difference in the blood collection times between the two groups on a daily basis (t = 0.66, P = 0.54, Table 1). The shortest individual blood collection times were 78 s and 89 s, respectively.
The average numbers of attempts required for a successful single blood collection were 1.21 and 1.17 for groups A and B, respectively. There was no significant difference in the number of attempts between the two groups (t = 0.58, P = 0.60, Table 2). The overall success rates were 93.8% (45/48) and 95.8% (46/48) for groups A and B, respectively, with no significant difference in the overall success rates between the two groups (P > 0.05, Table 1). There was no significant difference in the time of blood collection in group B at each time point. In group A, the blood collection time on the second day was less than that on the fourth day (105.75 ± 14.22s vs 144.5 ± 25.45 s, t = 12.39, P < 0.01; Table 1). Additionally, more attempts and longer puncture times often indicate higher failure rates (Figure 3A–C). On the third day, Group B encountered one failure attributed to hemolysis. On the fourth day, Group A encountered three failures: one due to hemolysis and the other two due to the inability to obtain blood samples. Group B also experienced one failure due to the inability to obtain a blood sample.
Over the course of 4 consecutive days of observation, both groups of rats displayed steady weight gain. Water and food intake remained relatively constant among rats of the same sex. Throughout the entire blood collection process, there were no instances of rat mortality, nor were any significant complications observed, such as clavicle fractures, pneumothorax, or puncture site hematomas (Figure 3D–F and Table 3).
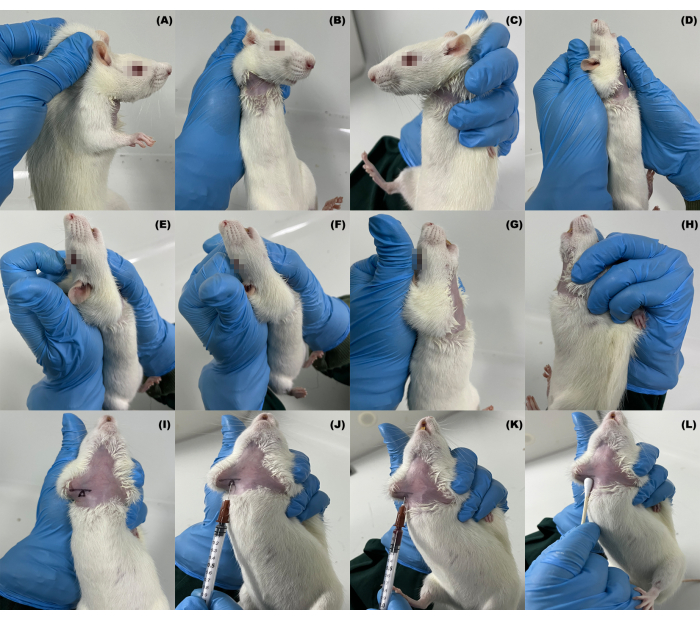
Figure 1: Fixation and blood collection methods of the subclavian vein in rats. (A–H) Manipulation of fixation; (I) location of the collarbone and blood collection site; (J–L) the process of blood collection and hemostasis. Please click here to view a larger version of this figure.
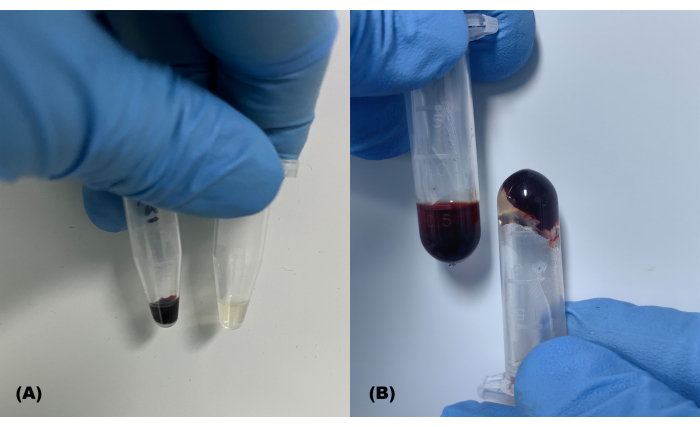
Figure 2: Successfully and unsuccessfully collected blood samples. (A) Typical blood samples and isolated plasma; (B) hemolyzed (left) and coagulated (right) blood samples Please click here to view a larger version of this figure.
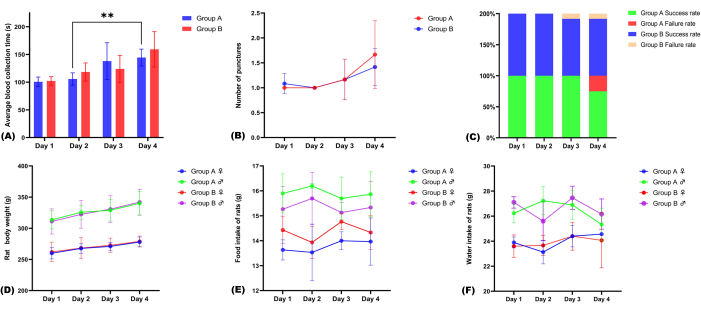
Figure 3: Evaluation of the effectiveness and safety of blood collection. (A) Average time of blood collection per day; (B) average number of punctures per day; (C) the success and failure rates of blood collection in both groups; (D–F) changes in body weight, food intake, and water intake during blood collection in both groups of rats. Please click here to view a larger version of this figure.
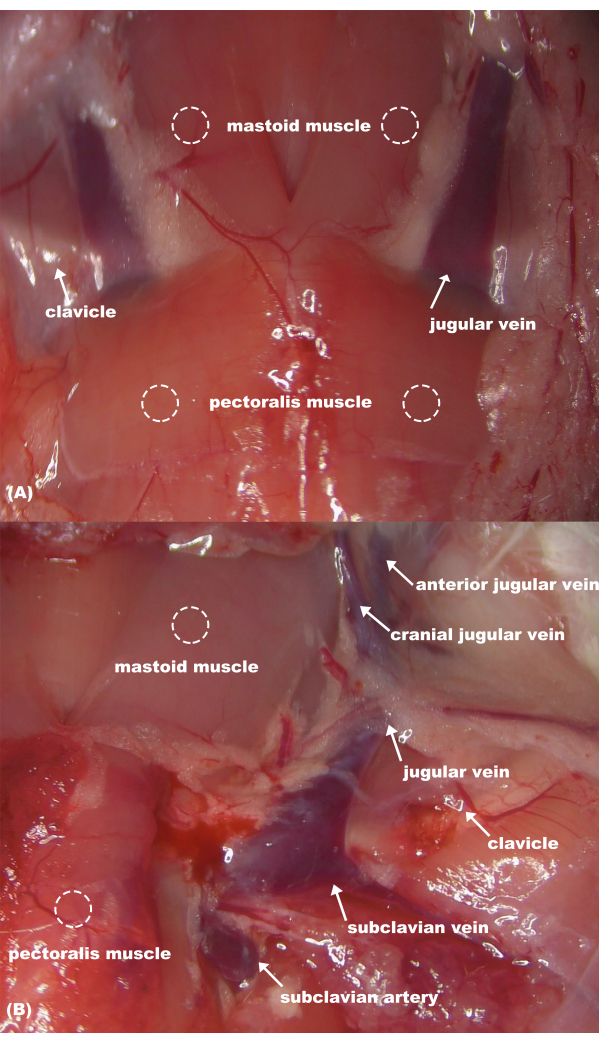
Figure 4: Anatomy of rat neck vessels. (A) Superficial anatomical structures; (B) deep anatomical structures. Please click here to view a larger version of this figure.
| Group | Time | Average blood collection time (s) | |||
| Day 1 | Day 2* | Day 3 | Day 4 | ||
| A | 10:00 AM | 92.83 ± 7.38 | 100.5 ± 17.36 | 117.83 ± 12.02 | 146.6 ± 24.76 |
| 10:00 PM | 108.67 ± 10.86 | 111.00 ± 6.95 | 158.33 ± 60.47 | 142.40 ± 25.96 | |
| Average time | 100.75 ± 12.20 | 105.75 ± 14.22 | 138.08 ± 48.07 | 144.5 ± 25.45 | |
| Success rate | 100% (12/12) | 100% (12/12) | 100% (12/12) | 75% (9/12) | |
| Overall success rate | 93.8% (45/48) | ||||
| Overall average time | 119.87 ± 33.62 | ||||
| B | 10:00 AM | 98.17 ± 7.24 | 110.17 ± 14.33 | 123.67 ± 30.99 | 147.2 ± 17.47 |
| 10:00 PM | 106.00 ± 14.35 | 126.67 ± 17.12 | 123.17 ± 17.50 | 165.67 ± 49.70 | |
| Average time | 102.08 ± 12.02 | 118.42 ± 17.82 | 123.92 ± 25.16 | 157.27 ± 39.63 | |
| Success rate | 100% (12/12) | 100% (12/12) | 91.7% (11/12) | 91.7% (11/12) | |
| Overall success rate | 95.8% (46/48) | ||||
| Overall average time | 123.28 ± 30.96 | ||||
Table 1: Blood collection times and success rates of the two groups of rats. *The blood collection time of the second day was less than that of the fourth day in group A (t = 12.39 P < 0.01).
| Group | Time | Mean number of punctures | |||
| Day 1 | Day 2 | Day 3 | Day 4 | ||
| A | 10:00 AM | 1 | 1 | 1 | 1.67 |
| 10:00 PM | 1 | 1 | 1.33 | 1.67 | |
| Average | 1 | 1 | 1.17 | 1.67 | |
| Overall average | 1.21 | ||||
| B | 10:00 AM | 1 | 1 | 1.17 | 1.5 |
| 10:00 PM | 1.17 | 1 | 1.17 | 1.33 | |
| Average | 1.08 | 1 | 1.17 | 1.42 | |
| Overall average | 1.17 | ||||
Table 2: Mean number of punctures for blood collection in rats.
| Gender | Group | Weight (g) | Food intake (g) | Water intake (g) | |||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 1 | Day 2 | Day 3 | Day 4 | Day 1 | Day 2 | Day 3 | Day 4 | ||
| ♀ | A | 260 ± 7.5 | 267.7 ± 6.3 | 271 ± 5.4 | 278 ± 6.5 | 13.3 ± 0.79 | 13.5 ± 0.93 | 14.0 ± 0.29 | 14.0 ± 0.77 | 23.9 ± 0.36 | 23.1 ± 0.77 | 24.4 ± 0.70 | 24.6 ± 0.12 |
| B | 262 ± 12.8 | 268.3 ± 14.0 | 272.7 ± 9.4 | 279 ± 7.0 | 14.4 ± 0.45 | 13.9 ± 0.52 | 14.7 ± 0.26 | 14.3 ± 0.56 | 23.6 ± 0.73 | 23.7 ± 0.65 | 24.4 ± 0.91 | 24.1 ± 1.79 | |
| t/q | 0.35 | 0.09 | 0.38 | 0.23 | 2.44 | 1.22 | 2.34 | 1.12 | 0.43 | 0.76 | 0.00 | 0.71 | |
| Adjusted P Value | >0.99 | >0.99 | >0.99 | >0.99 | 0.68 | 0.98 | 0.71 | 0.99 | >0.99 | >0.99 | >0.99 | >0.99 | |
| ♂ | A | 313.7 ± 12.0 | 325.7 ± 9.1 | 329 ± 14.2 | 340 ± 15.6 | 15.9 ± 0.64 | 16.2 ± 0.08 | 15.7 ± 0.70 | 15.9 ± 0.73 | 26.2 ± 0.62 | 27.2 ± 0.9 | 26.9 ± 1.0 | 25.3 ± 1.1 |
| B | 311 ± 16.4 | 322.3 ± 18.0 | 330.7 ± 17.6 | 342 ± 16.9 | 15.3 ± 0.74 | 15.7 ± 0.85 | 15.1 ± 0.33 | 15.3 ± 0.86 | 27.1 ± 0.37 | 25.6 ± 1.27 | 27.5 ± 0.76 | 26.2 ± 0.99 | |
| q | 1.50 | 1.88 | 0.94 | 1.13 | 2.34 | 1.85 | 2.10 | 1.97 | 1.90 | 3.57 | 1.24 | 1.82 | |
| Adjusted P Value | 0.94 | 0.86 | >0.99 | 0.99 | 0.71 | 0.87 | 0.79 | 0.83 | 0.86 | 0.33 | 0.98 | 0.88 | |
Table 3: Changes in daily body weight, food intake, and water intake of rats.
Video 1: Calming and handling of rats. Please click here to download this Video.
Video 2: Restraining procedures for rats. Please click here to download this Video.
Video 3: The blood collection procedure for rats. Please click here to download this Video.
Video 4: Hemostatic compression at the puncture site. Please click here to download this Video.
Discussion
Although tail vein blood collection is the most common method for repeated blood sampling in rats, it might be influenced by anesthesia drugs, and due to the small size of the tail vein, the amount of blood that can be collected in a single instance is limited, leading to a longer blood collection duration4,5. Although high-performance liquid chromatography (HPLC) -tandem mass spectrometry (MS/MS) systems combined with capillary microsampling (CMS) of rat tail veins can reduce the amount of blood used in rats11, not all institutions are equipped with this expensive equipment. Blood sampling from the retrobulbar plexus/sinus often causes anxiety and pain in rats, and improper operation can even damage the vision and health of rats. Therefore, this method is not recommended for blood sampling in rats9.
The subclavian vein is situated between the pectoralis major and deltoid muscle of the rat and drains into the jugular vein at a third of the internal clavicle (Figure 4). In the study by Yang et al., the success rate of blood collection from the subclavian vein in rats under anesthesia was approximately 90% by a skilled operator, whose minimum time from the start to the end of the puncture was 65 s7. In Wang et al.'s study, blood samples were collected from the rat subclavian vein using a vertical approach. Although their method did not involve anesthesia, it did require the collaboration of two individuals to securely restrict the rat6. This study protocol shows a good advantage of blood collection. This protocol does not require any special restraint devices or anesthesia facility. With proper handling, rats generally exhibit minimal resistance. The median time from rat restraint to the completion of blood collection was only 2 min, achieving an impressive overall success rate of 95%. This method significantly conserves human resources and reduces the time required for blood collection. Further, the obtained plasma samples are clear and transparent, with minimal occurrence of hemolysis and clotting events, thus minimizing experiment repetition. Proficiency in this technique is particularly valuable for managing large-scale rat pharmacokinetics and toxicology experiments requiring repetitive blood collection.
In our study, the main occurrence of blood draw failure was on day 4, which might be related to the venous damage caused by repeated punctures. Repeated punctures can lead to damage of the vascular wall and provoke an inflammatory response, causing the vascular wall to thicken and harden, and even induce vascular narrowing. If hemostasis is inadequate after puncture, the extravasated blood can further cause tissue edema and inflammation, subsequently leading to the formation of scar tissue. These scar tissues are tough to penetrate and can also pull and cause blood vessels to shift position, all of which make the blood vessels more difficult to locate and puncture. In our study, a 26G syringe (0.45mm) was used for blood collection, which is fine relative to human veins but still causes considerable damage to rat veins. This is evidenced by the clear sensation of penetration when the needle passes through the vessel during the first blood draw, which diminishes as the number of blood draws increases, with longer blood collection times and higher failure rates. Therefore, we recommend using a finer insulin needle for blood collection, and adequate pressure should be applied after blood collection to prevent hematoma formation, and alternate blood draws should be performed to allow sufficient venous repair. In our experience, a well-trained phlebotomist can use a 26G needle to alternately draw blood from the bilateral subclavian veins of the same rat 8-10 times within 24 hours, with an average interval of 2-3 hours between each blood draw. However, the maximum number of blood draws a rat can tolerate, the recovery period, and the blood draw cycle may be influenced by the needle gauge used, the blood draw intervals required by different experiments, and the proficiency of the phlebotomist. These factors need to be further explored in future research. For intensive blood sampling required for pharmacokinetics experiments, it is better to alternately collect blood from the left and right subclavian veins. In cases where it is truly unavailable, other methods of blood collection may be complemented.
Body weight, water consumption, and food intake are the most basic and straightforward indicators for assessing the health status of rats16. An early study had shown that blood collection via the jugular vein of less than 0.9 mL per day did not affect the hemodynamics of rats and did not result in any significant weight loss. However, when blood collection exceeds 1.5 mL, it can lead to weight loss17. In the study by Yokoya et al., repeated microsampling from the jugular vein (50 µL each time, 6-7x within 24 h) did not affect rat body weight or food intake10. In addition, the manner of blood collection may affect the body weight and food intake of rats. In a previous study using sublingual vein blood collection, a 24 h collection of 0.5-1.0 mL of blood on the first day resulted in a reduction in rat body weight and decreased food intake, although the weight loss was not significant18. In this study, the body weight of the rats still increased steadily during the blood collection period, and there were no significant changes in food and water intake, blood collection-related complications, and death of the rats, indicating that this method is safe and reliable.
It is crucial to emphasize that acclimating the rats to the restraint process prior to performing blood collection will likely decrease stress in the rat and improve the success rate of the blood collection. Inadequate fixation and insufficient vein exposure can lead to blood collection failures and even cause local vein rupture due to the rats' struggling in pain. In milder cases, this could result in noticeable subcutaneous hematomas, while in severe cases, it might lead to rat fatalities. Furthermore, poor fixation might lead to rats escaping and causing harm to individuals. Therefore, we strongly recommend mastering the handling technique thoroughly before proceeding with the blood collection procedure. Moreover, it is important to be cautious with the force applied for outwardly rotating the shoulder joint, as excessive pressure might lead to clavicle fractures in the rat.
A limitation of this study is that we did not systematically evaluate the changes in stress induced by this blood collection method in rats by measuring the changes in corticosterone level or by cage-side monitoring, which needs to be explored in future research. Another limitation of this article is the absence of alternative blood collection methods as a control. Comparisons with other blood collection methods for their advantages and disadvantages will be addressed in future research. In all, this study introduces a method for single-person blood collection from rats without the need for anesthesia. This approach offers a straightforward, rapid, and safe means of obtaining blood samples from rats.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This study was supported by Cuiying Plan Project of Lanzhou University Second Hospital (Grant No. PR0121015) and Gansu Provincial Key Laboratory of Urinary System Disease Research (Grant No. 0412D2).
Materials
| 0.75% normal saline | Gansu Fuzheng Pharmaceutical Technology Co., Ltd. | —— | Prepared heparin sodium solution |
| 1 mL Pasteur pipette | Biosharp | BS-XG-01-NS | Blood collection |
| 1 mL syringe (26 G, 0.45 mm x 12 mm) | Shinva Medical Instrument Co.,Ltd. | 0.45*12RWLB | Blood collection |
| 1.5 mL Eppendorf tube | Biosharp | BS-15-M | Blood storage and collection |
| 75% medical alcohol | Shandong Lircon Medical Technology Co., Ltd. | —— | Disinfection of rat blood collection site |
| Centrifuge tube holder | Biosharp | BS-05/15-SM60 | —— |
| Electronic scale | Shanghai PUCHUN Measure Instrument Co., Ltd. | JE1002 | Weigh |
| Heparin sodium injection | Hebei Changshan Biochemical Pharmaceutical Co., Ltd. | —— | Rinse the syringe and EP tube; dilute with normal saline to 25 U/mL |
| Low temperature centrifuge | HuNan Xiang Yi Centrifuge Instrument Co., Ltd. | H1750R | Separation of serum |
Referencias
- . Blood collection: The rat IACUC Guideline Available from: https://iacuc.ucsf.edu/sites/g/files/tkssra751/f/wysiwyg/GUIDELINE%20-%20Blood%20Collection%20-%20Rat.pdf (2022)
- Hattori, N., Takumi, A., Saito, K., Saito, Y. Effects of serial cervical or tail blood sampling on toxicity and toxicokinetic evaluation in rats. The Journal of Toxicological Sciences. 45 (10), 599-609 (2020).
- Liu, X., et al. Modified blood collection from tail veins of non-anesthetized mice with a vacuum blood collection system and eyeglass magnifier. Journal of Visualized Experiments. (144), e65513 (2019).
- Zou, W., et al. Repeated blood collection from tail vein of non-anesthetized rats with a vacuum blood collection system. Journal of Visualized Experiments. (130), e55852 (2017).
- Charlès, L., et al. Modified tail vein and penile vein puncture for blood sampling in the rat model. Journal of Visualized Experiments. (196), e65513 (2023).
- Wang, L., et al. Repetitive blood sampling from the subclavian vein of conscious rat. Journal of Visualized Experiments. (180), e63439 (2022).
- Yang, H., et al. Subclavian vein puncture as an alternative method of blood sample collection in rats. Journal of Visualized Experiments. (141), e58499 (2018).
- Tochitani, T., et al. Effects of microsampling on toxicity assessment of hematotoxic compounds in a general toxicity study in rats. The Journal of Toxicological Sciences. 47 (7), 269-276 (2022).
- Harikrishnan, V. S., Hansen, A. K., Abelson, K. S., Sørensen, D. B. A comparison of various methods of blood sampling in mice and rats: Effects on animal welfare. Laboratory Animals. 52 (3), 253-264 (2018).
- Yokoyama, H., et al. Lack of toxicological influences by microsampling (50 µL) from jugular vein of rats in a collaborative 28-day study. The Journal of Toxicological Sciences. 45 (6), 319-325 (2020).
- Korfmacher, W., et al. Utility of capillary microsampling for rat pharmacokinetic studies: Comparison of tail-vein bleed to jugular vein cannula sampling. Journal of Pharmacological and Toxicological Methods. 76, 7-14 (2015).
- Lu, W., et al. Microsurgical skills of establishing permanent jugular vein cannulation in rats for serial blood sampling of orally administered drug. Journal of Visualized Experiments. (178), e63167 (2021).
- National Research Council of the National Academies, Committee for the Update of the Guide for the Care and Use of Laboratory Animals. . Guide for the Care and Use of Laboratory Animals. , (2011).
- Perciedu Sert, N., et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. The Journal of Physiology. 598 (18), 3793-3801 (2020).
- Charan, J., Kantharia, N. D. How to calculate sample size in animal studies. Journal of Pharmacology & Pharmacotherapeutics. 4 (4), 303-306 (2013).
- Turner, P. V., Pang, D. S., Lofgren, J. L. A review of pain assessment methods in laboratory rodents. Comparative Medicine. 69 (6), 451-467 (2019).
- Kurata, M., Misawa, K., Noguchi, N., Kasuga, Y., Matsumoto, K. Effect of blood collection imitating toxicokinetic study on rat hematological parameters. The Journal of Toxicological Sciences. 22 (3), 231-238 (1997).
- Zeller, W., Weber, H., Panoussis, B., Bürge, T., Bergmann, R. Refinement of blood sampling from the sublingual vein of rats. Laboratory Animals. 32 (4), 369-376 (1998).

