Functional Magnetic Resonance Imaging (fMRI) of the Visual Cortex with Wide-View Retinotopic Stimulation
Summary
We have developed techniques for mapping the visual cortex function utilizing more of the visual field than is commonly used. This approach has the potential to enhance the evaluation of vision disorders and eye diseases.
Abstract
High-resolution retinotopic blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) with a wide-view presentation can be used to functionally map the peripheral and central visual cortex. This method for measuring functional changes of the visual brain allows for functional mapping of the occipital lobe, stimulating >100° (±50°) or more of the visual field, compared to standard fMRI visual presentation setups which usually cover <30° of the visual field. A simple wide-view stimulation system for BOLD fMRI can be set up using common MR-compatible projectors by placing a large mirror or screen close to the subject’s face and using only the posterior half of a standard head coil to provide a wide-viewing angle without obstructing their vision. The wide-view retinotopic fMRI map can then be imaged using various retinotopic stimulation paradigms, and the data can be analyzed to determine the functional activity of visual cortical regions corresponding to central and peripheral vision. This method provides a practical, easy-to-implement visual presentation system that can be used to evaluate changes in the peripheral and central visual cortex due to eye diseases such as glaucoma and the vision loss that may accompany them.
Introduction
Functional magnetic resonance imaging (fMRI) is a valuable method to assess changes in regional neurovascular function within the visual cortex in response to stimuli, as changes in regional blood flow correlate to the activation of brain regions1,2. High-resolution retinotopic blood oxygenation level-dependent (BOLD) signal measurements represent changes in deoxyhemoglobin, which are driven by localized changes in blood flow and blood oxygenation within the brain1,2. BOLD activity patterns collected from fMRI data can be used to functionally map the peripheral and central visual cortex, as well as detect changes in the retinotopic map in response to visual impairment and neurodegeneration3.
Most previous fMRI studies made use of narrow-view (around ±12° of the central visual field) non-retinotopic stimuli or simple retinotopic stimuli with narrow-view visual stimuli, which provided limited functional parcellation of the retinotopic representation in the visual cortex and limited assessment to only the central visual field, excluding the periphery3. Consequently, narrow-view fMRI data has reported inconsistent BOLD percent changes in glaucoma patients4,5,6. There is therefore a need for improved fMRI approaches to assessing the peripheral and central visual field, particularly in the evaluation of diseases such as glaucoma.
Glaucoma is the leading cause of irreversible blindness, affecting 10% of people by the age of 807. Glaucoma is caused by the progressive, irreversible neurodegeneration of retinal ganglion cells, which are responsible for transmitting visual stimuli to the brain through the optic nerve. In primary open-angle glaucoma (POAG), the most common form of glaucoma, increased intraocular pressure causes thinning of the retinal nerve fiber layer (RNFL), leading to the loss of peripheral vision followed by peripheral and central blindess8,9,10,11. Histological evidence from animal studies suggests that glaucoma additionally results in progressive neurodegeneration of the optic nerve, optic tract, lateral geniculate nucleus, optic radiation, and visual cortex12,13. MRI technology offers a minimally invasive method of assessing both blood oxygenation and neurodegeneration in the visual cortex. In patients with glaucoma, MRI has found evidence of gray-matter atrophy in the visual pathway13,14,15,16 and abnormal white matter in the optic chiasm, optic tract, and optic radiation1,17,18.
To further explore the effects on visual processing, fMRI can be used to detect brain function in response to visual cues. The protocol herein describes a novel method to obtain a low-cost, wide-view retinotopic map using high-resolution retinotopy fMRI with wide-field (>100°) stimuli, as described by Zhou et al3. Visual stimuli of expanding rings and rotating wedges were used to elicit retinotopic mapping of the eccentricity and polar angle for fMRI. BOLD fMRI percent changes were analyzed as a function of eccentricity to evaluate brain function, corresponding to both central and peripheral vision. The BOLD fMRI percent change may be used to visualize activation throughout the visual cortex. These fMRI measures provide a reliable new method to evaluate neurodegenerative changes and their functional effects on the visual cortex found in eye diseases involving visual field defects, such as glaucoma.
Protocol
Research with human participants was performed in compliance with institutional guidelines at the University of Texas Health Science Center and Stony Brook University, with informed consent obtained from participants for these studies and use of their data.
1. Setup of MRI scanner and imaging protocols
- For fMRI, use a 3T MRI scanner with multi-channel receiver head coils. Different field strengths can also be used but may present difficulties with signal-to-noise ratio (SNR) or distortion artifacts, so adjust accordingly. Use only the posterior half of the head coil for fMRI to allow for a larger viewing angle unobstructed by the anterior half of the coil.
- Set up a T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence with a repetition time (TR) of 2.2 s, echo time (TE) of 2.8 ms, field of view (FOV) of 176 mm x 256 mm x 208 mm, spatial resolution of 1 mm x 1 mm x 1 mm, bandwidth of 190 Hz/pixel, flip angle of 13°, and a scan duration of 3.1 min3.
- Set up a gradient-echo, echo-planar imaging (EPI) sequence with a TR of 2 s, TE of 30 ms, FOV of 220 mm x 220 mm, in-plane resolution of 1.7 mm x 1.7 mm, 29 slices with a thickness of 3 mm, and a bandwidth of 1,500 Hz/pixel3.
- Measure the dimensions of the head coil and scanner bore, and then construct a simple frame by cutting a polyvinyl chloride (PVC) pipe into suitable lengths and connecting them with PVC elbows. Obtain a mirror that is at least 25 cm wide and 15 cm tall and attach it to a plastic rod with screws (small holes can be drilled into the mirror).
- Attach the ends of the plastic rod to the PVC frame with nylon screws (Figure 1A). Make sure that the nylon screws are slightly loose to allow the mirror to be rotated by hand to optimize the angle for each participant.
- Make a screen to go inside the MRI bore. Cut a segment of a rear-projection screen that is approximately the size of the MRI bore. Construct a frame that is the size of the bore and attach the screen to the frame with screws. Place the screen inside the scanner just behind the head coil to minimize the distance between the screen and mirror and maximize the FOV.
NOTE: If the scanner bore is large enough, a single screen can be used for the participant to view directly instead of the mirror and rear projection screen setup. A projection screen attached to a thin sheet of wood for backing or a sheet of thin matte white plastic can be used as a screen and placed on the frame instead of a mirror. The projector should then be positioned and focused, such that it fills the screen and is in focus.
2. Participant preparation
- Inform the participant about the procedure, risks, and benefits of the fMRI scan. Obtain their informed consent.
- Ensure that the participant does not have any contraindications to MRI. This includes screening for pacemakers, metal implants, or claustrophobia. If you have any uncertainty, consult with a qualified radiologist or researcher, and exclude the participant from the study if any uncertainty remains.
- Explain the visual stimulation protocol and the need for the participants to fixate on the central cross during the fMRI scans. Show the participant a short demonstration of the visual stimulation for instructional purposes to familiarize them with the procedure.
- Carefully position the participant on the table of the MRI scanner to ensure that they are comfortable and relaxed. Provide earplugs and/or a sound-dampening headset to reduce the acoustic noise the participant will hear to protect their hearing.
- Immobilize the participant's head in the posterior half of the head coil array, using foam padding on the sides of the head to ensure that the head is properly immobilized to reduce motion artifacts. Use the scanner's positioning system and move the table into the scanner bore.
- Place the wide-view screen or mirror 10 cm from the patient's eyes (Figure 1B). Place the bore-sized screen from the back of the scanner bore just behind the head coil. Adjust the position and angle of the mirror/screen for each participant to achieve a consistent viewing angle.
- Ensure that the participant is comfortable throughout the scan via communication through the intercom.
3. fMRI scanning of participant
- Run a localizer scan with three orthogonal planes and scanner adjustments and calibrations for frequency adjustment and shimming.
- Run an MP-RAGE anatomical scan to help position the EPI slices.
- Create visual stimuli, as described in the following steps, using a program for running behavioral or psychological experiments.
- At the start of the fMRI protocol, instruct the participant to fixate on the white cross (3° x 3°), which should be on top of a gray background at the center of the stimuli for 10 s.
NOTE: The white cross will be shown before and after each visual stimulation paradigm for 10 s. Thus, the total fMRI stimulation test for each paradigm is 200 s. - Present the first visual stimulation paradigm (a series of rotating wedges) for a period of 30 s (giving an angular velocity of 6°/s) and cycle through six periods. The wedge stimuli should include 12 frames of rotating wedges (one scan with clockwise rotation and one with counterclockwise), extending to the edge of the screen/mirror (>100° visual field), with an 8 Hz contrast-reversing black and white (100% contrast) checkerboard pattern (Figure 2A).
- Present the white cross once again for 10 s.
- Repeat steps 3.4-3.6 with the second visual stimulation paradigm (a series of expanding and contracting rings) for a period of 30 s (expanding or contracting at 1.8°/s of the visual field) and cycle through six periods. The ring stimuli should include eight frames of expanding or contracting rings (>100° visual field), with an 8 Hz contrast-reversing black and white (100% contrast) checkerboard pattern (Figure 2B).
- After completing the fMRI, move the table out of the scanner bore while instructing the participant to remain still. Remove the mirror/screen, place the anterior portion of the head coil in addition to the posterior, and move the table back into the center of the scanner.
- Acquire a quick localizer scan in case of any movement and acquire an MP-RAGE scan with the full head coil.
NOTE: An anatomical image with the whole head coil is needed for accurate registration for group analyses and reconstruction purposes.
4. Analysis of retinotopic fMRI data
- Download and install the FreeSurfer application for MRI analysis (https://surfer.nmr.mgh.harvard.edu)20.
NOTE: FreeSurfer version 5.3.0 was used herein. - Obtain images in Digital Imaging and Communications in Medicine (DICOM) format from the MRI scanner. Convert the DICOM files to nifti format using the dcm2niix application (https://www.nitrc.org/projects/mricrogl)21.
- Process the T1-weighted scan to provide a cortical surface reference, as described in the following two steps. Use FreeSurfer to convert structural data from nifti format to .mgz format (mri_convert command).
- Use the recon-all command in a shell environment to perform automated segmentation and cortical reconstruction of the structural data.
NOTE: This step can take over 20 h to complete. - Use the graphical user interface tksurfer to view the inflated hemisphere and virtually cut the visual cortex along the calcarine fissure, and select the occipital lobe. Use the mris_flatten command to flatten the visual cortex patch. Repeat this step for both hemispheres.
- For the fMRI data, first remove the rest periods, with only the fixation cross presented, from the start and end of the data. Screen the fMRI data for artifacts or large movements.
- Preprocess the functional data for spatial smoothing and motion correction. Model the retinotopic stimulus paradigm and apply a canonical hemodynamic response function to construct the response function.
- Perform retinotopic phase-encoded analysis of the fMRI data using the FreeSurfer functional analysis stream (mkanalysis-sess, selxavg3-sess, and fieldsign-sess commands) to correlate the BOLD fMRI time series with a modeled response function and obtain phase-encoded retinotopic maps, with a significance level of p < 0.01 (Figure 3).
- Visualize the results of the retinotopic maps with color-coded activation maps overlaid on the virtually flattened visual cortex using the tksurfer-sess command, and display using the rtview command.
- Use the phase-encoded retinotopic maps from the wedge stimuli to help define the boundaries of the primary visual cortex (V1) and other extra striate areas (V2 and V3) by field sign maps (Figure 3A), along with anatomical landmarks and FreeSurfer atlases.
- To calculate the BOLD response at different eccentricities, first use FSL Feat (http://fsl.fmrib.ox.ac.uk/fsl) to calculate statistical maps using a general linear model for each size of ring stimuli with a z-score threshold of Z > 2.322,23. If group analysis is being performed, calculate the second level analysis for statistical maps of group differences with FSL Feat to help determine the BOLD response at different eccentricities.
- Co-register the fMRI images onto the reconstructed cortical surface using the FreeSurfer bbregister and tkregister2 commands to align the participant's fMRI data to the anatomical structural image of their brain and ensure accurate spatial alignment.
- Group the ring stimuli by eccentricity for each of the eight frames. Manually draw regions of interest for different eccentricities based on the activated voxel regions for each frame. Take the BOLD percent changes and plot them as a function of eccentricity. Also, bin the eccentricity data into central (< ±12°) and peripheral (> ±12°) regions, where a ±12° visual stimulus is typical for retinotopic fMRI studies.
Representative Results
Nine participants diagnosed with POAG (four males, 36-74 years old) and nine age-matched healthy volunteers (six males, 53-65) were evaluated using the aforementioned wide-view fMRI protocol, as previously described by Zhou et al3. POAG was confirmed clinically in patients with an open angle by assessment of the presentation of visual field defects consistent with glaucoma, optic disc cupping, and/or an intraocular pressure (IOP) greater than 21 mmHg3. A wide-view visual presentation (±55°) was used to evaluate central and peripheral vision in each group3.
Figure 3 depicts the retinotopic fMRI maps for polar (wedge) and eccentricity (ring) stimuli from a POAG and healthy control participant. The polar maps (Figure 3A) revealed no obvious differences between the POAG and healthy participants. The eccentricity maps (Figure 3B) showed that the central region of the parafovea that was activated by the smaller ring stimuli appeared larger in the POAG patient compared to the healthy participant. The enlarged parafoveal region in the visual cortex of POAG participants suggests cortical changes in response to peripheral vision disturbances.
BOLD percent changes for central (<24°) and peripheral (>24°) visual fields between POAG subgroups and the healthy control group were compared (Figure 4). BOLD percent changes at different eccentricities were reduced in POAG patients compared to healthy control participants, primarily at more peripheral eccentricities (Figure 4A). The BOLD percent changes were significantly reduced between the two groups, more so at larger eccentricities (p < 0.05, two-way ANOVA with Bonferroni post hoc test). The BOLD percent changes averaged for central vision (all stimuli <24°) were only slightly and not significantly reduced in POAG patients, while the BOLD response for peripheral vision (all stimuli >24°) was significantly reduced (Figure 4B). These results indicate this protocol's potential utility to assess changes in visual cortex function localized to peripheral or central vision, which is relevant to visual disorders such as glaucoma.
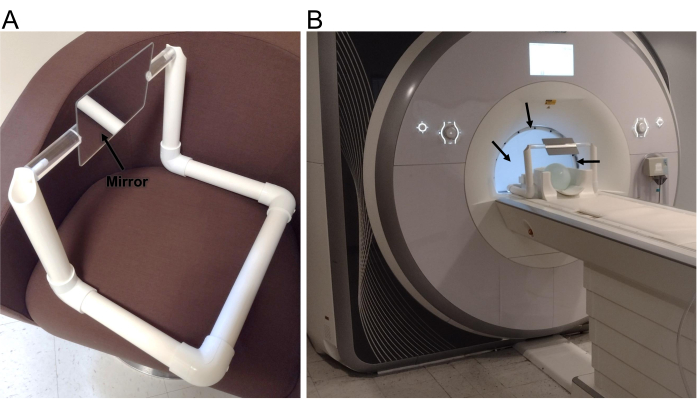
Figure 1: Experimental setup. (A) The 25 cm wide by 15 cm tall mirror held in place with a frame constructed from PVC pipe. (B) Setup of the presentation system on an MRI scanner, showing the posterior portion of a head array coil, the mirror and frame, and the back-projection screen (arrows) in the bore directly behind the head coil. Please click here to view a larger version of this figure.
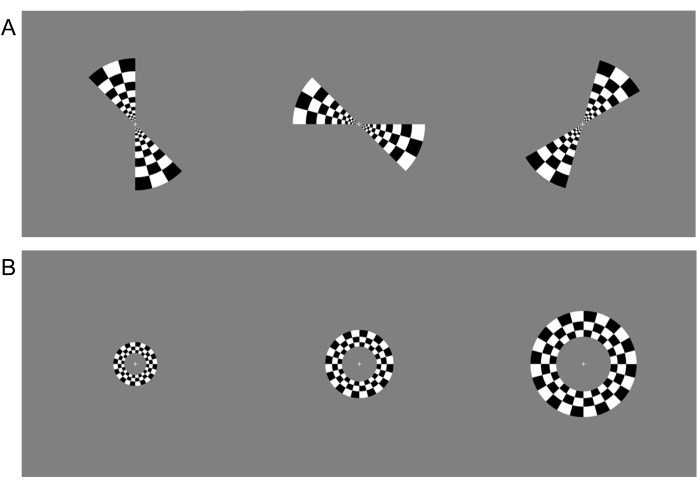
Figure 2: Visual stimulation paradigms. (A) Three frames from the polar retinotopic visual stimulation paradigm, which consist of clockwise and counterclockwise spinning wedges with a contrast-alternating checkerboard pattern. (B) Three frames from the eccentricity paradigm, which consist of expanding and contracting rings with a contrast-alternating checkerboard pattern. Please click here to view a larger version of this figure.
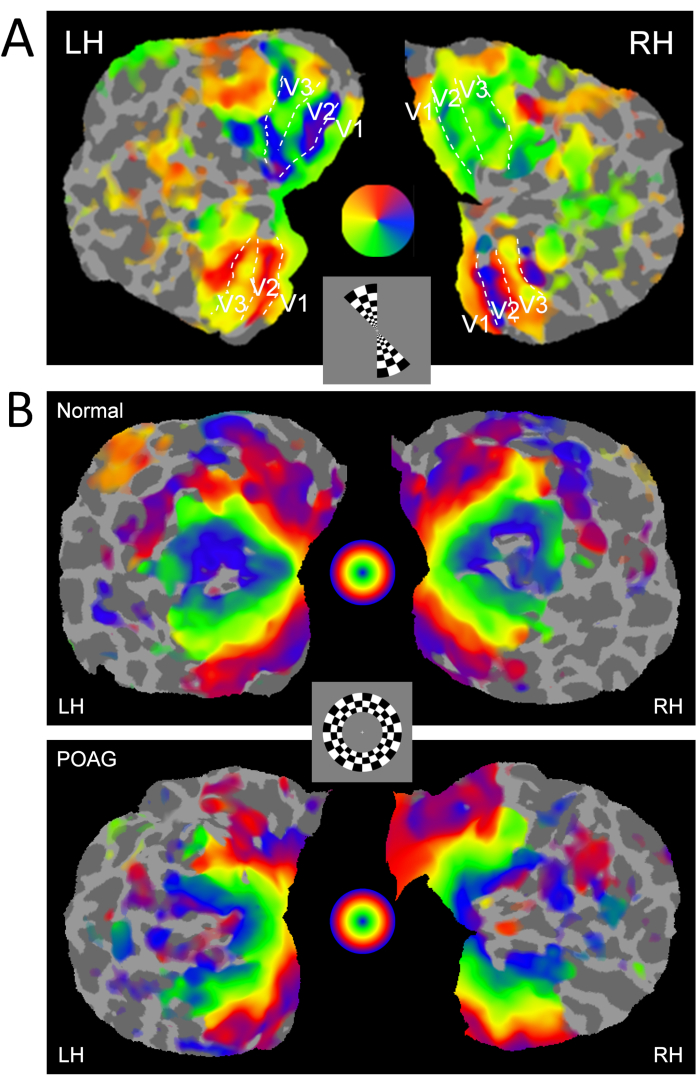
Figure 3: Retinotopic polar and eccentricity maps. Representative (A) polar map using a rotating wedge from a normal control and (B) eccentricity maps using expanding/contracting rings from a normal control and a POAG participant. Both the left and right hemispheres (LH and RH) are shown with defined visual cortical boundaries (V1, V2, and V3). One frame from each paradigm is shown in the central inset. The color scales map to the corresponding regions of the visual field, as indicated by the color wheels, with A) mapping to the polar angle of the wedge stimuli, and B) mapping to the eccentricity of the ring stimuli. Please click here to view a larger version of this figure.
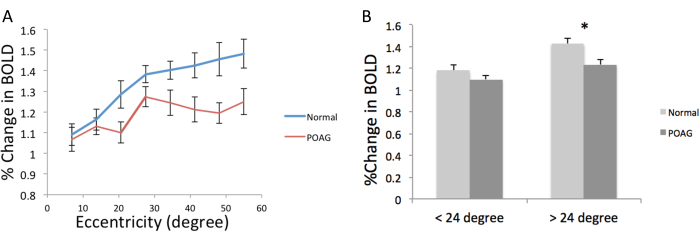
Figure 4: BOLD percent changes as a function of eccentricity and central or peripheral visual fields. (A) Group-averaged BOLD percent changes from the ring stimuli in healthy controls and POAG patients as a function of eccentricity. The BOLD percent change for each size of the ring stimuli were calculated to give the data at each eccentricity. (B) BOLD percent changes between healthy control and POAG patients of central (< ±12°) and peripheral (> ±12°) of the visual field, by binning data from all the eccentricities. Data are mean ± standard error of the mean. *p < 0.05, two-factor ANOVA with post hoc correlation. This figure has been modified from Zhou et al.3 with permission. Please click here to view a larger version of this figure.
Discussion
The above protocol for utilization of wide-view retinotopic fMRI is an innovative method to evaluate the effects of vision loss and eye diseases on the brain. Through wide-field retinotopic mapping of the visual cortex with the use of a wider-view screen, this approach allows for a more comprehensive understanding of the visual system’s functional organization. This could lead to a better understanding of abnormalities in the brain’s visual processing system, which occurs in neurodegeneration, such as in glaucoma24,25. This technology can also be used to detect and analyze brain degeneration and reorganization in other conditions that cause blindness, such as age-related macular degeneration26,27.
A single human eye has about a 100° visual angle. Previous techniques used in most visual fMRI studies used a FOV of less than 30°, limiting the portion of the visual cortex that could be activated and analyzed by the fMRI28. Consequently, peripheral vision could not be visualized, forcing all analyses to be focused solely on the central visual field. Clinically, this prevented clinicians from accurately performing preoperative cortical mapping, crucial for avoiding vital locations when performing brain surgeries29. With the wide-view retinotopic fMRI technique described in this protocol, the visual angle has been increased to up to 100° (±50°)3,30,31. To allow for a wide-view image and decrease visual obstruction caused by the head coil, only the posterior half of the head coil is used. Head coils usually have a relatively small window, with bars across that impede the ability to fully see the wide-view retinotopic stimuli. However, using only the posterior portion of the head coil causes large signal inhomogeneity across the brain and reduces the SNR in the anterior and central regions. The image quality and SNR of the posterior occipital lobe should not be heavily impacted32. However, the exact effects of using only the posterior portion of the coil likely depend on a specific coil design (number and size of arrayed coils), so testing the SNR or signal-to-noise fluctuation ratio in a few subjects with and without the anterior portion can be done if there is a concern of significant SNR loss with a given coil32.
Proper setup of the T1-weighted MP-RAGE sequence is essential to properly register functional images to high-resolution brain structural images and for anatomical registration to templates or for group studies. As such, we acquire the T1-weighted image using the entire head coil, which may result in slight movement of the participant relative to the fMRI scan. Alignment of the fMRI to the anatomical scan is a routine analysis step, so this should not be an issue. Alternatively, the acquisition of the T1-weighted image without the anterior coil could be done, but the image inhomogeneity may impact the quality of registration to a reference template. To avoid motion artifacts, it is crucial to properly immobilize the participant’s head within the head coil. Motion artifacts can naturally occur without proper stabilization, which will negatively affect the quality of the fMRI data collected, leading to poorer results from the analysis. While post-processing motion correction is routine for fMRI analysis, large movements can still impact the results, so it is important to check functional scans for data quality and discard studies with major artifacts. In this protocol, participants were instructed to focus on a white cross for 10 s, both before and after each visual stimulation paradigm, to obtain baseline BOLD data. This helped reduce the variability of the fMRI at baseline, and also allowed the subject’s brain to adjust to the scanner sounds and background screen brightness before the actual visual data tests began.
There are a variety of alternative approaches that could be considered for wide-view fMRI. The approach described herein, using a large screen/mirror with only the posterior half of the head coil, can provide a moderate wide-view up to around a 100° FOV3,30. The cost to make the mirror/screen is very low (potentially <US $100), assuming a standard projector is already available. Greco et al. used a slightly different approach, with the screen placed after the mirror in the optical path, directly in front of the subject’s face (7.5 cm away), providing an 80° visual angle28. MR-compatible glasses were needed for the participant to be able to focus on the screen. Ellis et al. also used a similar approach, but with the projector tilted down onto a mirror on the bottom of the bore, which reflected the stimuli directly onto the top of the bore above the subject’s face, providing a 115° visual angle32. The view is distorted by the curved bore, which requires the images for the stimuli to be warped to correct. An extension of this approach was recently reported with a custom curved screen at the top of the scanner bore and two mirrors that were able to provide an ultra-wide FOV of 175°34. Some of these reported methods used the anterior portion of the head coil and others did not; however, any of these methods could be used either way, with potentially a slightly higher SNR using the anterior coil, but with the tradeoff of reduced visual angle and portions of the visual field being blocked. A potential limitation with all methods using a projector is that, for a screen with custom size and location, the projector has to be adjusted for the focus and size of the projected image by adjusting the projector/lens, moving the projector, or getting custom lenses if the previous methods are not sufficient.
Another approach used a transparent plastic rod with a curved end as the screen, with a projector to provide a slightly larger visual angle of 120°, which is compatible with using the anterior head coil without limiting the FOV. However, only monocular stimulation can be performed. A special lens for the projector is needed, which increases the cost, and contact lenses must be worn for the participant to be able to focus on the screen, which complicates the setup31. A similar approach used fiber-optic bundles to directly transmit and present images from a screen to a participant’s eye, which provided a visual angle of up to 120°33. Contact lenses must also be worn, and only a single eye could be stimulated at a time. This method requires a long and high-density fiber optic bundle, which can have relatively low resolution for the presentation and may be moderately expensive33.
Visual impairment and eye disease can affect the structure and function of the visual cortex. BOLD fMRI can be used to visualize retinotopic cortical function, but most visual presentation systems used for fMRI only stimulate the central visual field. This protocol describes the implementation of a wide-view presentation system for fMRI that can be used to functionally map the peripheral and central visual cortex. This system can be set up easily and at a low cost utilizing common MR-compatible projectors. Although with some limitations, the protocol described has the potential to analyze functions of the visual cortex corresponding to central and peripheral vision at a level that balances cost and precision. The data collected through this method can be analyzed to determine selective activation based on different types of visual stimuli and brain communication amongst different areas for visual processing. This method could be used to evaluate changes in the function of peripheral and central visual cortex due to vision loss and eye diseases such as glaucoma. This technology therefore has applications in the diagnosis, management, and treatment of eye diseases.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institutes of Health [R01EY030996].
Materials
| 1/4"-20 nylon machine screws, knurled head thumb screw | to attach rod to PVC frame | ||
| 1-1/4 inch PVC pipe | length of ~5-10 ft is needed | ||
| 3T MRI scanner | Siemens | ||
| 6-32 nylon machine screws, rounded head | to attach mirror/screen to rod | ||
| 8-channel head array coil | Siemens | ||
| 90 degree PVC elbow, 1-1/4 inch fitting | |||
| Acrylic mirror | Width and length of 25-30cm | ||
| Acrylic rod | 1 inch width, ~ 2 ft long depening on size of scanner bore and head coil | ||
| E-Prime | Psychology Software Tools | to prepare and present visual stimuli paradigms | |
| Plywood sheet, 1/2 inch thick | Size should be at least as large as the scanner bore. Cut as bore-sized frame for the projection screen | ||
| Rear projection screen | Size should be at least as large as the scanner bore |
References
- Kwong, K. K., et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences. 89 (12), 5675-5679 (1992).
- Ogawa, S., et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proceedings of the National Academy of Sciences. 89 (13), 5951-5955 (1992).
- Zhou, W., et al. Retinotopic fMRI reveals visual dysfunction and functional reorganization in the visual cortex of mild to moderate glaucoma patients. Journal of Glaucoma. 26 (5), 430-437 (2017).
- Duncan, R. O., Sample, P. A., Weinreb, R. N., Bowd, C., Zangwill, L. M. Retinotopic organization of primary visual cortex in glaucoma: a method for comparing cortical function with damage to the optic disk. Investigative Ophthalmology & Visual Science. 48 (2), 733-744 (2007).
- Duncan, R. O., Sample, P. A., Weinreb, R. N., Bowd, C., Zangwill, L. M. Retinotopic organization of primary visual cortex in glaucoma: Comparing fMRI measurements of cortical function with visual field loss. Progress in Retinal and Eye Research. 26 (1), 38-56 (2007).
- Gerente, V. M., et al. Evaluation of glaucomatous damage via functional magnetic resonance imaging, and correlations thereof with anatomical and psychophysical ocular findings. PLoS One. 10 (5), e0126362 (2015).
- Allingham, R. R., Damji, K., Freedman, S., Moroj, G., Shafranov, . Shields’ textbook of glaucoma. 5th ed. , (2005).
- Kerrigan-Baumrind, L. A., Quigley, H. A., Pease, M. E., Kerrigan, D. F., Mitchell, R. S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investigative Ophthalmology & Visual Science. 41 (3), 741-748 (2000).
- Quigley, H. A., Addicks, E. M., Green, W. R. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Archives of Ophthalmology. 100 (1), 135-146 (1982).
- Smith 3rd, E. L., Hung, L. F., Harwerth, R. S. Developmental visual system anomalies and the limits of emmetropization. Ophthalmic and Physiological Optics. 19 (2), 90-102 (1999).
- Limb, G. A., Martin, K. R. Sixth ARVO/Pfizer Ophthalmics Research Institute Working Group. Current prospects in optic nerve protection and regeneration: sixth ARVO/Pfizer Ophthalmics Research Institute Conference. Investigative Ophthalmology & Visual Science. 52 (8), 5941-5954 (2011).
- Gupta, N., Yucel, Y. H. Glaucoma as a neurodegenerative disease. Current Opinion in Ophthalmology. 18 (2), 110-114 (2007).
- Yucel, Y. H., Zhang, Q., Weinreb, R. N., Kaufman, P. L., Gupta, N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Progress in Retinal and Eye Research. 22 (4), 465-481 (2003).
- Zikou, A. K., et al. Voxel-based morphometry and diffusion tensor imaging of the optic pathway in primary open-angle glaucoma: a preliminary study. American Journal of Neuroradiology. 33 (1), 128-134 (2012).
- Chen, W. W., et al. Structural brain abnormalities in patients with primary open-angle glaucoma: a study with 3T MR imaging. Investigative Ophthalmology & Visual Science. 54 (1), 545-554 (2013).
- Yu, L., et al. Morphologic changes in the anterior and posterior subregions of V1 and V2 and the V5/MT + in patients with primary open-angle glaucoma. Brain Research. 1588, 135-143 (2014).
- Hernowo, A. T., Boucard, C. C., Jansonius, N. M., Hooymans, J. M. M., Cornelissen, F. W. Automated morphometry of the visual pathway in primary open-angle glaucoma. Investigative Ophthalmology & Visual Science. 52 (5), 2758-2766 (2011).
- Dai, H., et al. Whole-brain voxel-based analysis of diffusion tensor MRI parameters in patients with primary open angle glaucoma and correlation with clinical glaucoma stage. Neuroradiology. 55 (2), 233-243 (2013).
- Zhou, W., Muir, E. R., Chalfin, S., Nagi, K. S., Duong, T. Q. MRI study of the posterior visual pathways in primary open angle glaucoma. Journal of Glaucoma. 26 (2), 173-181 (2017).
- Dale, A. M., Fischl, B., Sereno, M. I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 9 (2), 179-194 (1999).
- Li, X., Morgan, P. S., Ashburner, J., Smith, J., Rorden, C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. Journal of Neuroscience Methods. 264, 47-56 (2016).
- Smith, S. M., et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 23, 208-219 (2004).
- Woolrich, M. W., Ripley, B. D., Brady, M., Smith, S. M. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 14 (6), 1370-1386 (2001).
- Murphy, M. C., et al. Retinal structures and visual cortex activity are impaired prior to clinical vision loss in glaucoma. Scientific Reports. 6, 31464 (2016).
- Chan, R. W., et al. Relationships between cerebrovascular reactivity, visual-evoked functional activity, and resting-state functional connectivity in the visual cortex and basal forebrain in glaucoma. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. , 4037-4040 (2021).
- Murphy, M. C., et al. Top-down influence on the visual cortex of the blind during sensory substitution. Neuroimage. 125, 932-940 (2016).
- Bang, J. W., Hamilton-Fletcher, G., Chan, K. C. Visual plasticity in adulthood: perspectives from Hebbian and homeostatic plasticity. The Neuroscientist. 29 (1), 117-138 (2023).
- Greco, V., et al. A low-cost and versatile system for projecting wide-field visual stimuli within fMRI scanners. Behavior Research Methods. 48 (2), 614-620 (2016).
- DeYoe, E. A., Raut, R. V. Visual mapping using blood oxygen level dependent functional magnetic resonance imaging. Neuroimaging Clinics of North America. 24 (4), 573-584 (2014).
- Pitzalis, S., et al. Wide-field retinotopy defines human cortical visual area v6. The Journal of Neuroscience. 26 (30), 7962-7973 (2006).
- Wu, J., et al. Development of a method to present wide-view visual stimuli in MRI for peripheral visual studies. Journal of Neuroscience Methods. 214 (2), 126-136 (2013).
- Ellis, C. T., et al. Re-imagining fMRI for awake behaving infants. Nature Communications. 11 (1), 4523 (2020).
- Yan, T., Jin, F., He, J., Wu, J. Development of a wide-view visual presentation system for visual retinotopic mapping during functional MRI. Journal of Magnetic Resonance Imaging. 33 (2), 441-447 (2011).
- Park, J., Soucy, E., Segawa, J., Konkle, T. Full-field fMRI: a novel approach to study immersive vision. Journal of Vision. 22 (14), 4018 (2022).

