A Quantitative Measurement of Reactive Oxygen Species and Senescence-associated Secretory Phenotype in Normal Human Fibroblasts During Oncogene-induced Senescence
Summary
Intracellular ROS has been shown to play an important role in the induction of cellular senescence. Here, we describe a sensitive assay for quantifying ROS levels during cellular senescence. We also provide protocols for assessing the senescence-associated secretory phenotype, which reportedly contributes to various age-related dysfunctions.
Abstract
Cellular senescence has been considered a state of irreversible growth arrest upon exhaustion of proliferative capacity or exposure to various stresses. Recent studies have extended the role of cellular senescence to various physiological processes, including development, wound healing, immune surveillance, and age-related tissue dysfunction. Although cell cycle arrest is a critical hallmark of cellular senescence, an increased intracellular reactive oxygen species (ROS) production has also been demonstrated to play an important role in the induction of cellular senescence. In addition, recent studies revealed that senescent cells exhibit potent paracrine activities on neighboring cells and tissues through a senescence-associated secretory phenotype (SASP). The sharp increase in interest regarding therapeutic strategies against cellular senescence emphasizes the need for a precise understanding of senescence mechanisms, including intracellular ROS and the SASP. Here, we describe protocols for quantitatively assessing intracellular ROS levels during H-Ras-induced cellular senescence using ROS-sensitive fluorescent dye and flow cytometry. In addition, we introduce sensitive techniques for the analysis of the induction of mRNA expression and secretion of SASP factors. These protocols can be applied to various cellular senescence models.
Introduction
More than 50 years ago, Hayflick and Moorhead revealed that normal cells enter irreversible growth arrest upon the exhaustion of their proliferative potential after a certain number of cell divisions1. This phenomenon is now known as replicative senescence and is believed to strongly correlate with organismal aging2. Although the progressive erosion of telomeres is considered a major cause of replicative senescence, various cellular stresses, such as DNA damage, oncogenic activation, and oxidative stress, have been reported to induce another type of cellular senescence called "premature senescence" or "stress-induced senescence". Interestingly, premature senescence plays a potent tumor-suppressive role upon the activation of oncogenes such as H-Ras and BRAF. Studies of mouse models and human tissues have produced strong evidence that biomarkers of cell senescence were predominantly present in premalignant lesions where oncogenic Ras and BRAF are activated but were diminished in malignant cancers that developed from these lesions3,4,5. Beyond its role in aging and tumor suppression, cellular senescence has been shown in previous studies to play a role in various physiological processes, including wound healing, tissue repair, immune surveillance, and embryonic development6.
Although growth arrest has been extensively studied as a hallmark of cellular senescence7, a significant body of evidence suggests that intracellular reactive oxygen species (ROS) also contributes to cellular senescence8. The elevation of ROS levels during various types of cellular senescence, including replicative senescence and oncogene-induced senescence (OIS), was originally reported decades ago9,10. A more directly, exogenous treatment with a sublethal dose of H2O2 induces senescence11,12. The inhibition of ROS-scavenging enzymes, such as SOD1, also causes premature senescence13. In contrast, low ambient oxygen conditions and increasing ROS scavenging delay the onset of senescence10,14,15. These results undoubtedly indicate that ROS are important mediators or determinants of cellular senescence induction. However, how ROS contribute to the induction of cellular senescence and how ROS levels are elevated during cellular senescence require further investigation.
Recent studies have revealed that senescent cells have potent paracrine activities on neighboring cells and tissues through an SASP16,17. In aged tissue, senescent cells promote age-related tissue dysfunctions via many pathways through SASP in addition to an autonomous depletion of proliferative cells. Various proinflammatory factors, such as IL-6, IL-8, TGFβ, and matrix metalloproteinases (MMPs), secreted by senescent cells, cause age-related tissue dysfunctions through the impairment of tissue homeostasis, destruction of the tissue architecture, senescence of neighboring cells, and sterile inflammation18,19. However, SASPs can have beneficial effects depending on the biological context. In addition, the heterogenetic nature of SASPs depends on the senescent cell type and the cell stage, emphasizing the need for further research19.
Here, we describe rapid and sensitive cytometry-based techniques for assessing intracellular ROS levels during OIS. In addition, methods for the analysis of SASP factors using quantitative real-time polymerase chain reaction (qPCR) and ELISA are introduced.
Protocol
1. Inducing Oncogene-induced Senescence
- Preparing an H -RasV12 retrovirus
- Coat the 100 mm culture dish by adding 2 mL of 0.001% poly-L-lysine/phosphate buffered saline (PBS) for 5 min at room temperature.
- Remove the poly-L-lysine solution using a glass pipette connected to a vacuum and wash the culture dish by adding 2 mL of 1x PBS.
- Plate 3 x 106 ecotropic BOSC-23 packaging cells on the coated culture dish with Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin, and culture them at 37 °C in a CO2 tissue culture incubator for 1 d.
- The next day, transfect 2 µg of pBabe puro-H-RasV12 DNA together with retroviral packaging DNA (2 µg of pGAG/pol and 0.25 µg of pVSVG) using a transfection reagent for 8 h according to the manufacturer's instructions.
- Remove the transfection media and add 8 mL of fresh media.
- After 48 h, collect the media containing the viral particles and remove any cellular debris by centrifugation at 500 x g for 5 min.
- Filter the H-Ras virus-containing media with a 0.45 µm syringe filter.
NOTE: Avoid any freezing and thawing of the virus supernatants for an efficient viral infection. To obtain the best efficiency, use a virus that was harvested on the same day as infection.
- Infecting WI-38 normal human fibroblasts with the H-RasV12 retrovirus
- Plate 5 x 104 WI-38 cells in a 60 mm culture dish with DMEM containing 10% FBS and 1% penicillin/streptomycin 1 d before harvesting the H-RasV12 retrovirus, and culture them for 1 day at 37 °C.
- Remove the growth medium the next day and add 1 mL of H-RasV12 retrovirus media. Add an additional 1 mL of growth medium containing 2 µL of a polybrene stock solution (8 mg/mL in distilled water). Incubate the sample for 1 day in a humidified 37 °C, 5% CO2 tissue culture incubator.
- Remove the H-RasV12 retrovirus mixture 24 h later and wash the cells 2x with 1x PBS. Add growth medium and treat the cells with 2 µg/mL of puromycin for 2 days in a humidified 37 °C, 5% CO2 tissue culture incubator.
- After 2 d of puromycin selection, remove the growth medium, and wash the cells 2x with 1x PBS. Add growth medium and culture the cells in a humidified 37 °C, 5% CO2 incubator until the indicated time point (see Figure 1A for a schematic diagram).
2. Monitoring Senescence via Senescence-associated β-galactosidase Staining
- Prepare the following stock solutions.
- Prepare 20 mg/mL of 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-gal) in dimethyl formamide (DMF). Store the solution at -20 °C.
- Prepare a citric acid/sodium phosphate buffer by dissolving 3.377 g of Na2HPO4.7H2O (126 mM) and 0.708 g of citric acid (36.8 mM) in 100 mL of distilled water. Adjust the pH to 6.0, if necessary.
- Prepare 0.5 M potassium ferrocyanide. Store the solution in the dark at 4 °C.
- Prepare 0.5 M potassium ferricyanide. Store the solution in the dark at 4 °C.
NOTE: The pH of the senescence-associated β-galactosidase (SA β-gal) staining solution is critical for accurate results.
- Make a fresh SA β-gal staining solution by diluting the stock solution containing 150 mM NaCl, 2 mM MgCl2, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 20% (v/v) citric acid/sodium phosphate buffer, and 1 mg/mL of X-gal.
- Remove the culture media and wash the cells 1x with 1x PBS. Fix the cells with 3% formaldehyde for 5 min at room temperature.
NOTE: Cells that are not infected with the retrovirus should be used as a negative control. The uninfected control cells should continue to be passaged to maintain their proliferating status. - Remove the fixation solution from the cells and wash them with 1x PBS. Add freshly prepared 1x SA β-gal staining solution (1.5 mL in a 60 mm culture dish). Seal the dish with a paraffin film to prevent drying and incubate it for 24 h at 37 °C. Alternatively, incubate the dishes together in a humidity chamber.
NOTE: The fixative solution containing 3% formaldehyde should be disposed of as hazardous waste. An incubator without CO2 is desirable, to prevent pH changes during the incubation. - Check the staining status of the cells under a microscope on the next day; SA β-gal-positive cells appear blue in the perinuclear region.
NOTE: The proliferating control cells show a low frequency of SA β-gal-positive cells. If the staining is too light, continue the incubation for a slightly longer period (see Figure 1B for representative examples). - Acquire an SA β-gal staining image with a CCD camera using a 10X or greater objective lens. Capture a sufficient number of images to calculate the staining percentage (>200 cells).
- Calculate the percentage of SA β-gal-stained cells by quantifying the number of the SA β-gal-positive cells relative to the total number of cells.
NOTE: SA β-gal staining should be repeated independently at least 3x, and the mean values should be calculated based on the results of replicate experiments (Figure 1C). The appropriate cell density is critical for the SA β-gal staining experiment. A high confluency can interfere with the change to a senescence-associated morphology and induce a false-positive signal in the control cells.
3. Quantifying a Reactive Oxygen Species Induction During H-Ras-induced Senescence
- Plate 2 x 105 WI-38 cells on a 60 mm culture dish and provoke H-Ras-induced senescence as described in step 1.
NOTE: This protocol can be applied to other types of cellular senescence models. - Remove the growth medium at the indicated time point (2, 4, or 6 days), and wash the cells with 1x PBS. Detach the cells by treating them with 1 mL of 0.05% trypsin/EDTA solution and inactivate the trypsin by adding 1 mL of growth medium containing 10% FBS. Determine the cell count.
- Transfer 1 x 105 cells into a 15 mL conical tube, and collect the cells by centrifugation at 500 x g for 5 min.
- Prepare fresh 2',7'-dichlorofluorescin diacetate (DCF-DA) staining media by adding 50 µL of 10 mM DCF-DA to 10 mL of culture medium (the working concentration of DCF-DA is 50 µM).
NOTE: DCF-DA is light sensitive. Light exposure should be avoided.The concentration of DCF-DA and the staining time can be adjusted depending on the cell type. - Remove the growth medium carefully from the 15 mL conical tube and add 1 mL of freshly prepared DCF-DA staining medium. Carefully resuspend the cell pellet and incubate it at 37 °C for 30 min in the dark. Include a non-stained sample as a negative staining control.
NOTE: Proliferating cells not stained with DCF-DA should be prepared as a negative control. - Collect the cells by centrifugation at 500 x g for 5 min and wash them 1x with 2 mL of 1x PBS. Resuspend the cells with 1 mL of 1x PBS and transfer the sample to the appropriate fluorescence-activated cell sorting (FACS) tube.
- Measure the 2′,7′-Dichlorodihydrofluorescein diacetate (DCF-DA) fluorescence signal using a flow cytometer equipped with an appropriate laser source. Excite the samples with a blue laser (488 nm) and detect the emitted fluorescence with a 530 ± 30 nm detector (FITC) using a logarithmic scale.
- Analyze the non-stained negative control cells first. In a dot plot of forward scatter (FSC) versus side scatter (SSC), select live cells using a gating tool and eliminate dead cells and cellular debris.
NOTE: The cell size change should be carefully monitored in the dot plot of FSC versus SSC. If the size of the senescent cells is significantly increased, the DCF-DA intensity should be compared in cell populations of the same size after gating in the dot plot of FSC versus SSC. - In a single-parameter histogram displaying DCF-DA (488 nm) fluorescence on the logarithmic scale of the x-axis and the number of events (cell number) on the linear scale of the y-axis, adjust the voltage of the 488 nm laser to place the peak from the non-stained cells at the left edge.
- Analyze the DCF-DA stained samples and record at least 10,000 events for each sample. Acquire the mean fluorescence value of each sample using analysis software specific to the flow cytometer.
NOTE: The ROS measurements should be repeated independently at least 3x, and the mean values should be calculated from these results. Representative H-Ras-induced senescence results are shown in Figure 2.
- Analyze the non-stained negative control cells first. In a dot plot of forward scatter (FSC) versus side scatter (SSC), select live cells using a gating tool and eliminate dead cells and cellular debris.
4. Quantifying IL-6 and IL-8 mRNA expression for Senescence-associated Secretory Phenotype Analysis Using a Real-time Polymerase Chain Reaction
- Total RNA preparation
- Plate 3 x 105 WI-38 cells on a 100 mm culture dish in DMEM containing 10% FBS and 1% penicillin/streptomycin, and culture them at 37 °C in a tissue culture incubator for 1 day. Prepare duplicate cultures for each time point.
- Induce senescence by infecting the cells with the H-RasV12 retrovirus as described in step 1.
- At 1 day before the harvest, wash the cells 2x with 1x PBS, and add 3 mL of DMEM without FBS.
NOTE: This step is conducted to produce the conditioned medium used for ELISA as described in step 5. Serum starvation may induce an expression of IL-6 and IL-8 mRNAs in some sensitive cells. Thus, the IL-6 and IL-8 mRNA expression in cells cultured with and without serum starvation should be compared initially. If serum starvation induces significant changes in the expression of the IL-6 or IL-8 mRNA, the total RNA for qPCR and the conditioned medium for ELISA should be prepared separately. - Collect the conditioned medium from each sample 24 h later and store the medium at -80 °C for ELISA.
- Detach the cells by treating them with 1 mL of 0.05% trypsin/EDTA solution and inactivate the trypsin by adding 1 mL of growth medium containing 10% FBS. Determine the cell count for the normalization of the ELISA results as described in step 5. Harvest the cells in a 1.5 mL microcentrifuge tube by centrifugation at 500 x g for 5 min.
- Remove the medium by gentle suction and add 1 mL of RNA extraction solution. Then, vortex the microcentrifuge tube vigorously for 15 s. Subsequently, add 200 µL of chloroform and vigorously vortex the mixture again for an additional 15 s.
- Centrifuge the sample tubes at 17,000 x g for 10 min at 4 °C. Then, carefully transfer the upper phase to a new 1.5 mL microcentrifuge tube.
NOTE: The interphase and the lower organic phase should not be disturbed during the transfer of the upper phase to a new tube. - Add 400 µL of isopropanol to precipitate the RNA and mix them well by gentle inversion. Then, incubate the tube at room temperature for 10 min.
- Centrifuge the tube at 17,000 x g for 10 min at 4 °C. Then, discard the supernatant, taking care to retain the RNA pellet.Wash the RNA by adding 1 mL of 75% ethanol and invert the tube 2–3x. Centrifuge the tube for 5 min at 17,000 x g at 4 °C and discard the supernatant.
- Dry the RNA pellet at room temperature and dissolve the RNA in 50 µL of diethyl pyrocarbonate (DEPC)-treated distilled water. Using 1 µL of RNA solution, quantify the total RNA concentration with a spectrophotometer.
NOTE: Overdrying will reduce the solubility of the RNA; therefore, avoid overdrying the RNA.
- cDNA preparation
- Prepare the reverse-transcription reaction mixture for each sample by adding the following into a thin-walled PCR tube: 2 µg of total RNA (adjust the volume to 10 µL with RNase-free water), 2 µL of 10x RT buffer, 1 µL of a random primer (50 pmol), 0.8 µL of 25x dNTP mix (100 mM), 1 µL of MMLV reverse transcriptase (50 U/µL), and 5.2 µL of RNase-free water.
- Set up the reverse transcription reaction program on the thermal cycler with the following conditions: 42 °C for 60 min and 95 °C for 5 min. Place the PCR tubes in the thermal cycler and start the program.
- Real-time polymerase chain reaction
- Prepare the real-time PCR master mix for all reactions. The reaction mixture for each sample is as follows: 25 µL of 2x real-time PCR Master Mix, 1 µL each of the forward and reverse primers for IL-6 or IL-8, and 21 µL of ultra-pure distilled water (DNase- and RNase-free). See Table 1.
NOTE: Prepare the real-time PCR reaction mixture for each sample containing actin RNA primers as an internal control. GAPDH can also be used as an internal control for normalization. - Add 48 µL of master mix and 2 µL of 3-fold diluted cDNA product with water to each well in a 96-well optical qPCR plate. Perform each reaction in triplicate. Prepare a control well that does not contain the cDNA template as a PCR control.
- Set up the real-time PCR reaction program on the thermal cycler with the following conditions: 10 min at 95 °C, 40 cycles of15 s at 95 °C (denaturation), and 1 min at 60 °C (annealing and extension).
- Perform real-time PCR and record the threshold cycle (CT) value after the completion of the PCR cycles. Calculate the mRNA levels for IL-6 or IL-8 using the 2-ΔΔCΤ method20.
NOTE: The relative fold change in expression is determined after the normalization to the actin levels by using the following formula:
Fold change = 2-Δ(ΔCT)
Here,
ΔCT = CT, IL-6 or IL-8-CT, actin; and
Δ(ΔCT) = ΔCT, stimulated-ΔCT, control. - Calculate the mean value for each duplicate sample.
NOTE: qPCR should be repeated independently at least 3x, and the mean values should be calculated based on these results. Representative H-Ras-induced senescence results are shown in Figure 3. Serum starvation may induce the expression of the IL-6 and IL-8 mRNAs in some sensitive cells. Thus, the IL-6 and IL-8 mRNA expression in cells cultured with and without serum starvation should be compared initially. If serum starvation induces significant changes in the expression of IL-6 or IL-8 mRNA, the total RNA for qPCR and the conditioned medium for ELISA should be prepared separately.
- Prepare the real-time PCR master mix for all reactions. The reaction mixture for each sample is as follows: 25 µL of 2x real-time PCR Master Mix, 1 µL each of the forward and reverse primers for IL-6 or IL-8, and 21 µL of ultra-pure distilled water (DNase- and RNase-free). See Table 1.
5. Quantifying the Levels of Secreted IL-6 and IL-8 Proteins for a Senescence-associated Secretory Phenotype Analysis Using ELISA
- Thaw the 3 mL of conditioned medium harvested from senescent WI-38 cells as described in step 4. Remove cell debris by centrifugation at 500 x g for 5 min.
- Concentrate the conditioned medium 10-fold by centrifugation at 3,000 x g for 20 min using a centrifugal filter unit.
NOTE: The concentration of the conditioned medium may not necessarily depend on the sample type. - Perform the ELISA using 50 µL of each concentrated sample of conditioned medium with the appropriate IL-6 and IL-8 ELISA kits.
NOTE: Test each sample in two ELISA wells. Because each time point is duplicated in step 4, this analysis allows us to monitor variations in both the ELISA assay and the sample preparation technique.- For ELISA plate coating, dilute the IL-6 or IL-8 antibody with 1x PBS to a concentration of 0.5 µg/mL for IL-6 or 0.125 µg/mL for IL-8. Add 100 µL of the diluted antibodies to each well of the ELISA plate. Seal the plate with an ELISA plate sealing film and incubate without shaking overnight at room temperature.
- Remove the antibody solution by aspiration. Wash each well 4x with 300 µL of 1x wash buffer (0.05% Tween-20 in PBS). Add 300 µL of blocking buffer (1% BSA in PBS) to each well. Incubate at room temperature for 1 h.
- Remove the blocking solution by aspiration. Wash each well 4x with 300 µL of 1x wash buffer. Prepare serially diluted ELISA standards in a dilution buffer (0.05% Tween-20 and 0.1% BSA in PBS) to generate a standard curve.
NOTE: The serial dilution for IL-6 is 31.25, 62.5, 125, 250, 500, 1,000, and 2,000 pg/mL. The serial dilution for IL-8 is 2.34, 4.69, 9.38, 18.75, 37.5, 75, and 150 pg/mL. - Add either 100 µL of the standard solution or 100 µL of the sample solution (50 µL of concentrated medium with 50 µL of dilution buffer) to each well. Incubate the wells at room temperature for 2 h.
- Remove the standard or sample solution by aspiration. Wash each well 4x with 300 µL of 1x wash buffer. Dilute the detection antibody with the dilution buffer to a concentration of 0.1 µg/mL for IL-6 or 0.25 µg/mL for IL-8. Add 100 µL of the diluted antibody per well. Incubate the wells at room temperature for 2 h.
- Remove the antibody solution by aspiration. Wash each well 4x with 300 µL of 1x wash buffer. Dilute streptavidin-horseradish peroxidase (HRP) in the dilution buffer to a concentration of 0.05 µg/mL. Add 100 µL of streptavidin-HRP solution per well. Incubate the wells at room temperature for 30 min.
- Remove the antibody solution by aspiration. Wash each well 4x with 300 µL of 1x wash buffer. Add 100 µL of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution to each well. Incubate the wells at room temperature for 20 min to allow for color development. Stop the reaction by adding 100 µL of a 1 M HCl stop solution.
- Read the absorbance of each well with an ELISA reader at 450 nm.
- Plot the standard curve for the generated standards. Calculate the concentrations of IL-6 and IL-8 in the conditioned medium according to the standard curves. Plot the results after a normalization to the cell count. Calculate the mean values for duplicate samples.
NOTE: ELISAs should be repeated independently at least 3x, and the mean values should be calculated based on these results (Figure 4).
Representative Results
An example of H-Ras-induced senescence is shown in Figure 1. An infection of WI-38 normal human fibroblasts with the H-RasV12 retrovirus induced dramatic morphological changes (Figure 1B). In addition, as shown in Figure 1C, SA β-gal staining activity was remarkably increased upon H-RasV12 expression. More than 70% of the cells showed SA β-gal staining activity 6 d after the H-RasV12 retrovirus infection, indicating that an expression of H-RasV12 successfully induces cellular senescence in WI-38 cells, as we and other groups reported previously21,22.
Figure 2 shows representative results of the DCF-DA staining analysis for monitoring ROS levels during H-Ras-induced cellular senescence. Increased intracellular ROS levels are observed as early as 2 days after the H-Ras expression and are maintained until the 6-day time point. Meanwhile, the induction of the SASP shows different kinetics. Increases in the mRNA levels of IL-6 and IL-8, representative SASP factors, are observed from 4 days after the H-Ras expression and peak on the 8-day time point (Figure 3). As illustrated in Figure 4, the secreted IL-6 and IL-8 levels show similar increases, consistent with the real-time PCR results. These results suggest different roles of ROS and SASP in the induction of cellular senescence.
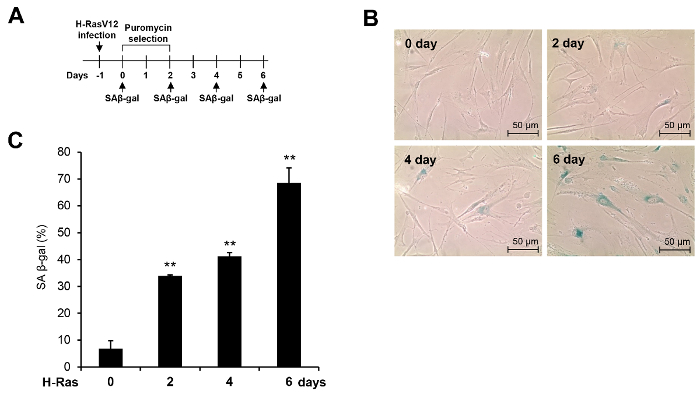
Figure 1: Morphological changes and increase in SA β-gal staining during H-Ras-induced senescence. (A) This panel shows the experimental procedure for the induction of H-Ras-induced senescence in WI-38 cells. (B) SA β-gal staining was performed at the indicated time point after the infection of WI-38 cells with the H-RasV12 retrovirus; here, representative images of the SA β-gal staining are shown. (C) SA β-gal-positive cells were counted in three independent experiments. The results are presented as the mean values, and the error bars represent standard deviations (SD). ** P <0.01, Student's t-test. Please click here to view a larger version of this figure.
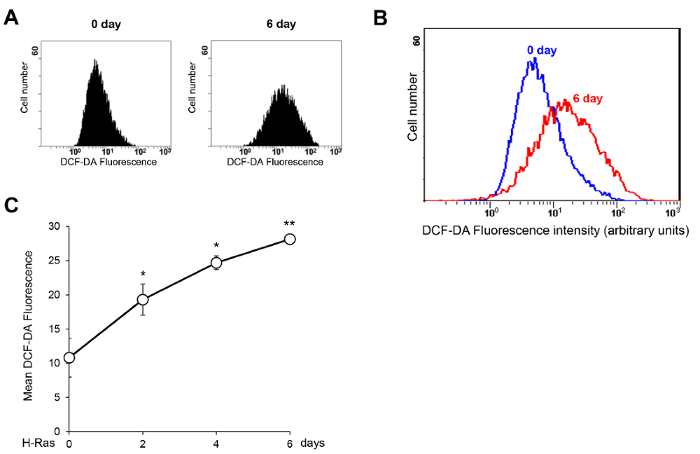
Figure 2: Increase in intracellular ROS levels during H-Ras-induced senescence. (A) WI-38 cells were infected with the H-Ras retrovirus and stained with DCF-DA (50 µM) at the indicated time points. DCF-DA fluorescence intensities were quantified using a flow cytometer. Representative histograms of the DCF-DA fluorescence intensity at the 0- and 6-d time points are shown. (B) Representative histograms of the DCF-DA fluorescence intensity at the 0- and 6-day time points are plotted together for easier comparison. (C) The DCF-DA fluorescence intensity during H-Ras-induced senescence was measured at the indicated time points 3x. The results are presented as the mean values, and the error bars represent standard deviations (SD). * P <0.05 and ** P <0.01, Student's t-test. Please click here to view a larger version of this figure.
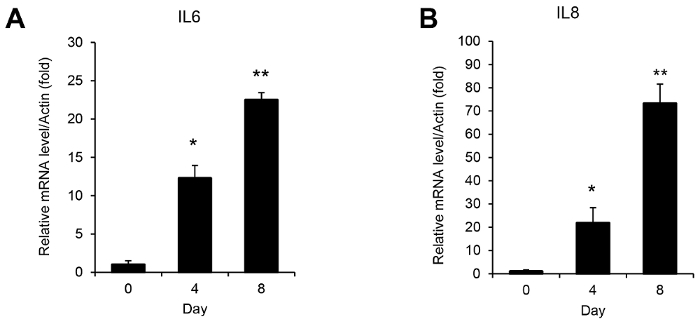
Figure 3: Quantification of SASP-related mRNAs during H-Ras-induced senescence. RNA was prepared from WI-38 cells at the indicated time points after the H-RasV12 retrovirus infection. These panels show the induction of the (A) IL-6 and (B) IL-8 expression analyzed by qPCR using the specific primers listed in Table 1. The actin mRNA levels were used for normalization. The normalized IL-6 or IL-8 mRNA levels measured in the 0-day sample were set to 1 and the relative fold changes were calculated. The experiments were independently repeated 3x. The results are presented as the mean values, and the error bars indicate standard deviations (SD). * P <0.05 and ** P <0.01, Student's t-test. Please click here to view a larger version of this figure.
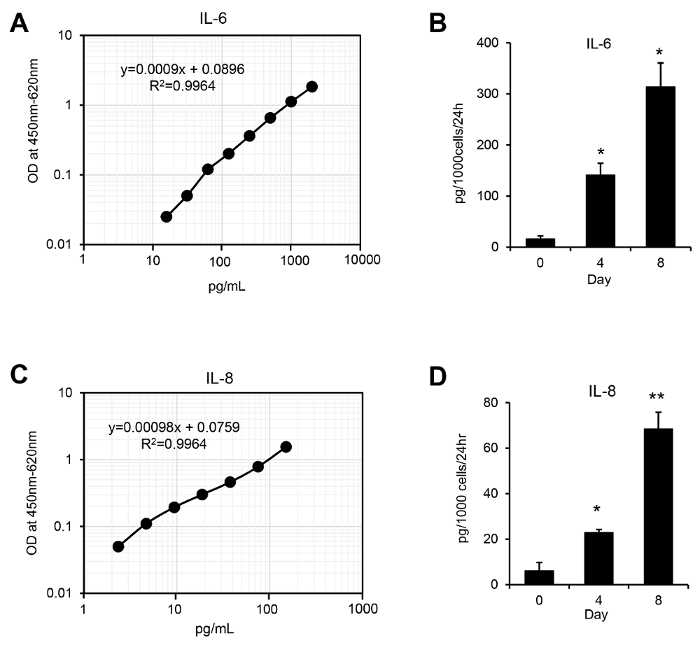
Figure 4: Quantification of secreted SASP factors during H-Ras-induced senescence. These panels show standard curves generated with IL-6 (A) and IL-8 (C) standards. Conditioned media were harvested from WI-38 cells at the indicated time points after H-RasV12 retrovirus infection. The secreted IL-6 (B) and IL-8 levels (D) were analyzed with an IL-6 and IL-8 ELISA kit, respectively. The experiments were independently repeated 3x. The results are presented as the mean values, and the error bars indicate standard deviations (SD). * P <0.05 and ** P <0.01, Student's t-test. Please click here to view a larger version of this figure.
| Gene name | Forward primer | Reverse primer |
| IL-6 | 5'-ACTCACCTCTTCAGAACGAATTG-3' | 5'-CCATCTTTGGAAGGTTCAGGTTG-3' |
| IL-8 | 5'-ACTGAGAGTGATTGAGAGTGGAC-3' | 5'-AACCCTCTGCACCCAGTTTTC-3' |
| actin | 5'-CAAGAGATGGCCACGGCTGCT-3' | 5'-TCCTTCTGCATCCTGTCGGCA-3' |
Table 1: Real-time PCR primers for SASP factors.
Discussion
Here, we have presented methods for monitoring intracellular ROS levels during H-Ras-induced senescence in WI-38 normal human fibroblasts. Intracellular ROS levels in live cells can be measured quantitatively using the cell-permeable reagent DCF-DA and flow cytometry. Upon cellular uptake, DCF-DA is deacetylated by intracellular esterases and, subsequently, oxidized by ROS to form highly fluorescent 2',7'-dichlorofluorescein (DCF). DCF fluorescence can be detected by flow cytometry using an FL1 detector (green fluorescence). Using the DCF-DA staining method, we successfully detected an increase in ROS levels during H-Ras-induced cellular senescence in WI-38 normal human fibroblasts. This method can be used in various models of cellular senescence to monitor the changes in ROS levels. In addition to DCF-DA staining, additional methods that have been used to measure the ROS induction include monitoring the levels of oxidized proteins23 and a confocal microscopy analysis using ROS-dependent fluorescent dyes24. Thus, further confirmation of changes in ROS levels using additional methods is desirable.
Recently, we have shown that ROS levels are increased in cancer cells during p53-induced senescence as well as H-Ras-induced senescence21. Increased intracellular ROS levels are observable prior to the increase in SA β-gal staining activity, suggesting the causative role of ROS in the induction of cellular senescence. Interestingly, the increase in ROS levels is separately controlled by the Akt-NF-κB-NOX4 pathway, while cell cycle arrest is mediated by p2121. Whether Akt also regulates the ROS increase in other types of cellular senescence would be interesting to explore.
We have also introduced methods for analyzing the mRNA and secreted protein levels of the SASP factors IL-6 and IL-8 during H-Ras-induced senescence. Using qPCR, we quantified the induction of IL-6 and IL-8 mRNA in senescent WI-38 cells. We examined actin mRNA levels as an internal control for normalization, but GAPDH mRNA can also be used as an internal control. The 18S ribosomal RNA (rRNA) is another housekeeping gene that is commonly used as an internal control in qPCR experiments. However, because rRNA transcription and ribosome maturation are regulated during cell cycle arrest and cellular senescence25,26, 18S rRNA may not be a good internal control for cellular senescence studies. ELISA, using a conditioned medium from senescent WI-38 cells, showed similar increases in secreted IL-6 and IL-8 levels. Consistent with the notion that the SASP is developed at the "mature" senescence stage19, increases in IL-6 and IL-8 secretion are detectable at a later time point than the increase in ROS levels. Notably, SASP factors vary depending on the cell types and senescence induction conditions27. Therefore, the appropriate SASP factors should be selected according to the cellular senescence model.
Although we confirmed H-Ras-induced senescence by monitoring morphological changes and the induction of SA β-gal staining activity, cellular senescence should be further verified by monitoring additional senescence characteristics, including the irreversible loss of proliferation potential, senescence-associated heterochromatin foci (SAHF), SASP factors, the activation of tumor suppressors such as p53 and p16INK4a, enhanced DNA damage signals, and persistent nuclear foci, defined as DNA segments with chromatin alterations reinforcing senescence (DNA-SCARS). However, it is important to remember that there is a heterogeneity of senescence characteristics in different senescence types. Certain senescence biomarkers can be observable only in some specific types of cellular senescence. For example, SAHF are usually evident in OIS28,29.
Cellular senescence was initially considered to represent autonomous growth arrest caused by artificial culture conditions. However, studies in the past half-century have demonstrated the importance of cellular senescence under various pathophysiological conditions, including aging and cancer. The causal link between cellular senescence and age-related tissue deterioration has been proven previously in BubR1 progeroid mouse models30,31. Thus, controlling cell senescence through either the elimination of senescent cells or the modulation of the SASP is currently considered as a promising strategy for age-related disease. Indeed, recent studies demonstrated that the elimination of senescent cells would be a promising treatment for age-related pathologies including stem cell depletion, bone loss, hair loss, and osteoarthritis32,33,34,35,36. A deeper understanding of the molecular mechanisms of senescence induction and senescence phenotypes, including SASPs, will provide improved strategies for targeting senescence by reducing possible drawbacks and selectively targeting only deleterious functions of senescent cells.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea (2015R1D1A1A01060839) (to Young Yeon Kim) and by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2016R1A2B2008887, No. 2016R1A5A2007009) (to Jeanho Yun).
Materials
| REAGENTS | |||
| poly-L-lysine | Sigma-Aldrich | P2636 | |
| BOSC 23 | ATCC | CRL-11269 | |
| FBS | GIBCO | 16000-044 | |
| penicillin/streptomycin | wellgene | LS202-02 | |
| PBS | Hyclone | SH30013.02 | |
| DMEM | GIBCO | 12800-082 | |
| OPTI-MEM | GIBCO | 31985-070 | |
| pBabe puro-H-RasV12 | Addgene | 1768 | |
| pGAG/pol | Addgene | 14887 | |
| pVSVG | Addgene | 1733 | |
| Turbofect | Thermo Fisher Scientific | R0531 | |
| polybrene | Sigma-Aldrich | H9268 | 8 mg/ml |
| puromycin | Sigma-Aldrich | P8833 | 2 mg/ml |
| formaldehyde | Sigma-Aldrich | F8775 | |
| 5-bromo-4-chloro-3-indolyl β D-galactopyranoside (X-gal) | Sigma-Aldrich | B4252 | |
| potassium ferrocyanide | Sigma-Aldrich | B4252 | |
| potassium ferricyanide | Sigma-Aldrich | P9387 | |
| trypsin-EDTA | wellgene | LS015-01 | |
| DCF-DA | Sigma-Aldrich | D6883 | 10 mM |
| Trizol | Thermo Fisher Scientific | 15596026 | |
| MMLV Reverse transcriptase | Promega | M1701 | |
| SYBR Green PCR master 2X mix | Takara | PR820A | |
| Random Primer | Promega | C118A | |
| Tween-20 | Sigma-Aldrich | P9416 | |
| Ultra-pure distilled water | Invitrogen | 10977015 | |
| Human IL-6 ELISA assay | PeproTech | #900-TM16 | |
| Human IL-8 ELISA assay | PeproTech | #900_TM18 | |
| EQUIPMENTS | |||
| 0.45 μm syringe filter | sartorius | 16555 | |
| Parafilm | BEMIS | PM-996 | |
| Microscope | NIKON | TS100 | |
| Flow cytometer | BD Bioscience | LSR Fortessa | |
| Amicon Ultra-4ml | Merk Millipore | UFC800324 | |
| NanoDrop spectrophotometer | BioDrop | 80-3006-61 | |
| Real-time PCR System | Applied Biosystems | ABI Prism 7500 | |
| ELISA Reader | Molecular Devices | EMax microplate reader |
References
- Hayflick, L., Moorhead, P. S. The serial cultivation of human diploid cell strains. Experimental Cell Research. 25, 585-621 (1961).
- Campisi, J. Aging, cellular senescence, and cancer. Annual Review of Physiology. 75, 685-705 (2013).
- Braig, M., et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 436 (7051), 660-665 (2005).
- Michaloglou, C., et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 436 (7051), 720-724 (2005).
- Collado, M., et al. Tumour biology: senescence in premalignant tumours. Nature. 436 (7051), 642 (2005).
- Malaquin, N., Martinez, A., Rodier, F. Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Experimental Gerontology. 82, 39-49 (2016).
- Kuilman, T., Michaloglou, C., Mooi, W. J., Peeper, D. S. The essence of senescence. Genes & Development. 24 (22), 2463-2479 (2010).
- Lu, T., Finkel, T. Free radicals and senescence. Experimental Cell Research. 314 (9), 1918-1922 (2008).
- Furumoto, K., Inoue, E., Nagao, N., Hiyama, E., Miwa, N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sciences. 63 (11), 935-948 (1998).
- Lee, A. C., et al. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. The Journal of Biological Chemistry. 274 (12), 7936-7940 (1999).
- Chen, Q., Ames, B. N. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proceedings of the National Academy of Sciences of the United States of America. 91 (10), 4130-4134 (1994).
- Dumont, P., et al. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radical Biology & Medicine. 28 (3), 361-373 (2000).
- Blander, G., de Oliveira, R. M., Conboy, C. M., Haigis, M., Guarente, L. Superoxide dismutase 1 knock-down induces senescence in human fibroblasts. The Journal of Biological Chemistry. 278 (40), 38966-38969 (2003).
- Packer, L., Fuehr, K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 267 (5610), 423-425 (1977).
- Serra, V., von Zglinicki, T., Lorenz, M., Saretzki, G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. The Journal of Biological Chemistry. 278 (9), 6824-6830 (2003).
- Rodier, F., Campisi, J. Four faces of cellular senescence. The Journal of Cell Biology. 192 (4), 547-556 (2011).
- Munoz-Espin, D., Serrano, M. Cellular senescence: from physiology to pathology. Nature Reviews. Molecular Cell Biology. 15 (7), 482-496 (2014).
- Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J., Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. The Journal of Clinical Investigation. 123 (3), 966-972 (2013).
- van Deursen, J. M. The role of senescent cells in ageing. Nature. 509 (7501), 439-446 (2014).
- Livak, K. J., Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25 (4), 402-408 (2001).
- Kim, Y. Y., et al. Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell. 16 (5), 1094-1103 (2017).
- Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D., Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 88 (5), 593-602 (1997).
- Wu, D., Yotnda, P. Production and detection of reactive oxygen species (ROS) in cancers. Journal of Visualized Experiments. (57), (2011).
- Wojtala, A., et al. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods in Enzymology. 542, 243-262 (2014).
- Duncan, F. E., et al. Age-associated dysregulation of protein metabolism in the mammalian oocyte. Aging Cell. 16 (6), 1381-1393 (2017).
- Yang, L., Song, T., Chen, L., Soliman, H., Chen, J. Nucleolar repression facilitates initiation and maintenance of senescence. Cell Cycle. 14 (22), 3613-3623 (2015).
- Coppe, J. P., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biology. 6 (12), 2853-2868 (2008).
- Kosar, M., et al. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a). Cell Cycle. 10 (3), 457-468 (2011).
- Sharpless, N. E., Sherr, C. J. Forging a signature of in vivo senescence. Nature Reviews. Cancer. 15 (7), 397-408 (2015).
- Baker, D. J., et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nature Cell Biology. 10 (7), 825-836 (2008).
- Baker, D. J., et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 479 (7372), 232-236 (2011).
- Baar, M. P., et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 169 (1), 132-147 (2017).
- Farr, J. N., et al. Targeting cellular senescence prevents age-related bone loss in mice. Nature Medicine. 23 (9), 1072-1079 (2017).
- Chang, J., et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine. 22 (1), 78-83 (2016).
- Yosef, R., et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nature Communications. 7, 11190 (2016).
- Jeon, O. H., et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature Medicine. 23 (6), 775-781 (2017).

