Carrier-assisted One-pot Sample Preparation for Targeted Proteomics Analysis of Small Numbers of Human Cells
Summary
A protein carrier-assisted one-pot sample preparation coupled with liquid chromatography (LC) – selected reaction monitoring (SRM) termed cLC-SRM is a convenient method for multiplexed targeted proteomics analysis of small numbers of cells, including single cells. It capitalizes on using excessive exogenous protein as a carrier and high-specificity LC-SRM for targeted quantification.
Abstract
Protein analysis of small numbers of human cells is primarily achieved by targeted proteomics with antibody-based immunoassays, which have inherent limitations (e.g., low multiplex and unavailability of antibodies for new proteins). Mass spectrometry (MS)-based targeted proteomics has emerged as an alternative because it is antibody-free, high multiplex, and has high specificity and quantitation accuracy. Recent advances in MS instrumentation make MS-based targeted proteomics possible for multiplexed quantification of highly abundant proteins in single cells. However, there is a technical challenge for effective processing of single cells with minimal sample loss for MS analysis. To address this issue, we have recently developed a convenient protein carrier-assisted one-pot sample preparation coupled with liquid chromatography (LC) – selected reaction monitoring (SRM) termed cLC-SRM for targeted proteomics analysis of small numbers of human cells. This method capitalizes on using the combined excessive exogenous protein as a carrier and low-volume one-pot processing to greatly reduce surface adsorption losses as well as high-specificity LC-SRM to effectively address the increased dynamic concentration range due to the addition of exogeneous carrier protein. Its utility has been demonstrated by accurate quantification of most moderately abundant proteins in small numbers of cells (e.g., 10-100 cells) and highly abundant proteins in single cells. The easy-to-implement features and no need for specific devices make this method readily accessible to most proteomics laboratories. Herein we have provided a detailed protocol for cLC-SRM analysis of small numbers of human cells including cell sorting, cell lysis and digestion, LC-SRM analysis, and data analysis. Further improvements in detection sensitivity and sample throughput are needed towards targeted single-cell proteomics analysis. We anticipate that cLC-SRM will be broadly applied to biomedical research and systems biology with the potential of facilitating precision medicine.
Introduction
Recent technological advances in genomics (transcriptomics) allow for comprehensive and precise analysis of the genome (transcriptome) in single cells1,2,3. However, single-cell proteomics technologies are lagging far behind but are just as important as genomics (transcriptomics) technologies4,5,6,7,8. Furthermore, protein abundance cannot necessarily be inferred from mRNA abundance9, and the proteome is more complex and dynamic than the transcriptome10. Given these challenges, a large number of mixed populations of cells (i.e., bulk cells) are generally used to generate comprehensive proteome data11,12,13. However, such bulk measurements average out stochastic variations of individual cells, thus obscure important cell-to-cell variability (i.e., cell heterogeneity)4,14. Limitations of such bulk measurements become even more severe when the cells of interest only account for a small portion of the total populations of cells (e.g., cancer stem cells within tumors at an early-stage cancer). Therefore, there is a huge knowledge gap between single-cell proteomics and genomics (transcriptomics).
Antibody-based immunoassays (e.g., flow or mass cytometry) are predominantly used for targeted proteomic analysis of single cells6,7,15,16,17,18. However, they suffer from low multiplex, limited specificity, and unavailability of antibodies for new proteins of interest. Mass spectrometry (MS)-based targeted proteomics has emerged as an alternative for accurate protein quantification because of its being antibody-free, high multiplex (≥150 proteins in a single analysis19), high quantitation accuracy (absolute amounts or concentrations), and high specificity and reproducibility (≤10% CV)20,21,22,23. Recent significant progress in sample preparation has made MS-based single-cell proteomics possible for quantitative analysis of highly abundant proteins from single human cells. However, MS-based single-cell proteomics is still at the early infancy stage. For example, the most advanced MS platform coupled with ultralow-flow RPLC flow rates can only allow label-free MS detection and quantification of ~670-870 proteins out of the total ≥12,000 proteins in single HeLa cells24,25.
Currently, there are six MS-based single-cell proteomics approaches available for analysis of single mammalian cells, in which four are for global proteomics (nanoPOTS: nanowell-based Preparation in One pot for Trace Samples26; iPAD-1: integrated proteome analysis device for single-cell analysis27; OAD (oil-air-droplet) chip-based single cell proteomic analysis28; SCoPE-MS: single cell proteomics by mass spectrometry29) and the other two are for targeted proteomics (cLC-SRM: carrier-assisted liquid chromatography (LC) – selected reaction monitoring (SRM)30; cPRISM-SRM: carrier-assisted high pressure, high-resolution separations with intelligent selection and multiplexing coupled to SRM31). However, all these approaches have technical drawbacks. nanoPOTS, iPAD-1, and OAD downscale sample processing volume to 2-200 nL and are not ready for broad benchtop applications26,27,28. For SCoPE-MS, a TMT (tandem mass tag) carrier is added after single-cell processing, so it cannot effectively prevent surface adsorption losses during sample processing when a single tube is used for single-cell processing29, resulting in low reproducibility with a correlation coefficient of only ~0.2-0.4 between replicates32. For cLC-SRM and cPRISM-SRM, using exogenous proteins as a carrier is more suitable for targeted proteomics because peptides from excessive exogenous proteins are frequently sequenced by MS/MS, which greatly reduces the chance for sequencing low abundant endogenous peptides30,31. Unlike global proteomics for relative quantification, the two targeted proteomics approaches can provide accurate or absolute protein analysis of small numbers of cells with high reproducibility using heavy isotope-labeled internal standards at known concentrations. When compared to cPRISM-SRM that requires prior high-resolution PRISM fractionation, resulting in many fraction samples that need to be analyzed, cLC-SRM has a significant advantage in sample throughput without fractionation and can simultaneously quantify hundreds of proteins in a single analysis but with relatively lower detection sensitivity30. Therefore, cLC-SRM is more accessible and should have broader utilities for accurate multiplexed protein analysis of small numbers of cells as well as mass-limited samples.
Herein we describe a detailed protocol to perform cLC-SRM for convenient targeted proteomics analysis of small numbers of human cells, including single cells. The protocol consists of the following major steps: cell sorting by FACS (fluorescence activated cell sorting), cell lysis and digestion processed in low-volume single polymerase chain reaction (PCR) tubes, LC-SRM data collection, and SRM data analysis using publicly available Skyline software (Figure 1). Its broad utility was demonstrated along with our previously well-established SRM assays by absolute targeted quantification of EGFR/MAPK pathway proteins in 1-100 MCF7 or MCF10A cells and determination of pathway protein copies per cell at a wide dynamic range of concentrations30. We anticipate that with the detailed protocol most proteomics researchers can readily implement cLC-SRM in their laboratories for accurate protein analysis of ultrasmall samples (e.g., rare tumor cells) to meet their project needs.
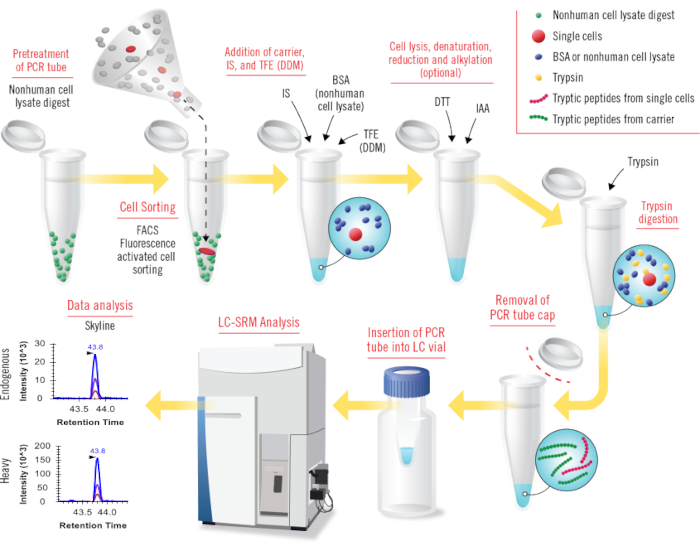
Figure 1: Overview of all steps in cLC-SRM (carried-assisted one-pot sample preparation coupled with liquid chromatography –selected reaction monitoring). Nonhuman cell lysate digests (e.g., Shewanella oneidensis) are used to pretreat PCR tubes for coating tube surface. Small numbers of human cells or single cells sorted by FACS are collected into pretreated PCR tubes. BSA protein (or nonhuman cell lysate proteome) carrier, heavy internal standard (IS), and TFE (or DDM) are added into sample tubes sequentially for facilitating cell lysis and reducing surface adsorption losses. Conceptually, the combined DDM and nonhuman cell lysate proteome carrier will work well for cLC-SRM. Cell lysis is conducted by sonication, and protein denaturation is achieved by heating at high temperature. DTT and IAA reagents are used for reduction and alkylation, respectively (this step is optional). Trypsin is added for digestion with much higher ratios of trypsin over protein amount than that for standard trypsin digestion. The cap of sample tube is removed and then PCR tube is inserted into LC vial for direct LC-SRM analysis. Collected SRM data are analyzed by using publicly available Skyline software. Please click here to view a larger version of this figure.
Protocol
NOTE: The step-by-step cLC-SRM analysis is shown in Figure 1.
1. Pretreatment of PCR tubes
- Add 100 µL of nonhuman (e.g., Shewanella oneidensis) cell lysate digests at 0.2 µg/µL to PCR tubes. Incubate at room temperature for overnight to coat PCR tube surface.
- Remove the cell lysate digests by pipetting, rinse PCR tubes with HPLC-grade water 3 times, and then airdry PCR tubes in a fume hood.
- Store coated PCR tubes in a 4 ˚C freezer till further use.
2. FACS sorting
- Align a fluorescence-activated cell sorter (FACS) (see Table of Materials) into a 96-well PCR plate using fluorescent beads with dyes that can be excited by the laser source to provide reliable reference signals.
- Verify that the FACS machine is well aimed for sorting cells into the bottom of PCR plates.
- Set the angle of the sort stream to the minimum possible to maximize the success rate that the cells will be sorted into the bottoms of wells rather than hit the sides.
- To aim the machine, use the test stream to 'sort' droplets of PBS buffer (e.g., 100 droplets) onto the well of an empty plate to make sure that the collected droplets are on the bottom. If the droplets do not land in the right position, recalibrate the cell sorter and repeat till the droplets in the sort stream are collected correctly.
- Place the coated PCR tubes into a 96-well PCR tube rack.
- Sort desired numbers of human breast cells (MCF7 or MCF10A) into precoated PCR tubes.
- Immediately centrifuge the sorted breast cells at 100 x g for 10 min at 4 °C to keep them at the bottom of the tube to avoid potential cell loss.
- Store the sorted cells in a -80 °C freezer until further analysis.
3. Addition of protein carrier, heavy internal standards, and TFE
- Add 4 µL of 25 mM NH4HCO3 into PCR tubes containing collected cells.
- Add 1 µL of 10 ng/µL bovine serum albumin (BSA) in 25 mM NH4HCO3 into sample tubes.
- Add 0.3 µL of 100 fmol/µL crude heavy peptide standards (total 30 fmol) into sample tubes.
NOTE: Aliquots of crude heavy peptide standards for EGFR/MAPK pathway proteins are made at a nominal concentration of 100 fmol/µL in 25 mM NH4HCO3. Dispense the small volume of crude heavy peptide standards at 100 fmol/µL into the middle of the solution and make sure there is no left-over liquid in the pipet tip. - Add 9 µL of 100% TFE into sample tubes with the final concentration of ~60% TFE.
- Centrifuge at 1500 x g for 5 min and then gently vortex at 100 x g for 3 min.
4. Cell lysis and protein denaturation
- Sonicate FACS-sorted breast cells on ice for 1 min with 1-min intervals for a total of 5 cycles at the 70% amplitude and 0.5 Hz.
- Incubate samples at 90 °C for 1 h for protein denaturation using a PCR thermocycler with the heated-lid option.
NOTE: TFE is a volatile solvent with a boiling point of 74 °C, and thus the heated-lid option is selected to avoid complete drying with substantial sample loss. - Cool samples to room temperature with centrifugation at 1500 x g for 3 min.
5. Reduction and alkylation (optional)
- Add 0.6 µL of 50 mM DTT for a final concentration of 2 mM.
NOTE: DTT solution needs to be freshly prepared each time. Weigh 77.5 mg of DTT and then dissolve into 1 mL of 25 mM NH4HCO3 to make 500 mM DTT. 10 µL of 500 mM DTT is then added into 90 µL of 25 mM NH4HCO3 to make 50 mM DTT. - Centrifuge at 1500 x g for 5 min and then gently mix at 850 rpm for 3 min.
- Incubate at 56°C for 1 h with gentle shaking at 100 x g.
- Cool samples to room temperature and centrifuge at 1500 x g for 3 min.
- Add 0.5 µL of 60 mM IAA to the RT-PCR tube with the final concentration of ~2 mM.
NOTE: IAA solution needs to be freshly prepared each time. Weigh 74.3 mg of IAA and then dissolve into 1 mL of 25 mM NH4HCO3 to make 400 mM IAA. 15 µL of 400 mM IAA is added into 85 µL of 25 mM NH4HCO3 to make 60 mM IAA. - Incubate in the dark at room temperature for 30 min with gentle shaking at 100 x g.
NOTE: The reduction and alkylation steps are optional when there are no cysteine-containing peptides selected for target proteins.
6.Trypsin digestion
- Reduce the sample volume to ~4 µL using a SpeedVac concentrator.
NOTE: TFE is a volatile solvent with boiling point at 74°C. High percentage TFE inhibits trypsin digestion. - Add 9 µL of 25 mM NH4HCO3 and 1-3 µL of 15 ng/µL trypsin for digestion with the final trypsin concentration of 1-3 ng/µL.
NOTE: For 10 FACS-sorted human breast cells (~1 ng), the ratio of trypsin enzyme over protein is ≥15:1, 750-fold higher than that in standard trypsin digestion (the ratio of 1:50). - Mix gently at 100 x g for 3 min and incubate overnight (~16 hours) at 37 °C.
- Add 0.5 µL of 5% formic acid to stop the enzymatic reaction.
- Centrifuge for 1 h at 1500 x g.
- Store in -80 °C freezer until further LC-SRM analysis or in 4 °C freezer for immediate analysis.
7. Preparation for direct LC-SRM analysis
- Remove the cap of the PCR tube that was used for collection (Step 2) and processing (Step 6) of FACS-sorted breast cells.
- Insert PCR tube into LC vial.
- Close the LC vial for LC-SRM analysis.
NOTE: For small numbers of cells processed in 96-well PCR plate, the mat cover will be added to seal the plate for direct LC-SRM analysis.
8. LC-SRM analysis
- Analyze samples using UPLC coupled to a triple quadrupole mass spectrometer.
- Connect capillary C18 columns (75 µm i.d. × 20 cm for standard gradient and 100 µm i.d. × 10 cm for short gradient) to a chemically etched 20 µm i.d. fused silica electrospray emitter via a stainless metal union.
- Use a 20 µL sample loop for directly loading all of the sample (~15 µL in total) into the LC column to maximize detection sensitivity.
- Use 0.1% formic acid in water and 0.1% formic acid in 90% acetonitrile as mobile phases A and B for capillary RPLC separation.
- Use the binary LC gradient at flow rates of 300 nL/min for a standard gradient when large numbers of target proteins (>10) are measured. Standard gradient: 5-20% B in 26 min, 20-25% B in 10 min, 25-40% B in 8 min, 40-95% B in 1 min and at 95% B for 7 min for a total of 52 min and the analytical column re-equilibrated at 99.5% A for 8 min.
- Use the binary LC gradient at flow rates of 400 nL/min for a short gradient when small numbers of target proteins (≤10) are measured. Short gradient for fast separation: 5-95% B in 5 min and the analytical column re-equilibrated at 99.5% A for 5 min with a total 10 min LC run time.
- Operate the QQQ mass spectrometer with ion spray voltages of 2,400 ± 100 V, a capillary offset voltage of 35 V, a skimmer offset voltage of -5 V and a capillary inlet temperature of 220° C.
- Obtain the other QQQ MS parameters from automatic tuning and calibration without further optimization.
- Spike different concentrations of crude heavy peptide standards into 20-50 ng/µL of nonhuman cell lysate digests before LC-SRM analysis of samples to determine peptide LC retention time (RT) for building a scheduled SRM method and potential interference transitions for accurate quantification.
- Use the RT scheduled SRM mode for multiplexed quantification with the scan window of ≥6 min.
NOTE: The scan window will be adjusted accordingly depending on the number of transitions in a certain time window. - Set the total cycle time to 1 s, and the dwell time for each transition is automatically adjusted depending on the number of transitions scanned at different retention time windows. A minimal dwell time of 10 ms is needed for each SRM transition. All target proteins can be simultaneously monitored in a single LC-SRM analysis
9. Data Analysis
- Import raw data files into publicly available Skyline software33 for data visualization of each target peptide to determine their detectability.
- Use two criteria to determine SRM peak detection and integration: 1) same LC retention time; 2) approximately the same relative SRM peak intensity ratios across multiple transitions between endogenous (light) peptides and their corresponding (heavy) isotope-labeled peptide internal standards.
- Inspect all the SRM data manually to ensure correct SRM peak assignment and SRM peak boundary for reliable quantification using the above two criteria.
- Use the best transition with a high SRM signal but without matrix interference for protein quantification, and use the other transitions as a reference.
- Use multiple replicates to obtain the standard deviation (SD) and coefficient of variation (CV).
- Calculate the signal-to-noise ratio (S/N) by the peak apex intensity over the average background noise within a retention time region of ±15 s for standard gradient and ±6 s for short gradient. The limit of detection (LOD) and the limit of quantification (LOQ) are defined as the lowest concentration points at which the S/N ratio is at least 3 and 7, respectively.
- Apply one additional criteria for conservatively determining the LOQ values besides S/N ≥7: surrogate peptide response against different cell numbers must be within the linear dynamic range.
- Use the Excel software to plot all calibration curves. Load the RAW data from QQQ MS into publicly available Skyline software to display graphs of extracted ion chromatograms (XICs) of multiple transitions of target proteins monitored.
Representative Results
Small amounts of MCF7 cell lysates (0.5-20 ng equivalent to 5-200 cells) were first used to evaluate the performance of cLC-SRM by targeted quantification of EGFR/MAPK pathway proteins because they are more uniform with less variations when compared to small numbers of cells sorted by FACS. As shown in Figure 2A, XICs clearly shows detection of SRM transitions for ATADDELSFK derived from GRB2 present at ~220,000 copies per MCF7 cell34. cLC-SRM enabled reproducible quantification of endogenous ATADDELSFK down to 5 MCF7 cell equivalents with a S/N ratio of 14 and ~1,800 zmol of quantification sensitivity. The resultant calibration curves displayed excellent linearity with LOQs of 5 cells for high-abundance GRB2 protein and 20 cells for moderate-abundance PTPN11 protein (Figure 2B). The median SRM technical CV for all target peptides across all the data points was ~9%, consistent with the technical reproducibility of standard LC-SRM with CV below 10%20,23,35,36,37,38.
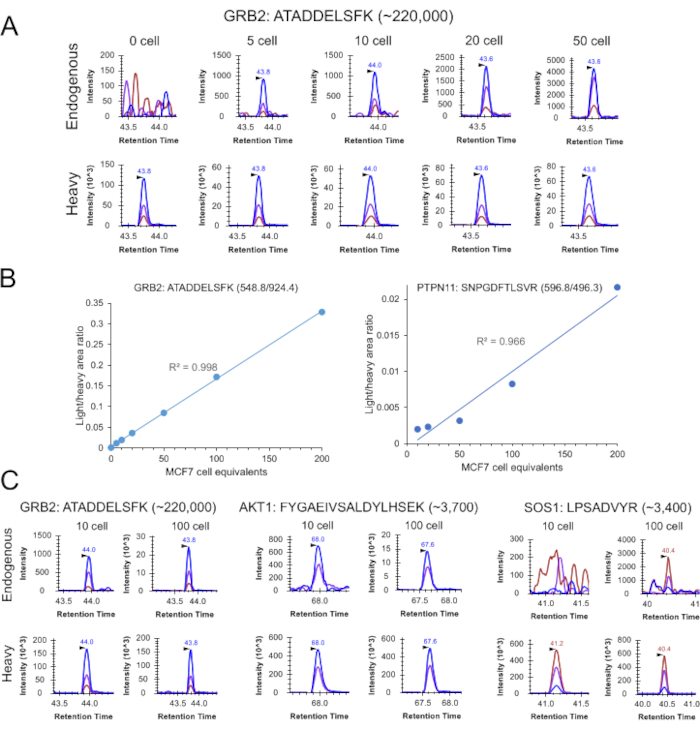
Figure 2: Sensitivity and accuracy of cLC-SRM for multiplexed quantification of EGFR/MAPK pathway proteins. (A) Extracted ion chromatograms (XICs) of transitions monitored for ATADDELSFK derived from GRB2 at different numbers of MCF7 cell equivalents: 548.8/924.4 (blue), 548.8/853.4 (purple), 548.8/738.4 (chestnut). (B) Calibration curves for quantifying high abundance GRB2 and moderate-abundance PTPN11 with the use of the best responsive interference-free transitions, ATADDELSFK (548.8/924.4) for GRB2 and SNPGDFTLSVR (596.9/496.3) for PTPN11. Three and two SRM replicates were performed for 0-10 and 20-200 MCF7 cell equivalents, respectively. (C) Comparison of SRM signal between 10 and 100 MCF7 cells sorted by FACS. Each sample consists of two biological replicates with the addition of ~30 fmol of heavy peptide standards per replicate. XICs of transitions monitored for ATADDELSFK derived from GRB2: 548.8/924.4 (blue), 548.8/853.4 (purple), 548.8/738.4 (chestnut); XICs of transitions monitored for FYGAEIVSALDYLHSEK derived from AKT1: 648.0/897.9 (blue), 648.0/816.4 (purple), 648.0/283.1 (chestnut); XICs of transitions monitored for LPSADVYR derived from SOS1: 460.7/807.4 (blue), 460.7/710.3 (purple), 460.7/404.2 (chestnut). This figure has been modified from Zhang et al30 with the explicit permission from ACS publisher. Please click here to view a larger version of this figure.
cLC-SRM was next applied to measure EGFR pathway proteins in 10 and 100 intact MCF7 cells sorted by FACS. cLC-SRM enabled detection of high- and moderate-abundance proteins in 10 intact MCF7 cells (Figure 2C). Interestingly, low-abundance AKT1 protein (~3700 copies per MCF7 cell) was also detected with the average S/N ratio of 7, suggesting ~60 zmol of absolute sensitivity of cLC-SRM. With the cell number increased to 100, most previously identified important EGFR pathway proteins (22 out of the total 32 proteins which have a wide dynamic concentration range)34 were reliably detected and quantified by cLC-SRM. Furthermore, we have recently tested whether a short gradient time (e.g., 5 min vs standard 45 min) is sufficient for rapid sensitive cLC-SRM analysis. As shown in Figure 3, XICs clearly shows endogenous detection of VLTPTQVK peptide derived from PEBP1 at ~744,000 copies per cell34 in single MCF10A cells sorted by FACS with a S/N ratio of 5 and ~1,240 zmol of quantification sensitivity. As expected, with the cell number increased to 50 and 75, stronger SRM signal was observed with detection of all three transitions, which have the same pattern as its corresponding heavy internal standard (Figure 3A). The calibration curve has linearity with R2 of 0.89 (Figure 3B). Thus, a short gradient is feasible for cLC-SRM presumably because sample complexity for ≤20 ng of tryptic peptides (10 ng BSA and ≤100 human cells ≈ 10 ng proteins) from carrier-assisted small numbers of cells can be effectively addressed by high-resolution capillary RPLC separation with high loading capacity of ≥200 ng. Taken together, these results have shown that cLC-SRM can be used for multiplexed, sensitive, absolute quantification of target proteins in small numbers of human cells including single cells.
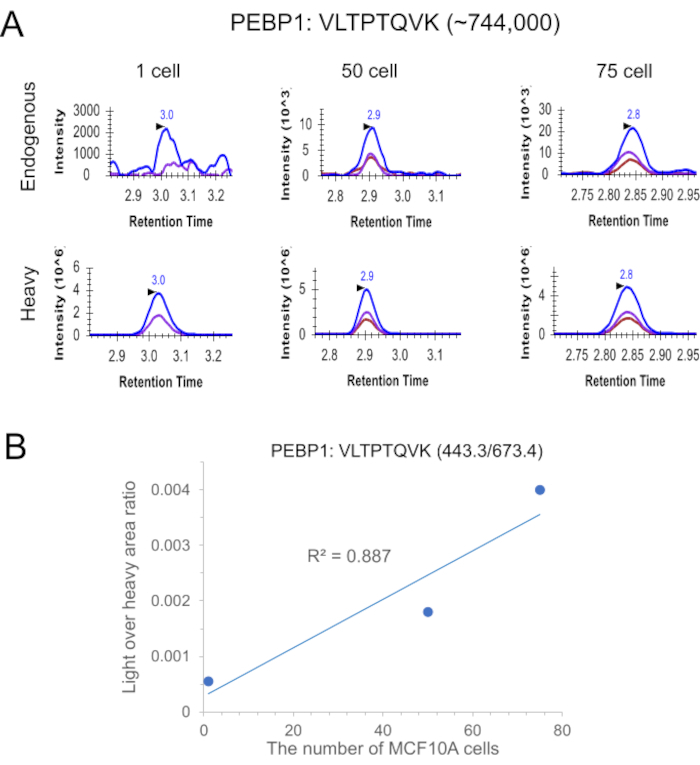
Figure 3: An example of targeted proteomics analysis of small numbers of MCF10A cells including single cells with cLC-SRM at short LC gradient. (A) Comparison of SRM signal among 1, 50 and 75 MCF10A cells sorted by FACS. ~30 fmol of internal standard was added to each sample. XICs of transitions monitored for VLTPTQVK derived from PEBP1: 443.3/673.4 (blue), 443.3/572.3 (purple), 443.3/213.2 (chestnut). For single cells, the transition 443.3/213.2 was removed due to severe matrix interference. (B) Calibration curve for quantifying PEBP1 with the use of the best responsive interference-free transition, VLTPTQVK (443.3/673.4). Please click here to view a larger version of this figure.
Discussion
cLC-SRM is a convenient targeted proteomics method that enables accurate multiplexed protein analysis of small numbers of cells including single cells. This method capitalizes on protein carrier-assisted one-pot sample preparation, in which all steps including cell collection, multistep cell lysis and digestion, and transfer of peptide digests to capillary LC column for MS analysis are performed in one pot (e.g., single tube or single well) (Figure 1). This 'all-in-one' low-volume one-pot processing effectively maximizes the recovery of small numbers of cells for targeted proteomics analysis by greatly reducing possible surface absorption losses.
There are two critical steps for cLC-SRM analysis: 1) after FACS sorting, immediate centrifugation is required to ensure that the collected cells are at the bottom of tubes or wells because a low volume (~15 µL) is used to process small numbers of cells; 2) prior to the addition of trypsin for digestion, the TFE levels need to be reduced down to ≤10% for effective digestion. To avoid drying out samples resulting in unrecoverable loss, frequently checking the sample volume (~4 µL of the remaining volume) is suggested during SpeedVac concentrating. One-pot sample preparation can be further simplified by removal of reduction and alkylation steps with negligible effect on digestion efficiency. One drawback is that cysteine containing peptides cannot be selected as surrogate peptides for target proteins. To avoid the SpeedVac concentrating step for TFE removal we considered replacing TFE with MS-compatible surfactants (e.g., DDM: n-Dodecyl β-D-maltoside) for cell lysis because frequently checking the sample volume severely prevents automation of sample processing with low throughput. This was evidenced by our recent data for DDM-assisted global single-cell proteomics analysis (unpublished). In addition, a single carrier protein (e.g., BSA) may not be sufficient to reduce surface adsorption losses for every protein without bias. This was evidenced by a few undetected proteins at >30,000 copies per cell (e.g., MAP2K1) in 10 MCF7 cells that should have been detected30. Non-human cell lysates presumably can be used as a more effective proteome carrier in contrast to our current BSA carrier because there are many different types of nonhuman proteins (e.g., >3000 proteins for Shewanella oneidensis) with less interference for SRM detection of human proteins. We are working on several different ways to further improve cLC-SRM performance with the potential of moving towards sensitive targeted single-cell proteomics.
When compared to other MS-based single-cell proteomics methods that require specific devices, cLC-SRM can be readily implemented in any MS proteomics laboratory without additional investment on specific devices for sample processing. But it can only be used for quantitative analysis of target proteins of interest. Unlike global single-cell proteomics methods for relative untargeted quantification, cLC-SRM can provide accurate or absolute quantification of target proteins in small numbers of cells including single cells. Furthermore, cLC-SRM has significant advantages over conventional targeted single-cell proteomics using antibody-based immunoassays in terms of multiplexing and quantitation specificity and accuracy. Future developments will focus on significant improvements in detection sensitivity and sample throughput for enabling rapid absolute quantification of most proteins (e.g., important negative feedback regulators) in single human cells. Automation of cLC-SRM is another direction to improve its robustness, reproducibility, and the overall sample throughput with commercially available liquid handlers. We anticipate that cLC-SRM will be broadly used for multiplexed accurate quantification of target proteins in small subpopulations of cells and rare tumor cells as well as mass-limited samples. In turn, it will greatly benefit current biomedical research and systems biology.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH Grant R21CA223715 (TS) and UG3CA256967 (TS and HL). The experimental work described herein was performed in the Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, a national scientific user facility sponsored by the United States of America Department of Energy under Contract DE-AC05-76RL0 1830.
Materials
| 2 mL glass LC vial | Microsolv | 9502S-WCV | Vessel to hold PCR tube for autosample injection |
| BSA | Sigma-Aldrich | P0834-10×1mL | Carrier protein for greatly reducing surface adsorption losses |
| DTT | Thermo Scientific | A39255 | Reagent for reduction |
| Formic acid | Thermo Scientific | 28905 | Reagent for stopping enzyme reaction |
| IAA | Thermo Scientific | A39271 | Reagent for alkylation |
| Peptide internal standards | New England peptide | Targeted quantification of EGFR/MAPK pathway proteins | |
| RT-PCR tube | GeneMate Bioexpress | T-3035-1 | 0.2 mL PCR tube for one-pot sample preparation |
| Skyline software | University of Washington | Publicly available for SRM data analysis | |
| Sonicator | Hielscher Ultrasound Technology | UTR200 | Sonication on ice for cell lysis |
| Speed Vac concentrator | Thermo Scientific | Reduction of the percentage of TFE for effective trypsin digestion | |
| TFE | Sigma-Aldrich | 18370-10×1mL | 60% TFE for cell lysis |
| Thermocycler w/ heated lid | Peltier Thermal Cycler | PTC-200 | Heating for protein denaturation |
| Trypsin Gold | Promega | V528A | Enzyme for protein digestion |
| Waters BEH C18 column | Waters | C18 column for peptide separation |
References
- Wang, Z., Gerstein, M., Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nature Reviews Genetics. 10 (1), 57-63 (2009).
- Miyamoto, D. T., et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 349 (6254), 1351-1356 (2015).
- Wang, Y., Navin, N. E. Advances and applications of single-cell sequencing technologies. Molecular Cell. 58 (4), 598-609 (2015).
- Macaulay, I. C., Ponting, C. P., Voet, T. Single-Cell Multiomics: Multiple Measurements from Single Cells. Trends in Genetics. 33 (2), 155-168 (2017).
- Gavasso, S., Gullaksen, S. E., Skavland, J., Gjertsen, B. T. Single-cell proteomics: potential implications for cancer diagnostics. Expert Review of Molecular Diagnostics. 16 (5), 579-589 (2016).
- Wu, M., Singh, A. K. Single-cell protein analysis. Current Opinion in Biotechnology. 23 (1), 83-88 (2012).
- Hughes, A. J., et al. Single-cell western blotting. Nature Methods. 11 (7), 749-755 (2014).
- Peterson, V. M., et al. Multiplexed quantification of proteins and transcripts in single cells. Nature Biotechnology. 35 (10), 936-939 (2017).
- Liu, Y., Beyer, A., Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell. 165 (3), 535-550 (2016).
- Choudhary, C., Mann, M. Decoding signalling networks by mass spectrometry-based proteomics. Nature Reviews Molecular Cell Biology. 11 (6), 427-439 (2010).
- Mertins, P., et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature. 534 (7605), 55-62 (2016).
- Zhang, H., et al. Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell. 166 (3), 755-765 (2016).
- Zhang, B., et al. Proteogenomic characterization of human colon and rectal cancer. Nature. 513 (7518), 382-387 (2014).
- Gaudet, S., Miller-Jensen, K. Redefining Signaling Pathways with an Expanding Single-Cell Toolbox. Trends in Biotechnology. 34 (6), 458-469 (2016).
- Giesen, C., et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nature Methods. 11 (4), 417-422 (2014).
- Di Palma, S., Bodenmiller, B. Unraveling cell populations in tumors by single-cell mass cytometry. Current Opinion in Biotechnology. 31, 122-129 (2015).
- Schulz, K. R., Danna, E. A., Krutzik, P. O., Nolan, G. P. Single-cell phospho-protein analysis by flow cytometry. Current Protocols in Immunology. , 11-20 (2012).
- Willison, K. R., Klug, D. R. Quantitative single cell and single molecule proteomics for clinical studies. Current Opinion in Biotechnology. 24 (4), 745-751 (2013).
- Lee, J. Y., et al. Detection of Head and Neck Cancer Based on Longitudinal Changes in Serum Protein Abundance. Cancer Epidemiology, Biomarkers & Prevention. 29 (8), 1665-1672 (2020).
- Shi, T., et al. Advancing the sensitivity of selected reaction monitoring-based targeted quantitative proteomics. Proteomics. 12 (8), 1074-1092 (2012).
- Shi, T., et al. Advances in targeted proteomics and applications to biomedical research. Proteomics. 16 (15-16), 2160-2182 (2016).
- Lange, V., Picotti, P., Domon, B., Aebersold, R. Selected reaction monitoring for quantitative proteomics: a tutorial. Molecular Systems Biology. 4, 222 (2008).
- Picotti, P., Aebersold, R. Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nature Methods. 9 (6), 555-566 (2012).
- Zhu, Y., et al. Proteomic Analysis of Single Mammalian Cells Enabled by Microfluidic Nanodroplet Sample Preparation and Ultrasensitive NanoLC-MS. Angewandte Chemie International Edition. 57 (38), 12370-12374 (2018).
- Cong, Y., et al. Improved Single-Cell Proteome Coverage Using Narrow-Bore Packed NanoLC Columns and Ultrasensitive Mass Spectrometry. Analytical Chemistry. 92 (3), 2665-2671 (2020).
- Zhu, Y., et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10-100 mammalian cells. Nature Communications. 9 (1), 882 (2018).
- Shao, X., et al. Integrated Proteome Analysis Device for Fast Single-Cell Protein Profiling. Analytical Chemistry. 90 (23), 14003-14010 (2018).
- Li, Z. Y., et al. Nanoliter-Scale Oil-Air-Droplet Chip-Based Single Cell Proteomic Analysis. Analytical Chemistry. 90 (8), 5430-5438 (2018).
- Budnik, B., Levy, E., Harmange, G., Slavov, N. SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biology. 19 (1), 161 (2018).
- Zhang, P., et al. Carrier-Assisted Single-Tube Processing Approach for Targeted Proteomics Analysis of Low Numbers of Mammalian Cells. Analytical Chemistry. 91 (2), 1441-1451 (2019).
- Shi, T., et al. Facile carrier-assisted targeted mass spectrometric approach for proteomic analysis of low numbers of mammalian cells. Communications Biology. 1, 103 (2018).
- Vitrinel, B., Iannitelli, D. E., Mazzoni, E. O., Christiaen, L., Vogel, C. Simple Method to Quantify Protein Abundances from 1000 Cells. ACS Omega. 5 (25), 15537-15546 (2020).
- MacLean, B., et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 26 (7), 966-968 (2010).
- Shi, T., et al. Conservation of protein abundance patterns reveals the regulatory architecture of the EGFR-MAPK pathway. Science Signaling. 9 (436), 6 (2016).
- Addona, T. A., et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nature Biotechnology. 27 (7), 633-641 (2009).
- He, J., et al. Analytical platform evaluation for quantification of ERG in prostate cancer using protein and mRNA detection methods. Journal of Translational Medicine. 13, 54 (2015).
- Shi, T., et al. Antibody-free, targeted mass-spectrometric approach for quantification of proteins at low picogram per milliliter levels in human plasma/serum. Proceedings of the National Academy of Sciences. 109 (38), 15395-15400 (2012).
- Shi, T., et al. Targeted quantification of low ng/mL level proteins in human serum without immunoaffinity depletion. Journal of Proteome Research. 12 (7), 3353-3361 (2013).

.
