Macrophage Differentiation and Polarization into an M2-Like Phenotype using a Human Monocyte-Like THP-1 Leukemia Cell Line
Summary
M2-like tumor-associated macrophages (TAM) are associated with tumor progression and poor prognosis in cancer. This protocol serves as a detailed guide to reproducibly differentiate and polarize THP-1 monocyte-like cells into M2-like macrophages within 14 days. This model is the basis to investigate the anti-inflammatory effects of TAM within the tumor microenvironment.
Abstract
Tumor-associated macrophages (TAM) can switch their expression and cytokine profile according to external stimuli. This remarkable plasticity enables TAM to adapt to ongoing changes within the tumor microenvironment. Macrophages can have either primarily pro-inflammatory (M1-like) or anti-inflammatory (M2-like) attributes and can continually switch between these two main states. M2-like macrophages within the tumor environment are associated with cancer progression and poor prognosis in several types of cancer. Many different methods for inducing differentiation and polarization of THP-1 cells are used to investigate cellular and intercellular mechanisms and the effects of TAM within the microenvironment of tumors. Currently, there is no established model for M2-like macrophage polarization using the THP-1 cell line, and the results of expression and cytokine profiles of macrophages due to certain in vitro stimuli vary between studies. This protocol serves as detailed guidance to differentiate THP-1 monocyte-like cells into M0 macrophages and to further polarize cells into an M2-like phenotype within 14 days. We demonstrate the morphological changes of THP-1 monocyte-like cells, differentiated macrophages, and polarized M2-like macrophages using light microscopy. This model is the basis for cell line models investigating the anti-inflammatory effects of TAM and their interactions with other cell populations of the tumor microenvironment.
Introduction
Tumor-associated macrophages (TAM) and their role in chronic inflammation, the onset of cancer, and tumor development are important targets in recent research1,2. Peripheral blood monocytes that are recruited to the tissue microenvironment of the developing tumor differentiate into macrophages and can be polarized into two main subtypes of macrophages3. The classically activated macrophage represents the primarily pro-inflammatory M1-like phenotype and the alternatively activated M2-like subtype shows predominantly anti-inflammatory characteristics4. Macrophages can switch dynamically between these two main phenotypes depending upon their cellular metabolism, with intermediate subtypes having both inflammatory and anti-inflammatory attributes5. TAM represents a heterogeneous population of both phenotypes. A tumor-promoting function and poor prognosis in different types of cancers is, however, particularly associated with M2-like macrophages6,7,8.
The functional profiles of macrophages and their interaction with other cells within the tumor microenvironment are complex and challenging to capture in a continuously changing environment during ongoing tumor development. Cell lines can provide a homogenous cell population with stable viability in culture, which can facilitate the process of demonstrating defined cellular and intercellular mechanisms. The monocyte-like THP-1 cell line is a legitimate model system for primary human monocytes9. This spontaneously immortalized cell line has been obtained from the peripheral blood of a one-year-old infant with acute monocytic leukemia9,10. The differentiation and polarization of THP-1 cells have been reported by several studies and have been performed in multiple different ways11,12,13,14. Activation and, therefore, the polarization of macrophages into an M1-like phenotype is followed by a compensatory anti-inflammatory rebound mechanism, promoting an M2-like phenotype through cytokines produced by inflammatory macrophages, such as interleukin 6 (IL-6) or itaconate15,16. This might serve as a break mechanism to attenuate an overshooting inflammatory response following cell activation17. The process of differentiating and polarizing monocytes and THP-1 monocyte-like cells into an anti-inflammatory M2-like phenotype is itself also accompanied by pro-inflammatory stimuli that must be overcome. An inflammatory cytokine response can be caused by mechanical stress18, such as changing media to refeed the cells, or adding chemical compounds to differentiate THP-1 cells, such as phorbol 12-myristate 13-acetate (PMA), and induce production of tumor necrosis factor α (TNFα), interleukin 1β (IL-1β) or IL-619. This altered cytokine expression profile as a response to PMA can affect and prevent subsequent macrophage polarization20. Adequate resting periods, as reported before after PMA treatment, allow these inflammatory responses to decrease and facilitate cell polarization into a distinct M2-like phenotype21.
This protocol demonstrates a method to differentiate and polarize THP-1 monocyte-like cells into an M2-like phenotype of macrophages within 14 days.
Protocol
NOTE: An overview of the steps described in this protocol is shown in Figure 1. The human monocyte-like leukemia cell line THP-1 was purchased. Short tandem repeat analysis was performed to authenticate the THP-1 cell line. Perform all steps under sterile conditions. The THP-1 monocytic cell line grows in suspension and does not attach to cell culture surfaces. Adherence can be induced by differentiating monocytes into macrophage-like cells through, e.g., mechanical stress or specific treatment with PMA.
1. Culturing and maintenance of THP-1 monocyte-like cells
- Set a timer for 150 s. Remove the frozen vial containing the THP-1 cell line (Table of Materials) from the liquid nitrogen and thaw it immediately in a clean water bath (37 °C). Start the timer as soon as the vial is put into the water bath. Loosen the cap to release the pressure that is building up due to the thawing process but ensure that the tube opening does not contact the water, to avoid contamination. The optimal time period for thawing the cells lies between 120-150 s. Continue thawing the cell suspension until an ice chip of the size of about 4 mm is left within the vial; then, proceed to the next step immediately.
- Transfer the liquid phase of the cell suspension to a 15 mL tube containing 9 mL of warm (37 °C) growth media (Table of Materials). Then, transfer 1 mL of the warm medium-cell suspension into the THP-1 vial and back into the 15 mL tube to melt the remaining ice chip and flush the vial to assure that no cells are left behind.
- Mix the suspension gently by pipetting up and down with a 1000 µL pipette. Remove a small sample (approximately 10 µL) to count the cells for viability (using trypan blue for exclusion) while they are spun. Spin down the warm cell suspension at 200 x g for 7 min at 37 °C.
- Remove the supernatant completely and resuspend with a certain volume of warm growth medium to achieve a cell density of 5 x 105/mL. Mix the suspension gently and transfer 22 mL of volume into T-75 cell culture flasks (Table of Materials). Store flasks upright in an incubator at 37 °C with 5% carbon dioxide (CO2) concentration. Exchange the growth media every 3-4 days.
2. Seeding of THP-1 cells and differentiation into M0 macrophages
- Prepare the cell containing growth medium with the respective cell density to seed cells at a density of 3 x 105/mL/well into 24-well cell culture plates (Table of Materials). Mix the medium gently and prepare aliquots of 26 mL, each put into a 50 mL tube. Use each 26 mL aliquot for seeding the cells into a respective plate.
- Transfer 1 mL of the cell-containing medium into each well of a 24-well plate. Mix the media gently by pipetting up and down between transfers.
- Prepare a stock solution of PMA (dissolve 1 mg of PMA in 100 µL of Dimethyl Sulfoxide (DMSO) = ~16 mM solution of PMA in DMSO) and dilute it with cold Phosphate Buffered Saline (PBS) to a final working concentration of 10 ng/µL right before cell treatment (Table of Materials). Keep the solution on ice and use it immediately. Do not refreeze. Add 100 ng of PMA per well. Let each cell plate sit in the incubator without any further treatment for 72 h.
- After 72 h, remove the growth medium and replace it with 1 mL of fresh growth medium. Do not touch the bottom of the wells with pipette tips. Let the cells rest for another 96 h in the incubator.
- After 96 h, repeat step 2.4 (media change) and let the cells rest for another 24 h.
NOTE: The M0 macrophages are now ready to be used for experiments (Figure 2). Immediately prior to treating the cells as part of further experiments, consider a media change with RPMI only (Table of Materials) since growth media supplements can cause interference with reagents that are added for cell treatment. In case M2-like macrophages are needed, proceed with section 3.
3. Polarization of M0 macrophages into M2-like macrophages
- Prepare a stock solution of IL-4 and IL-13 (dissolve 20 µg of IL-4 or IL-13 in 200 µL of nuclease-free water) and dilute it to a final working concentration of 2 ng/µL with PBS immediately prior to cell treatment. Keep the solution on ice and use it immediately. Do not refreeze.
- Remove the growth medium and replace it with 1 mL of fresh growth medium. Add 20 ng of interleukin 4 (IL-4) and 20 ng of interleukin 13 (IL-13) per well. Let the cells rest for 48 h in the incubator.
- After 48 h, repeat step 3.2. Let the cells rest for another 48 h in the incubator.
- Remove the growth medium and replace it with 1 mL of fresh growth medium. Let the cells rest for 48 h in the incubator.
NOTE: M2-like macrophages are now ready to be used for experiments (Figure 2). Immediately prior to treating the cells as part of further experiments, consider a media change with RPMI only (Table of Materials) since growth media supplements can cause interference.
4. Detaching and harvesting macrophages for flow cytometry
NOTE: Use a mechanical method combining cold shocking and cell scraping to detach and harvest the polarized macrophages from plates for flow cytometry.
- Remove the warm cell medium and replace it with a mixture of ice-cold PBS (without calcium and magnesium) and 5% fetal bovine serum (FBS), 1 mL per well. Immediately after this, place the cell plate on ice for 45 min. Do not place the cell plate on ice before the warm cell medium is removed, since this will decrease cell viability significantly. Keep the cells on ice only after inducing cold shock with ice-cold PBS/5% FBS mixture.
- After 45 min on ice, scrape off the cells using mini cell scrapers (Table of Materials). Gently transfer the detached macrophages in cold PBS/5% FBS into a 15 mL tube. Keep the tube on ice at all times until cells are stained.
NOTE: Pool eight wells of cells to reach adequate cell counts for staining.
Representative Results
M2-like macrophages were characterized, and M2-polarization was validated using flow cytometry for Cluster of Differentiation markers (CD) CD14, CD11b, CD80 (M1-like marker), and CD206 (M2-like marker). Flow cytometry staining was performed according to the manufacturer's instructions. Macrophages were washed with PBS/5% FBS and incubated with Fcγ-receptor block to avoid unspecific binding. Cells were then stained with FITC-conjugated mouse anti-human CD14 and CD80 antibodies, with PE-conjugated mouse anti-human CD11b antibodies, and with PE-tandem-conjugated mouse anti-human CD206 antibodies and isotype-matched IgG (Table of Materials) for 30 min at 4 °C. Four-color flow cytometric analysis and fluorescence quantitation were performed. Gating of cells was carried out, excluding cell debris according to forward scatter and side scatter.
The monocyte and macrophage markers CD14 and CD11b were expressed in 70.9% and 74.7% of cells, respectively (Figure 3). Cells showed almost no positivity for the M1-like marker CD80 (0.2%) and high surface levels of the M2-like marker CD206 in 62.6% of cells (Figure 4).
The macrophages derived from the polarization method described in this protocol show the expression of CD14 as well as CD11b markers. Both markers can but do not have to be expressed in macrophages. A clear positivity for CD206 as an M2-like macrophage marker is expected, while the level of CD80 expression as an M1-like marker should be low. Raggi et al. demonstrated similar results using peripheral blood mononuclear cells (PBMC), that were polarized into M2-like macrophages22. The mean expression of CD206 varied between 50%-60%, while the mean expression of CD80 ranged between 20%-25%22.
Further characterization of the M2-like macrophages created by this protocol was performed using quantitative real-time PCR (qRT-PCR). M2-like macrophages showed an upregulation of IL-6 and C-X-C Motif Chemokine Ligand 10 (CXCL10) compared to THP-1 monocyte-like cells, as well as an upregulation of the anti-inflammatory markers CD206, Interleukin 10 (IL-10) and C-C Motif Chemokine Ligand 18 (CCL18) (results not shown, to be published).
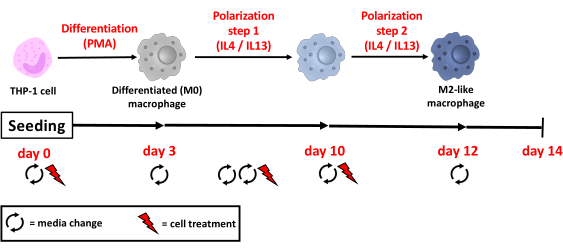
Figure 1: Overview of the M2-like macrophage cell line model. On day 0, cells are seeded into plates with a growth medium and incubated with PMA for 72 h. On day 3 and day 7 cell medium is changed, which lets the cells rest without PMA for a total of 120 h. On day 8, the growth medium is changed once more, and cells are incubated with IL-4 and IL-13 to induce M2-like polarization. This step is repeated after 2 days, on day 10. On day 12, the last medium change is performed, and M2-like cells rest in the growth medium for another 48 h before being used for experiments (PMA = phorbol 12-myristate-13-acetate; IL = interleukin). Please click here to view a larger version of this figure.
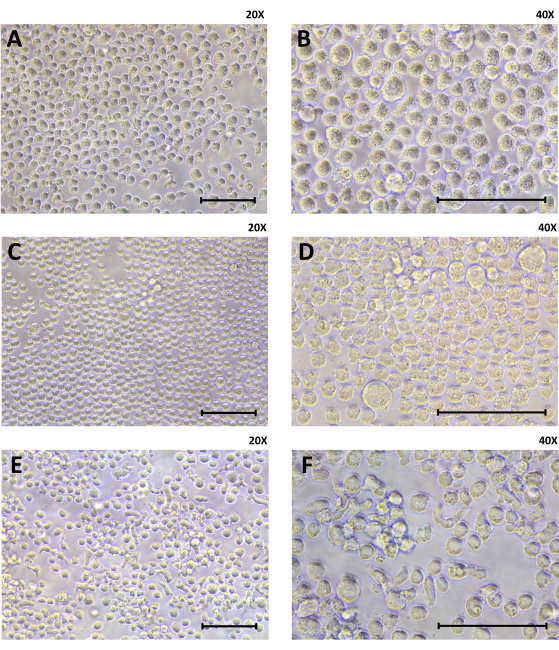
Figure 2: Cell morphology of THP-1 cells, differentiated (M0) macrophages, and M2-like macrophages using light microscopy. Cells were seeded at 3 x 105 per well in a 24-well plate. (A,B) THP-1 cells are shown at baseline. (C,D) The differentiated M0 macrophages received PMA treatment for 72 h, growth medium change and a 96-h-resting period. (E,F) The M2-like macrophages are shown after completed polarization treatment with IL-4 and Il-13 at day 14 of this cell model (20x and 40x magnification; scale = 100 µm). Please click here to view a larger version of this figure.
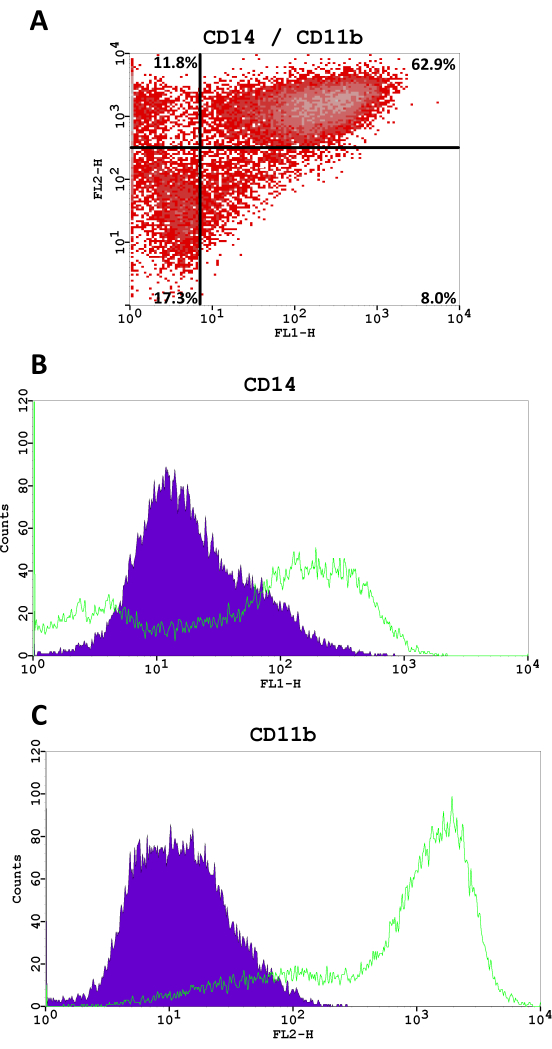
Figure 3: Flow cytometry fluorescence analysis for CD14 (FITC, FL1-H) and CD11b (PE, FL2-H). (A) Density scatter plot, percentages of cells are shown in each quadrant. (B,C) The histograms for CD14 and CD11b in M2-like macrophages compared to a negative staining control. Please click here to view a larger version of this figure.
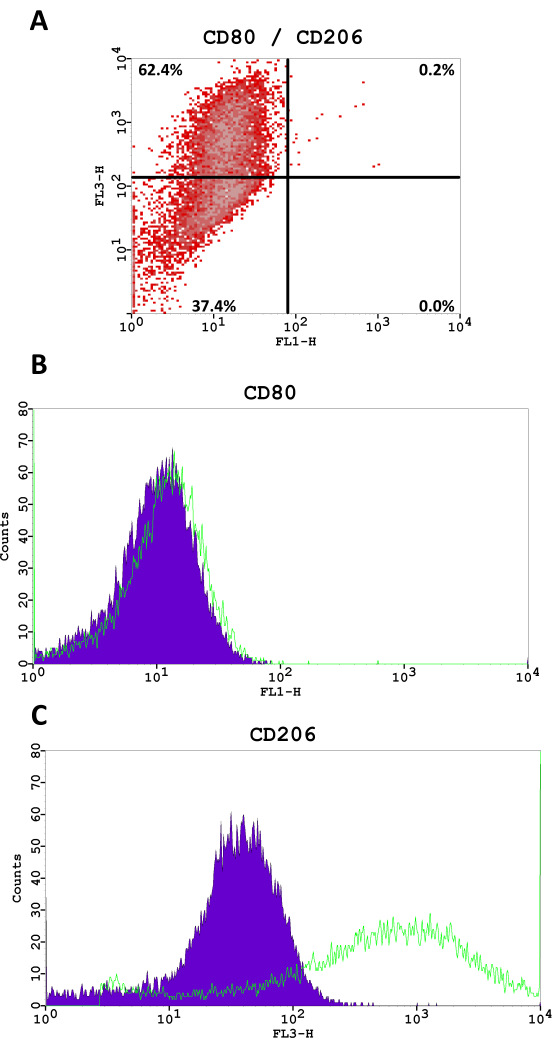
Figure 4: Flow cytometry fluorescence analysis for CD80 (FITC, FL1-H) and CD206 (PE-tandem conjugate, FL3-H). (A) Density scatter plot, percentages of cells are shown in each quadrant. (B,C) The histograms for CD80 and CD206 in M2-like macrophages compared to a negative staining control. Please click here to view a larger version of this figure.
Discussion
This protocol on differentiating and polarizing THP-1 monocyte-like cells within 14 days provides a method to obtain macrophages with a distinct M2-like phenotype due to long treatment incubation of cells with adequate resting periods between steps.
Certain steps are critical to this protocol. The doubling time of THP-1 monocytes is approximately 26 h. Cells can be split at a cell density of 9 x 105/mL and should be seeded at a density of 3 x 105/mL during every split. The split can be performed without removing all the used (old) cell medium – refeeding the cells with only 50% of the fresh medium can indeed lead to faster cell growth because growth factors in conditioned cell medium can enhance cellular proliferation. Not exchanging all the cell media, however, increases the risk of culture contamination and should therefore only be performed during the first passages of freshly cultured cells. Counting the cells is important to be able to culture cells at the right density and to determine the cellular viability after thawing. The proportion of dead cells after thawing cells properly should not exceed 15%.
The seeding of cells into plates requires gentle but thorough mixing of the cell suspension to obtain a consistent cell density in each well. Aliquots are prepared before seeding the cells into culture plates to assure that the volume of the cell-containing the medium is mixed properly. As THP-1 monocytes in the medium tend to sink to the bottom of a vial, the continuous gentle mixing of transfer volume is crucial to achieve a consistent density of cells.
Cell media should be warmed to 37 °C at all times to avoid cold shocks and a pro-inflammatory cellular stress response23. Therefore, also for media changes and cell treatment, plates should not be left out of the incubator for more than 15 min. Furthermore, the respective compounds for cell treatment, such as PMA, interleukins, and the PBS for diluting stock solutions prior to treating the cells, should be always kept on ice to avoid degradation.
Live macrophages that differentiated from monocytes by PMA treatment adhere to the surface of the wells. During media changes and other further treatment steps, pipette tips should not touch the bottom of a well within the plate to prevent cell damage.
For performing flow cytometry with differentiated or polarized macrophages, the cells that are attached to the plates must be harvested. There are either enzymatical or mechanical techniques to detach cells, including trypsinization, treatment with enzyme mixtures with proteolytic and collagenolytic activity, or ethylenediaminetetraacetic acid (EDTA), cold shock, cell scraping, or even detachment through acoustic pressure24,25. Enzymatic detachment of macrophages can lead to altered cell surface marker expression and is therefore not the first choice for phenotypic or functional analyses25. In contrast to other reports, the experiments performed here showed that the combination of cold shocking and scraping off macrophages showed good cell viability (>90%) using Trypan Blue dye exclusion. Therefore, it is recommended to use this technique to detach the cells, with the annotation that once a cold shock is induced, the cells have to be kept cold on ice at all times.
A limitation of this protocol is the use of a monocyte-like cell line as a basis to mimic macrophage mechanisms in vivo. Cell culture studies using primary macrophages, however, can show variable cellular responses and mechanisms can be masked due to cell heterogeneity26. The THP-1 cell line is an established model system for primary human monocytes9. Due to the homogenous THP-1 cell population in a controlled culture setting, cellular responses are potentially more precisely reproducible. Furthermore, certain techniques optimize THP-1 cells as a model to resemble primary monocytes. An important step is the resting period of 5 days after PMA treatment, which increases cytoplasmatic volume and cell surface adherence similar to that of differentiated monocyte-derived cells21.
Another limitation is the creation of a certain M2-like macrophage phenotype that has other characteristics than M2-like macrophages that were used in previous studies. Many different techniques to differentiate and polarize THP-1 cells have been reported, and a lack of baseline characterization complicates interstudy reproducibility11,12,13,14. Therefore, it is important to characterize the macrophages that are used in a study at baseline, depending on the mechanisms that are investigated. After that, the cellular responses after a respective treatment should be demonstrated.
The M2-like macrophages produced by following this protocol are a solid basis for the investigation of cellular responses in tumor onset and progression in different types of cancers, wound healing, or fibrosis. With this protocol, either M0-macrophages or M2-macrophages can be used in vitro, and the cells are suitable to be used in coculture models. This provides a wide variety of applications of a distinct M2-like macrophage phenotype that is robustly controlled in vitro over time.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The Price Institute of Surgical Research, University of Louisville, is financially supported by the John W. Price and Barbara Thruston Atwood Price Trust. The funding sources had no role in the design and conduct of the study as well as in the collection, management, analysis, and interpretation of the data.
Materials
| 0.4% trypan blue | VWR, Radnor, USA | 152-5061 | |
| 1.5 mL microcentrifuge tube | USA Scientific, Ocala, USA | 1615-5510 | |
| 10 mL serological pipet | VWR, Radnor, USA | 89130-898 | |
| 1000 μL TipOne pipet tips | USA Scientific, Ocala, USA | 1111-2821 | |
| 15 mL Centrifuge tube | VWR, Radnor, USA | 89039-664 | |
| 20 μL TipOne pipet tips | USA Scientific, Ocala, USA | 1120-1810 | |
| 200 μL TipOne pipet tips | USA Scientific, Ocala, USA | 1120-8810 | |
| 25 mL serological pipet | VWR, Radnor, USA | 89130-900 | |
| 5 mL serological pipet | VWR, Radnor, USA | 89130-896 | |
| 50 mL Centrifuge tube | VWR, Radnor, USA | 89039-662 | |
| Accutase solution 500 mL | Sigma, St. Louis, USA | A6964 | |
| Antibiotic Antimycotic Solution (100x), stabilized | Sigma, St. Louis, USA | A5955-100 mL | with 10,000 units penicillin, 10 mg of streptomycin and 25 μg of amphotericin B per mL, sterile-filtered, BioReagent, suitable for cell culture |
| Binder CO2 Incubator | VWR, Radnor, USA | C170-ULE3 | |
| CytoOne T-75cm flask with filter cap | USA Scientific, Ocala, USA | CC7682-4875 | |
| Dulbecco’s Phosphate Buffered Saline (PBS) | Sigma, St. Louis, USA | D8537-500 mL | PBS without calcium chloride and magnesium chloride should be used, since both can alter macrophage polarization |
| Eppendorf Centrifuge 5804 R (refrigerated) | Eppendorf, Enfield, USA | – | |
| Ethyl alcohol (70%) | – | – | |
| FACSCalibur flow cytometer | BD Biosciences, San Diego, USA | – | The flow cytometer operates with CellQuest software (BD Biosciences) |
| Falcon 24-well plate | VWR, Radnor, USA | 353504 | |
| Fetal Bovine Serum (FBS) | ATCC, Manassas, USA | 30-2020 | |
| FITC Mouse Anti-Human CD14 | BD Biosciences, San Diego, USA | 555397 | Flow cytometry, myeloid cell marker (100 tests) |
| FITC Mouse Anti-Human CD80 | BD Pharmingen, San Diego, USA | 557226 | Flow cytometry, M1 marker (100 tests) |
| FITC Mouse IgG1 κ Isotype Control | BD Pharmingen, San Diego, USA | 555748 | Flow cytometry, isotype control for CD80 (100 tests) |
| FITC Mouse IgG2a, κ Isotype Control | BD Biosciences, San Diego, USA | 553456 | Flow cytometry, isotype control for CD14 (100 tests) |
| Human BD Fc Block | BD Biosciences, San Diego, USA | 564220 | Flow cytometry, Fc block (0.25 mg) |
| Human interleukin 13 (IL-13) | R&D, Minneapolis, USA | IL-771-10 μg | |
| Human interleukin 4 (IL-4) | R&D, Minneapolis, USA | SRP3093-20 μg | |
| Labconco Biosafety Cabinet (Delta Series 36212/36213) | Labconco, Kansas City, USA | – | |
| L-Glutamine Solution, 200 mM | ATCC, Manassas, USA | 30-2214 | |
| Lipopolysaccharide (LPS) from E. coli 0111:B4 | Sigma, St. Louis, USA | L2630-100 mg | |
| Mini Cell Scrapers | Biotium, Fremont, USA | 22003 | |
| Neubauer hemocytometer | Fisher Scientific, Waltham, USA | 02-671-5 | |
| Nikon Eclipse inverted microscope TS100 | Nikon, Melville, USA | – | |
| Nuclease-free water | Invitrogen, Carlsbad, USA | AM9937 | |
| Olympus Light Microscope RH-2 | Microscope Central, Feasterville, USA | 40888 | |
| P10 variable pipet- Gilson | VWR, Radnor, USA | 76180-014 | |
| P1000 variable pipet-Gilson | VWR, Radnor, USA | 76177-990 | |
| P200 variable pipet- Gilson | VWR, Radnor, USA | 76177-988 | |
| PE Mouse Anti-Human CD11b | BD Biosciences, San Diego, USA | 555388 | Flow cytometry, myeloid cell marker (100 tests) |
| PE Mouse IgG1, κ Isotype Control | BD Biosciences, San Diego, USA | 555749 | Flow cytometry, isotype control for CD11b (100 tests) |
| PE-Cy 5 Mouse Anti-Human CD206 | BD Pharmingen, San Diego, USA | 551136 | Flow cytometry, M2 marker (100 tests) |
| PE-Cy 5 Mouse IgG1 κ Isotype Control | BD Pharmingen, San Diego, USA | 555750 | Flow cytometry, isotype control for CD206 (100 tests) |
| Phorbol 12-myristate 13-acetate (PMA) | Sigma, St. Louis, USA | P8139 | |
| Powerpette Plus pipettor | VWR, Radnor, USA | 75856-448 | |
| Precision Water bath (model 183) | Precision Scientific, Chicago, USA | 66551 | |
| RPMI-1640 Medium | ATCC, Manassas, USA | 30-2001 | |
| THP-1 cell line, American Type Culture Collection (ATCC) | ATCC, Manassas, USA | TIB-202 |
References
- Zhang, R., et al. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death and Disease. 10 (4), 273 (2019).
- Wang, J., Li, D., Cang, H., Guo, B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Medicine. 8 (10), 4709-4721 (2019).
- Soncin, I., et al. The tumor microenvironment creates a niche for the self-renewal of tumor-promoting macrophages in colon adenoma. Nature Communications. 9 (1), 582 (2018).
- Mosser, D. M., Edwards, J. P. Exploring the full spectrum of macrophage activation. Nature Reviews Immunology. 8 (12), 958-969 (2008).
- Mazzone, M., Menga, A., Castegna, A. Metabolism and TAM functions-it takes two to tango. Federation of European Biochemical Societies Journal. 285 (4), 700-716 (2018).
- Eum, H. H., et al. Tumor-promoting macrophages prevail in malignant ascites of advanced gastric cancer. Experimental and Molecular Medicine. 52 (12), 1976-1988 (2020).
- Qian, B. Z., Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell. 141 (1), 39-51 (2010).
- Scheurlen, K. M., Billeter, A. T., O’Brien, S. J., Galandiuk, S. Metabolic dysfunction and early-onset colorectal cancer – how macrophages build the bridge. Cancer Medicine. 9 (18), 6679-6693 (2020).
- Bosshart, H., Heinzelmann, M. THP-1 cells as a model for human monocytes. Annals of Translational Medicine. 4 (21), 438 (2016).
- . The American Type Culture Collection (ATCC) Available from: https://www.atcc.org/products/all/TIB-202.aspx#generalinformation (2021)
- Baxter, E. W., et al. Standardized protocols for differentiation of THP-1 cells to macrophages with distinct M(IFNgamma+LPS), M(IL-4), and M(IL-10) phenotypes. Journal of Immunological Methods. 478, 112721 (2020).
- Genin, M., Clement, F., Fattaccioli, A., Raes, M., Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 15, 577 (2015).
- Starr, T., Bauler, T. J., Malik-Kale, P., Steele-Mortimer, O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS One. 13 (3), 0193601 (2018).
- Lund, M. E., To, J., O’Brien, B. A., Donnelly, S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. Journal of Immunological Methods. 430, 64-70 (2016).
- Yin, Z., et al. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. Journal of Cellular Biochemistry. 119 (11), 9419-9432 (2018).
- O’Neill, L. A. J., Artyomov, M. N. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nature Reviews Immunology. 19 (5), 273-281 (2019).
- Luig, M., et al. Inflammation-induced IL-6 functions as a natural brake on macrophages and limits gn. Journal of the American Society of Nephrology. 26 (7), 1597-1607 (2015).
- Maruyama, K., Nemoto, E., Yamada, S. Mechanical regulation of macrophage function – cyclic tensile force inhibits NLRP3 inflammasome-dependent IL-1beta secretion in murine macrophages. Inflammation and Regeneration. 39, 3 (2019).
- Gatto, F., et al. PMA-Induced THP-1 Macrophage Differentiation is Not Impaired by Citrate-Coated Platinum Nanoparticles. Nanomaterials (Basel). 7 (10), (2017).
- Maess, M. B., Wittig, B., Cignarella, A., Lorkowski, S. Reduced PMA enhances the responsiveness of transfected THP-1 macrophages to polarizing stimuli. Journal of Immunological Methods. 402 (1-2), 76-81 (2014).
- Daigneault, M., Preston, J. A., Marriott, H. M., Whyte, M. K., Dockrell, D. H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 5 (1), 8668 (2010).
- Raggi, F., et al. Regulation of human macrophage M1-M2 polarization balance by hypoxia and the triggering receptor expressed on myeloid cells-1. Frontiers in Immunology. 8, 11097 (2017).
- Neutelings, T., Lambert, C. A., Nusgens, B. V., Colige, A. C. Effects of mild cold shock (25 degrees C) followed by warming up at 37 degrees C on the cellular stress response. PLoS One. 8 (7), 69687 (2013).
- Kurashina, Y., et al. Enzyme-free release of adhered cells from standard culture dishes using intermittent ultrasonic traveling waves. Communications Biology. 2, 393 (2019).
- Chen, S., So, E. C., Strome, S. E., Zhang, X. Impact of Detachment Methods on M2 Macrophage Phenotype and Function. Journal of Immunological Methods. 426, 56-61 (2015).
- Bailey, J. D., et al. Isolation and culture of murine bone marrow-derived macrophages for nitric oxide and redox biology. Nitric Oxide. 100-101, 17-29 (2020).

