Dissection and Immunohistochemistry of the Drosophila Adult Leg to Detect Changes at the Neuromuscular Junction for an Identified Motor Neuron
Summary
We describe a dissection technique that preserves the architecture of the neuromuscular junction and enables a detailed immunocytochemical study of motor neurons in the adult Drosophila leg.
Abstract
Drosophila melanogaster represents a genetically tractable model to study neuronal structure and function, and subsequent changes in disease states. The well characterized larval neuromuscular junction is often used for such studies. However, rapid larval development followed by muscle histolysis and nervous system remodeling during metamorphosis makes this model problematic for the study of slow age-dependent degenerative changes like those occurring in amyotrophic lateral sclerosis. Alternatively, adult flies live for 90 days and the adult leg can be used to study motor neuron changes over the course of adult lifespan using in vivo fluorescent imaging through the cuticle. Here, we describe a leg dissection technique coupled with immunocytochemistry, which allows for the study of molecular changes at the neuromuscular junction of identified adult leg motor neurons. These techniques can be coupled with a myriad of antibodies labeling both pre- and post-synaptic structures. Together these procedures allow a more complete characterization of slow age-dependent changes in adult flies and can be applied across multiple motor neuron disease models.
Introduction
Motor neuron (MN) diseases encompass a group of heterogenous conditions that include progressive degeneration leading to muscle wasting and paralysis as a primary clinical phenotype1. Although rare with a global prevalence of 4.5 per 100,000, this prevalence is expected to increase with an aging population2. Amyotrophic lateral sclerosis (ALS) is the most common MN disease (MND) and is typically fatal within a short time of diagnosis with no existing disease-modifying treatments available3. MNDs share in common a protracted presymptomatic phase with early molecular biomarker changes and functional imaging changes seen in patients4. Early presymptomatic cellular pathology is also observed in non-human disease models5,6,7,8. The study of early changes at the neuromuscular junction is important for understanding MN disease pathogenesis and may aid in developing early diagnostics and potential therapeutics.
A wealth of genetic and molecular tools exists in Drosophila to dissect the structure and function of the neuromuscular junction (NMJ, see9 for a review of the well-characterized larval NMJ). These tools combined with a short lifespan make Drosophila an excellent model to study neurodegenerative changes at the NMJ. Specifically, MNs innervating adult muscles are present throughout the ~90-day adult lifespan and are subject to normal aging processes10,11,12,13. The adult MNs therefore provide an opportunity to study slow degenerative changes in contrast to larval NMJs which exist for only a short ~1 week time-period prior to metamorphosis14,15.
Here, we describe a dissection procedure that allows us to conduct immunocytochemical analysis of MNs in the adult leg. Each adult leg is innervated by ~50 MNs, which synapse onto the associated leg muscular to drive locomotion. The leg anatomy, mechanical physiology, and neurobiology has been well described16,17,18. Axon arbors of leg MNs have previously been characterized by imaging through cuticle in back-filled or genetically labeled cell populations using the bipartite Gal4/UAS system and imaging methods have been published previously19. The dissection methods presented here preserves axon branching morphology and allows us to exploit a diverse range of antibodies to label different molecular components of the NMJ. Our previous work has focused on projections of a defined MN in the metathoracic (3rd) leg, which innervates the tibia levator muscle (tilm) and shows consistent arborization patterns and bouton numbers. Initially we studied age-dependent changes in Drosophila superoxide dismutase 1 (dsod1) mutants and found alterations consistent with dismantling of the NMJ20. These dissection methods offer the opportunity to better characterize slow degenerative changes at the NMJ for other ALS models, basic studies of aging and other MN associated diseases.
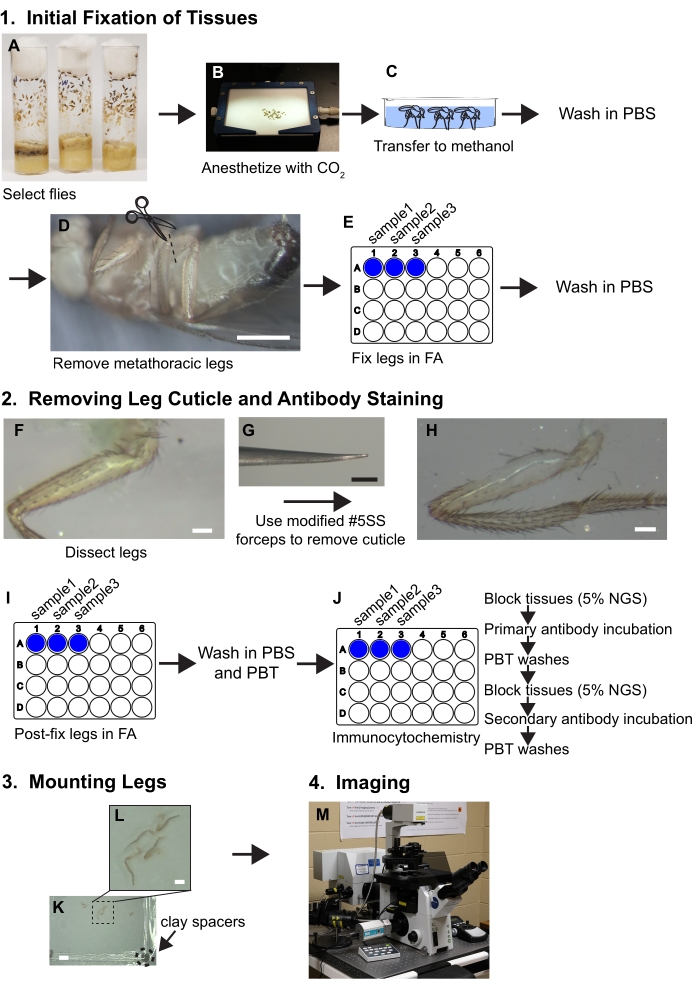
Figure 1. Workflow summary for dissecting legs. See protocol for detailed steps. (A,B) Flies are selected and anesthetized. (C) Flies are transferred to methanol and washed with PBS. (D) The metathoracic legs are removed at the base of the coxa while visualized with a dissecting microscope (~30x magnification); scale bar = 500 μm. (E) The legs are then fixed in 3.7% formaldehyde/PBS (FA) solution for 30 minutes within wells of 24-well plates and then FA is removed by washes with PBS. (F, G, H) Legs are transferred to silicone elastomer dissecting trays and a piece of cuticle is removed from the proximal femur using beveled forceps while visualized under a dissecting microscope at 80x; scale bar = 50 μm. (I) Legs are post-dissection fixed in FA and washed in PBS and then PBT (PBS+ 0.1% non-ionic surfactant). (J) Legs are subjected to immunocytochemical staining. (K,L) Legs are transferred to a glass slide, cleared in mounting media, and covered with a coverslip containing clay spacers; scale bars = 2 mm and 500 μm. (M) Legs are imaged by confocal microscopy. Please click here to view a larger version of this figure.
Protocol
The procedures for preparing working solutions used in conjunction with this protocol are described in Table 1.
| Reagent | Preparation | Storage | |||
| PBS | Make a working stock of 1x PBS from a 10x PBS stock solution by diluting in distilled water. The pH of the working PBS stock should be 7.2- 7.4 | 4 °C for 1+ months until bacterial contamination is visible. | |||
| PBT | 1x PBS solution with 0.1% non-ionic surfactant. | 4 °C for 1+ months until bacterial contamination is visible. | |||
| FA | 3.7% formaldehyde solution made from a 37% formaldehyde stock and diluted in 1x PBS. | Room temperature. Make fresh each day of dissection | |||
| CAUTION: Formaldehyde supplied as 37% stock solution is a potential carcinogen and should be diluted in a fume hood. | |||||
| 5% NGS | 5% normal goat serum diluted in PBT. The serum used should match the species of the secondary antibody to be used. | 4 °C for several weeks until bacterial contamination is visible | |||
Table 1. Solutions for performing immunocytochemistry of the adult Drosophila leg.
1. Initial fixation of tissues
- Select approximately 10 flies for each genotype and age. Anesthetize on a fly pad under carbon dioxide (Figure 1A, B).
NOTE: Begin with more flies than necessary to ensure a large enough sample size after dissection. - Using a paintbrush, transfer flies to cold methanol in a glass well or dish for approximately 30 seconds to 1 minute (Figure 1C). The methanol solubilizes the cuticular hydrocarbons and flies can now be submerged rather than float in aqueous solutions.
- With forceps, carefully transfer flies to PBS. Rinse 3x in ice-cold PBS to remove excess methanol and keep flies in PBS on ice until dissection and fixation. At this point, dissect flies and fix within the shortest time possible (<30 minutes).
- To isolate legs, transfer flies to a silicone elastomer dissection dish filled with cold PBS, remove the metathoracic legs at the coxa using two pairs of #5 Dumont forceps or cut the legs with Vanna scissors (Figure 1D). Transfer the legs to a well in a plastic 24 well plate filled with 1 mL of PBS and keep the plate on ice until all legs are removed and transferred to wells.
NOTE: Each well can hold at least 20 legs. - Replace the PBS solution with 1 mL of FA solution and rotate on a nutator for 30 minutes (Figure 1E). The nutator setting should be set at a medium speed (17 rpm). Ensure the legs are completely submerged in FA solution during this time for adequate fixation.
- To remove FA solution, wash in 1 mL of PBS 3x quickly followed by an additional 3 washes for 5 minutes each in 1 mL of PBS. Hold tissues in 1 mL of PBS on ice prior to, and during the dissection steps described below.
2. Removing the Leg Cuticle and Antibody Staining
- Removing the leg cuticle
- The dissecting forceps are critical for success. Introduce slight parallel bends in both prongs at the end of #5 super fine forceps to provide a bevel that allows the cuticle to be grabbed superficially rather than be poked, which can ruin the tissue (Figure 1F, G).
NOTE: Prongs bent in parallel should still make contact with each other throughout the length of the prong when closed (Figure 2). - Transfer legs to a silicone elastomer dish in PBS for dissection. Orient a leg so that the anterior side is facing up (see Figure 3 for leg anatomy and orientation information). Using one pair of forceps, hold the tibia segment against the silicone elastomer dish. Using the other forceps held bevel side down, grab a piece of cuticle on the distal end of the femur and pull in the proximal direction toward the trochanter.
- Keep methodically removing cuticle until the naked muscle is visible throughout the proximal end of the femur (Figure 1F, G, H).
NOTE: Only make superficial contact with the legs using the beveled side of the forceps to avoid pulling muscle. - Once all legs are dissected, replace PBS with FA to post-fix legs for 30 minutes with shaking on a nutator at medium speed (Figure 1I). Wash samples in 1 mL of PBS for 3 times quick and then 3 times for 5 minutes each in PBT (Figure 1J).
NOTE: If staining with monoclonal antibody NC82 (anti-bruchpilot) to label active zones, post-fix for 20 minutes as this antigen is sensitive to longer fixations.
- The dissecting forceps are critical for success. Introduce slight parallel bends in both prongs at the end of #5 super fine forceps to provide a bevel that allows the cuticle to be grabbed superficially rather than be poked, which can ruin the tissue (Figure 1F, G).
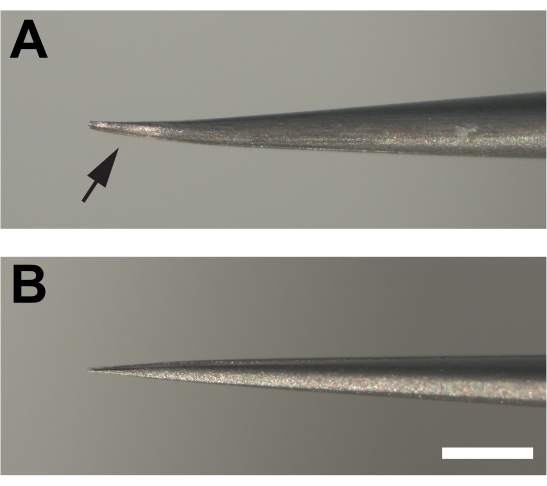
Figure 2. Modified forceps used for dissecting adult legs. (A) The ends of the forceps are bent and then flattened at the bottom (arrow) creating a bevel by filing on a sharpening stone. (B) In contrast, the prongs of unmodified forceps are not bent. Scale bar = 1 mm Please click here to view a larger version of this figure.
- Antibody staining
- To block tissues for antibody staining, replace 1 mL of PBT with blocking solution consisting of 1 mL of 5% NGS diluted in PBT. Incubate dissected legs for 4 hours at room-temperature or overnight at 4 °C while rocking on a nutator at medium speed (17 rpm). During all incubations, cover wells with sealing tape in addition to the plastic cover (Figure 1J).
NOTE: 24 well plates are used for immunocytochemistry rather than 1.5 mL or 2 mL microcentrifuge because previous attempts to use microcentrifuge tubes resulted in broken legs and damaged tissue. - Remove blocking solution and add 300 mL of primary antibodies diluted in fresh blocking solution. The small volume of antibody used should be sufficient to cover the tissues. Re-seal wells with laboratory sealing tape and plastic lid and incubate overnight at 4 °C with shaking on a nutator at medium speed (Figure 1J). The working antibody reagent information and concentrations used in for these studies are described in Table 2.
- Wash primary antibodies in 1 mL of PBT for 3 times briefly and then 3 times for 15 minutes each (Figure 1J).
NOTE: Diluted primary antibodies can be saved and reused if stored at 4 °C for up to 2 weeks. - Block tissues again in 1 mL of 5% NGS for at least 2 hours at room temperature or overnight at 4 C (Figure 1J).
- Remove the 5% NGS blocking solution and add 300 µL of the appropriate diluted fluorescent conjugated secondary antibodies. Additionally, add 1:2000 dilution of fluorescence-conjugated phalloidin to label muscle (Figure 1J).
- For secondary antibody incubations, seal wells with laboratory sealing tape and lid. Also, wrap plates in aluminum foil to protect fluorophores from light. Incubate for 6-8 hours at room-temperature or overnight at 4 °C.
- Wash secondary antibodies and phalloidin as described in step 2.2.3 above. Cover plates with aluminum foil in between washes to protect fluorophores from light.
- To block tissues for antibody staining, replace 1 mL of PBT with blocking solution consisting of 1 mL of 5% NGS diluted in PBT. Incubate dissected legs for 4 hours at room-temperature or overnight at 4 °C while rocking on a nutator at medium speed (17 rpm). During all incubations, cover wells with sealing tape in addition to the plastic cover (Figure 1J).
3. Mounting Legs
- Transfer legs to a slide using forceps and orient anterior side up. Cover the legs with mounting media (Figure 1K, L).
NOTE: The dissected legs can be aspirated with a P1000 pipette tip if the bottom bore is widened by cutting with a razor blade. - Add clay spacers to a 22×22 mm2 coverslip (#1.5 thickness) by scraping the coverslip corners across a small ball of modeling clay. Each corner should have a small amount of clay 1-2 mm thick (Figure 1K).
- To cover the slide, add the coverslip with the clay spacers facing towards the slide and carefully push on the corners until the coverslip just touches the surface of the femur.
- To prevent evaporation, seal the edges of the coverslip with nail polish and let dry in a dark place (about 10 minutes) before storing at 4 °C until imaging.
4. Imaging
- Image by confocal microscope (Figure 1M). Include a transmitted light channel to better assess the quality of the dissection and samples with visibly disrupted muscle fibers in the area of interest should be discarded.
- Begin imaging z-stacks with 20x magnification with 2x zoom and a total image depth of ~ 40 mm corresponding to the thickness of the femur. For fluorescent signal detection, capture images at resolutions consistent with Nyquist sampling (we are using 1024 x 1024 pixels with a dwell setting of 8-10 μs/pixel). The signal intensity should be in the linear range which is achieved by adjusting the high voltage gain settings. Once the gain settings are set for a series of samples within an experiment, they should not be altered so that signal intensities can be compared between samples.
- For imaging synaptic boutons and other subcellular structure, capture confocal images at ≥60x magnification. The detector settings should be in the linear range, while the pixel density, dwell settings and z-depth should be similar for images captured at lower magnification (step 4.1).
Representative Results
Figure 4 shows a representative example of a metathoracic leg stained with anti-hrp, anti-dlg, and phalloidin. For dissections that remove cuticle from the proximal portion of the femur, stereotyped arbors will be apparent near the tendon which is detected easily by autofluorescence. Note that antibody penetration into the leg occurs for a short distance beyond the region in which cuticle has been removed (Figure 4A). These regions can be imaged effectively when strong fluorescence signal is present. Imaging at low magnification (20x with a 2x zoom) allows an easy determination of 1) how much cuticle is removed, and 2) whether damage occurred during dissection. Increased magnification (60x) shows stereotyped projections onto the tilm (Figure 4B). Our work has focused on one MN, likely derived from the I- MN lineage which innervate the tilm in the proximal femur (box, Figure 4B and Figure 4C). Increasing magnification further (100x with 2x zoom) allows for effective visualization of synaptic boutons (Figure 4D).
To study morphological changes at the adult NMJ over time, we have previously used dsod1 mutants as a model of ALS. Bouton swelling occurs in aged dsod1H71Y mutants relative to dsod1null and dsod1+ (Figure 5A, B). At the larval NMJ, monoclonal antibody NC82 is often used to label active zones and this these structures can be easily visualized at the adult NMJ (Figure 5C). Weakly-positive HRP axon branches are abundant in dsod1H71Y mutants and these branches often show weak and diffuse BRP localization (arrows).
Important for neurodegeneration, commercially available antibodies can detect ubiquitinated proteins often found in aggregates, and we have detected ubiquitinated aggregates within terminal axons of MNs in aged mutant dsod1 flies as well as within muscle (Figure 6A). Also, antibodies labeling mitochondria can also detect morphological changes with age (Figure 6B).
For some preparations, muscle damage during the dissection makes the sample unusable. At first, such damage can be a common occurrence, but improvement occurs with practice. Examples of good dissections are show Figure 7A, C while poor dissections are shown in Figure 7B, D. Poor dissections cause disorganization of muscle fibers as detected by phase contrast microscopy (Figure 7B) or missing muscle fibers visualized by fluorescent-conjugated phalloidin (Figure 7D, arrow). The combination of using phalloidin to label muscle, and transmitted light images can help to detect such muscle damage which may not be apparent when viewing under a dissecting microscope.
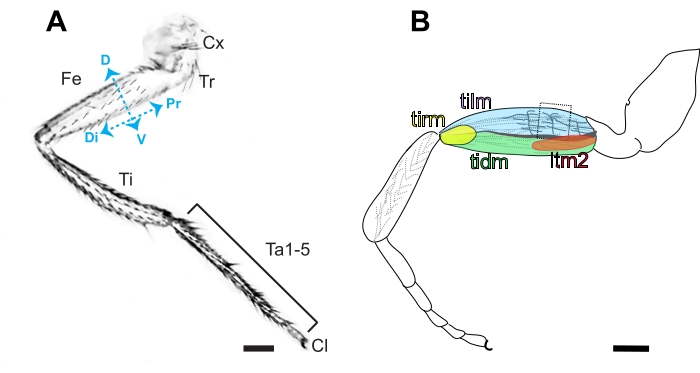
Figure 3. Anatomy of the Drosophila leg. (A) Diagram of the anterior side of a metathoracic leg, characterized by the presence of bristles in contrast to the prominent naked cuticle present on the posterior side. The segmented leg is comprised of the coxa (Cx), trochanter (Tr), femur (Fe), tibia (Ti), 5 tarsal segments (Ta1-5), and claw (Cl) in order from proximal (Pr) to distal (Di). Dorsal (D) and ventral (V) sides of the femur are also indicated. Scale bar =100 μm. (B) Diagram of the femur containing the tibia levator (tilm), tibia depressor (tidm), long tendon muscle 2 (ltm2) and tibia reductor (tirm) muscles and axon projections in the proximal femur innervating the tilm. These stereotyped axonal projections are presumed to be derived from I-lineage neuroblasts16, and the second arborization is the easiest to access by dissection (boxed). Scale bar = 100 μm. Please click here to view a larger version of this figure.
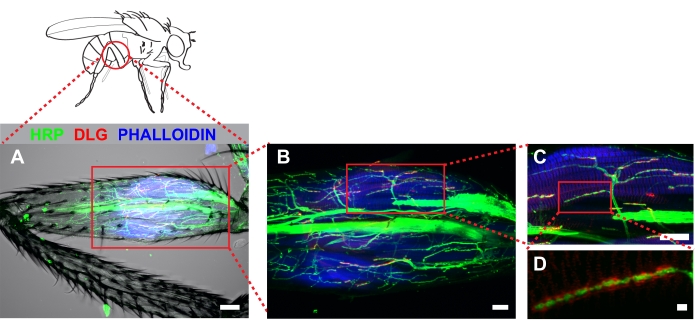
Figure 4. Dissected metathoracic femur revealed stereotyped MN architecture innervating the tilm. Immunocytochemistry was used to mark neurons (HRP), discs-large (DLG) and muscle (phalloidin). Z-stacks were captured by confocal microscopy imaging through the entire femur and shown as a maximum series projection. (A) A transmitted light channel was also included to illustrate no detectable muscle damage occurred during dissection. Image was captured at 20x magnification, scale bar =50 μm. (B) Identified arbors associated with the tilm (boxed area, center), scale bar =20 μm; and (C) at 60x magnification, scale bar = 20 μm. (D) DLG surrounding boutons was apparent in wild type animals when imaged at 100x magnification with 2x zoom; scale bar = 2 μm Please click here to view a larger version of this figure.
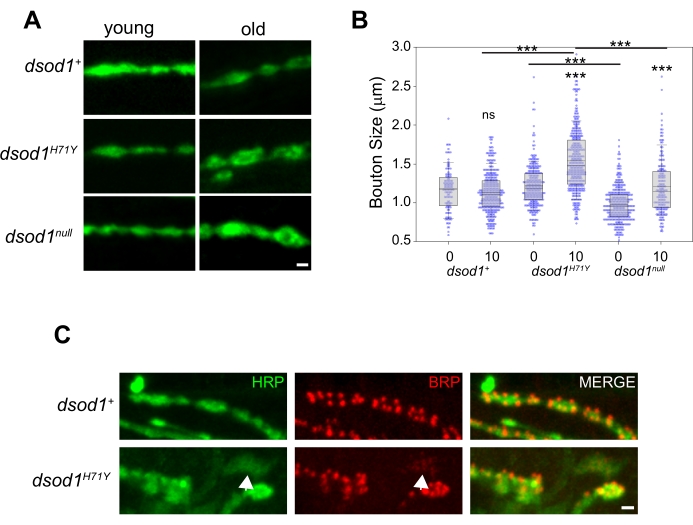
Figure 5. Example of age-dependent changes in bouton morphology which can be detected using the leg dissection technique. Bouton swelling is seen in aged dsod1H71Y mutants. (A) Representative images of HRP stained boutons from young (newly eclosed, day 0 adults) and old (day 10) flies, scale bar = 1 μm. (B) Bouton sizes were quantified from respective genotypes using the measure function within ImageJ. ***p<0.0001 (2-way ANOVA with post-hoc Tukey test). (C) Active zones within synaptic boutons labeled with monoclonal antibody NC82 (anti-bruchpilot); scale bar = 1 μm. Figure modified from20 Please click here to view a larger version of this figure.
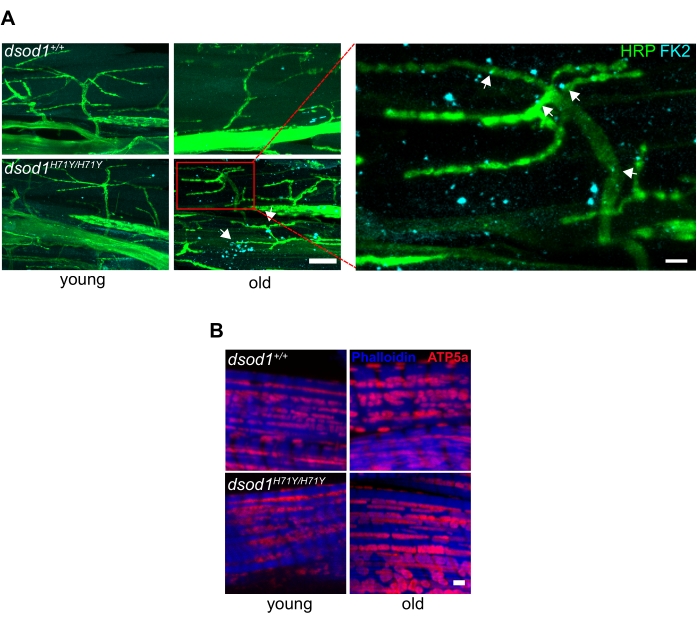
Figure 6. Common markers associated with neurodegenerative disease used in Drosophila leg preparations. (A) Subcellular polyubiquitinated aggregates are detected in terminal axons and muscle of aged dsod1H71Y flies. Immunocytochemistry using anti-polyubiquitin (FK2) detects puncta (arrows) in aged dsod1H71Y/H71Y, scale bar = 20 μm (left) and 5 μm (right). (B) Swollen mitochondria can be detected using anti-ATP5A, scale bar = 1 μm. Figure modified from20 Please click here to view a larger version of this figure.
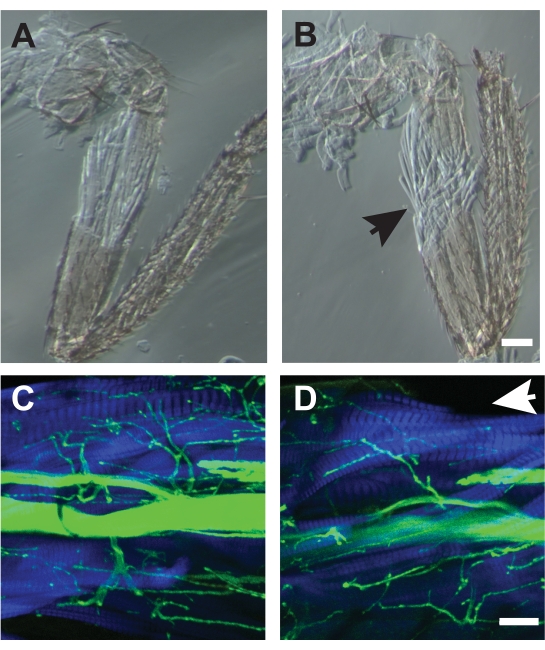
Figure 7. Examples of good and poor dissections. (A) Dissection which did not disrupt the underlying muscle architecture. (B) An example of a poor dissection which showed frayed muscle fibers (arrow) and could not be used for analysis. Both samples were imaged by phase-contrast microscopy. Scale bar = 50 μm. (C) Example of a good dissection imaged by confocal microscopy with HRP (green) and phalloidin (blue). (D) A poor dissection missing dorsal muscle fibers of the TILM missing (arrow). Scale bar = 50 μm Please click here to view a larger version of this figure.
| Antibody/Stain | Dilution* |
| Anti-ATP5A | 1:500 |
| Anti-bruchpilot (nc82) | 1:20 |
| Anti-cysteine string protein (ab49) | 1:50 |
| Anti-discs large (4F3) | 1:200 |
| Anti-hrp | 1:550 |
| Anti-polyubiquitin (FK2) | 1:1000 |
| Anti-repo (8D12) | 1:5 |
| Goat anti-mouse secondary antibody | 1:800 |
| Phalloidin | 1:2000 |
| *Dilute in 5% NGS |
Table 2. Antibodies and dilutions. The commercial suppliers of the antibodies used in these studies are listed in the Materials List.
Discussion
The Drosophila adult leg is an ideal model to study neurodegeneration given relative simplicity with well-characterized MNs mapped from neuroblast lineages and stereotyped arborization patterns. Several reports have previously used leg MNs for the study of neurodegenerative disease21,22. These studies utilized GFP-expressing lines combined with mosaic analysis with a repressible cell marker (MARCM) to image through the cuticle and documented a series of morphological changes. Imaging adult NMJs by immunocytochemistry with resected cuticle enables further characterization with the ability to track complex molecular changes using a toolbox of antibodies available.
The immunocytochemistry portion of this protocol is relatively standard and can be implemented independent of genotype (see23 for an excellent description of general antibody staining methods for use with Drosophila). Furthermore, parameters such as fluorescence intensity, axon branch length, and bouton numbers and size can be determined using a variety of available ImageJ macros once images are captured and detailed methods for quantitative analysis have been published (for example, see24,25,26). Thus, the dissection technique is the main innovation described here. Prior to dissection, flies are submerged in an alcohol to strip cuticular hydrocarbons. Both ethanol and methanol are commonly used for this purpose; however, we have only used methanol. Critical to dissection success are several factors: First, using modified forceps with a bevel allows for very superficial contact with the cuticle. Second, using a dissecting microscope capable of 60-100x total magnification so that the surface of the cuticle is clearly visible. For microscopes with lower maximum magnification, 2x objectives are available for most common brands and should be sufficient when combined with existing lenses. Third, the initial fixation step makes the cuticle brittle and easier to pull away without damaging muscle underneath. Over-fixation at this step makes the entire leg too stiff for effective dissection. Therefore, the initial fixation should be limited to 30 minutes. The formaldehyde fixative will not penetrate enough to effectively crosslink the underlying tissue during this short period and thus a second fixation step is necessary. Prior to the second fixation, tissues should be kept on ice to prevent degradation and changes in morphology. Fourth, we have found dissecting samples while cold is also important, likely for similar reasons in that cuticle is brittle and a small piece can be more easily removed.
With practice, we find ~50% of dissections will be usable within no discernable tissue damage. Although this percentage may seem low relative to some other tissues, the dissection procedure is rapid, and many legs can be processed in 30- 60 minutes. Therefore, even if success rates are low initially, it is feasible to obtain 4-5 good samples for each experimental group. However, a limitation may be the number of flies available at any given time if genotypes and/or age result in substantial lethality.
Another limitation is that we have not been able to dissect other areas of the leg beyond the proximal region of the femur due to size. Thus, we can study identified MN arbors innervating the TILM reliably and it is possible to dissect cuticle above the tibia depressor muscle with small changes to the way the leg is oriented when dissecting. However, accessing other regions of the leg have proved more difficult without disrupting axonal architecture during dissection.
Here, we present dissection methods to detect changes at the adult NMJ for defined MNs innervating the tilm using immunocytochemistry. The leg is useful as a simple system, innervated by only ~50 MNs and containing 14 muscles with well-described anatomy. The dissected leg preparation can be used across genotypes and a suite of antibodies are available for NMJ visualization without the need for building genotypically complex stocks of reporter gene constructs in mutant backgrounds. This approach will enable a more detailed characterization of changes at the NMJ for MN diseases and other age-related conditions.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Eric Roberts for advice on imaging. We also wish to thank Information Technology Services at Rhode Island College and in particular Michael Caine and Jake Douglas for videography. Monoclonal antibodies anti-dlg and anti-brp were developed by UC-Berkeley and Universitaetsklinikim Wuerzburg respectively, and were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, USA. The research reported here was supported fully by the Rhode Island Institutional Development Award (IDeA) Network of Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number [P20GM103430].
Materials
| 10x Phosphate Buffered Saline | Fisher Scientific | BP3991 | |
| 24 well plates | Corning | 3473 | Hydrophobic, ultra-low attachment surface |
| 2x objective accessory | Olympus | 110AL2X | Screw-on attachment |
| Anti-ATP5A primary antibody | Abcam | ab14748 | Mouse monoclonal |
| Anti-bruchpilot primary antibody | Developmental Studies Hybridoma Bank | nc82 | Mouse monoclonal |
| Anti-discs large primary antibody | Developmental Studies Hybridoma Bank | 4F3 | Mouse monoclonal |
| Anti-hrp primary antibody | Jackson Immuno Research | 123-605-021 | Alexa Fluor 647 conjugated polyclonal |
| Anti-polyubiquitin (FK2) primary antibody | Millipore Sigma | 04-263 | Mouse monoclonal |
| Confocal Microscope | Olympus | FV1000 | Objectives (NA): 10x (0.4), 20x (0.85), 40x (1.20), 60x (1.42), 100x (1.40) |
| Coverslips | Corning | 285022 | 160-190 mm thickness |
| Dissecting forceps | Fine Science Tools | 11252-00 | Dumont #5SF |
| Dissecting Microscope | Olympus | SZ61 | |
| Formaldehyde | Fisher Scientific | BP531-500 | 37% stock stabilized with methanol |
| Goat anti-mouse secondary antibody | Jackson Immuno Research | 115-545-146 | Alexa Fluor 488 conjugated |
| Goat Serum | Novus Biologicals | NB036768 | 0.2 mm filtered |
| Laboratory sealing tape | Fisher Scientific | 03-448-254 | Parafilm M |
| Methanol | Fisher Scientific | A413 | |
| Microscope Slides | Fisher Scientific | 12-550-123 | 76mm x 25mm |
| Mounting media | Molecular Probes | S36972 | Slowfade Diamond mounting media |
| Nonionic surfactant | Acros Organics | 215680010 | Triton-X 100 |
| Nutator | Fisher Scientific | S06622 | |
| Phalloidin | Invitrogen | A34055 | Alexa Fluor 555- conjugated |
| Sharpening stone | Fine Science Tools | 29008-01 | |
| Silicone elastomer | Electron Microscopy Sciences | 2423610 | Sylgard 184 |
References
- McDermott, C. J., Shaw, P. J. Diagnosis and management of motor neurone disease. BMJ. 336 (7645), 658-662 (2008).
- Global Collaborators, G. B. D. M. N. D. Global, regional, and national burden of motor neuron diseases 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology. 17 (12), 1083-1097 (2018).
- Foster, L. A., Salajegheh, M. K. Motor Neuron Disease: Pathophysiology, Diagnosis, and Management. American Journal of Medicine. 132 (1), 32-37 (2019).
- Bede, P., Pradat, P. F. Editorial: Biomarkers and Clinical Indicators in Motor Neuron Disease. Frontiers in Neurology. 10, 1318 (2019).
- Clark, J. A., Southam, K. A., Blizzard, C. A., King, A. E., Dickson, T. C. Axonal degeneration, distal collateral branching and neuromuscular junction architecture alterations occur prior to symptom onset in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Journal of Chemical Neuroanatomy. 76, 35-47 (2016).
- Martineau, E., Di Polo, A., Van de Velde, C., Robitaille, R. Dynamic neuromuscular remodeling precedes motor-unit loss in a mouse model of ALS. Elife. 7, (2018).
- Shahidullah, M., et al. Defects in synapse structure and function precede motor neuron degeneration in Drosophila models of FUS-related ALS. Journal of Neuroscience. 33 (50), 19590-19598 (2013).
- Tremblay, E., Martineau, E., Robitaille, R. Opposite Synaptic Alterations at the Neuromuscular Junction in an ALS Mouse Model: When Motor Units Matter. Journal of Neuroscience. 37 (37), 8901-8918 (2017).
- Harris, K. P., Littleton, J. T. Transmission, Development, and Plasticity of Synapses. Génétique. 201 (2), 345-375 (2015).
- Beramendi, A., Peron, S., Casanova, G., Reggiani, C., Cantera, R. Neuromuscular junction in abdominal muscles of Drosophila melanogaster during adulthood and aging. Journal of Comparative Neurology. 501 (4), 498-508 (2007).
- Banerjee, S., et al. Miniature neurotransmission is required to maintain Drosophila synaptic structures during ageing. Nature Communications. 12 (1), 4399 (2021).
- Liao, S., Broughton, S., Nassel, D. R. Behavioral Senescence and Aging-Related Changes in Motor Neurons and Brain Neuromodulator Levels Are Ameliorated by Lifespan-Extending Reproductive Dormancy in Drosophila. Frontiers in Cellular Neuroscience. 11, 111 (2017).
- Mahoney, R. E., Rawson, J. M., Eaton, B. A. An age-dependent change in the set point of synaptic homeostasis. Journal of Neuroscience. 34 (6), 2111-2119 (2014).
- Fernandes, J. J., Keshishian, H. Development of the adult neuromuscular system. International Review of Neurobiology. 43, 221-239 (1999).
- Truman, J. W. Metamorphosis of the central nervous system of Drosophila. Journal of Neurobiology. 21 (7), 1072-1084 (1990).
- Baek, M., Mann, R. S. Lineage and birth date specify motor neuron targeting and dendritic architecture in adult Drosophila. Journal of Neuroscience. 29 (21), 6904-6916 (2009).
- Enriquez, J., et al. Specification of individual adult motor neuron morphologies by combinatorial transcription factor codes. Neuron. 86 (4), 955-970 (2015).
- Soler, C., Daczewska, M., Da Ponte, J. P., Dastugue, B., Jagla, K. Coordinated development of muscles and tendons of the Drosophila leg. Development. 131 (24), 6041-6051 (2004).
- Guan, W., Venkatasubramanian, L., Baek, M., Mann, R. S., Enriquez, J. Visualize Drosophila Leg Motor Neuron Axons Through the Adult Cuticle. Journal of Visualized Experiments. (140), e58365 (2018).
- Agudelo, A., et al. Age-dependent degeneration of an identified adult leg motor neuron in a Drosophila SOD1 model of ALS. Biology Open. 9 (10), (2020).
- Fernius, J., Starkenberg, A., Thor, S. Bar-coding neurodegeneration: identifying subcellular effects of human neurodegenerative disease proteins using Drosophila leg neurons. Disease Models & Mechanisms. 10 (8), 1027-1038 (2017).
- Sreedharan, J., Neukomm, L. J., Brown, R. H., Freeman, M. R. Age-Dependent TDP-43-Mediated Motor Neuron Degeneration Requires GSK3, hat-trick, and xmas-2. Current Biology. 25 (16), 2130-2136 (2015).
- Patel, N. H. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods in Cell Biology. 44, 445-487 (1994).
- Guirado, R., Carceller, H., Castillo-Gomez, E., Castren, E., Nacher, J. Automated analysis of images for molecular quantification in immunohistochemistry. Heliyon. 4 (6), 00669 (2018).
- Castells-Nobau, A., et al. Two Algorithms for High-throughput and Multi-parametric Quantification of Drosophila Neuromuscular Junction Morphology. Journal of Visualized Experiments. (123), e55395 (2017).
- Brown, J. R., Phongthachit, C., Sulkowski, M. J. Immunofluorescence and image analysis pipeline for Drosophila motor neurons. Biology Methods and Protocols. 4 (1), (2019).

