Determination of 45 Pesticides in Avocado Varieties by the QuEChERS Method and Gas Chromatography-Tandem Mass Spectrometry
Summary
The present protocol describes the analysis of multiclass pesticide residues in avocado varieties using the Quick-Easy-Cheap-Effective-Rugged-Safe (QuEChERS) method with ammonium formate, followed by gas chromatography-tandem mass spectrometry.
Abstract
Gas chromatography (GC) tandem mass spectrometry (MS/MS) stands as a preeminent analytical instrument extensively employed for the surveillance of pesticide residues in food. Nevertheless, these methods are vulnerable to matrix effects (MEs), which can potentially affect accurate quantification depending on the specific combination of analyte and matrix. Among the various strategies to mitigate MEs, matrix-matched calibration represents the prevailing approach in pesticide residue applications due to its cost-effectiveness and straightforward implementation. In this study, a total of 45 representative pesticides were analyzed in three different varieties of avocado (i.e., Criollo, Hass, and Lorena) using the Quick-Easy-Cheap-Effective-Rugged-Safe (QuEChERS) method with ammonium formate and GC-MS/MS.
For this purpose, 5 g of the avocado sample was extracted with 10 mL of acetonitrile, and then 2.5 g of ammonium formate was added to induce phase separation. Subsequently, the supernatant underwent a cleanup process via dispersive solid-phase extraction employing 150 mg of anhydrous MgSO4, 50 mg of primary-secondary amine, 50 mg of octadecylsilane, 10 mg of graphitized carbon black, and 60 mg of a zirconium oxide-based sorbent (Z-Sep+). The GC-MS/MS analysis was successfully performed in less than 25 min. Rigorous validation experiments were carried out to assess the performance of the method. The examination of a matrix-matched calibration curve for each variety of avocado revealed that the ME remained relatively consistent and less than 20% (considered as a soft ME) for most pesticide/variety combinations. Furthermore, the method´s limits of quantification were lower than 5 µg/kg for all three varieties. Finally, the recovery values for most pesticides fell within the acceptable range of 70-120%, with relative standard deviation values below 20%.
Introduction
In chemical analysis, the matrix effect (ME) can be defined in various ways, but a widely accepted general definition is as follows: it refers to the change in the signal, particularly a change in the slope of the calibration curve when the sample matrix or portion of it is present during the analysis of a specific analyte. As a critical aspect, ME necessitates thorough investigation during the validation process of any analytical method, as it directly affects the accuracy of quantitative measurement for the target analytes1. Ideally, a sample pretreatment procedure should be selective enough to avoid extracting any components from the sample matrix. However, despite significant efforts, many of these matrix components still end up in the final determination systems in most cases. Consequently, such matrix components often compromise the recovery and precision values, introduce additional noise, and escalate the overall cost and labor involved in the method.
In gas chromatography (GC), ME arises due to the presence of active sites within the GC system, which interact with the target analytes through various mechanisms. On the one hand, the matrix constituents block or mask these active sites that would otherwise interact with the target analytes, resulting in frequent signal enhancement2. On the other hand, active sites that remain unobstructed may cause peak tailing or analyte decomposition due to strong interactions, leading to a negative ME. However, this can offer potential benefits in certain cases2. It is crucial to emphasize that achieving complete inertness in a GC system is exceedingly challenging, despite using highly inert components and proper maintenance. With continuous use, the accumulation of matrix components in the GC system becomes more pronounced, causing an increased ME. Nowadays, it is widely recognized that analytes containing oxygen, nitrogen, phosphorus, sulfur, and similar elements, exhibit a greater ME as they readily interact with these active sites. Conversely, highly stable compounds such as hydrocarbons or organohalogens do not undergo such interactions and do not show observable ME during analysis2,3.
Overall, ME cannot be fully eliminated, leading to the development of several strategies for compensation or correction when complete removal of matrix components is not feasible. Among these strategies, the utilization of deuterated internal standards (ISs), analyte protectants, matrix-matched calibration, the standard addition method, or the modification of injection techniques have been documented in scientific literature1,2,4,5. The SANTE/11312/2021 guidelines have also recommended these strategies6.
Regarding the application of matrix-matched calibration to compensate for MEs, sample sequences in practical situations encompass diverse types of foods or various samples from the same commodity. In this case, the assumption is made that employing any sample from the same commodity will effectively compensate for ME in all samples. However, there is a lack of sufficient studies in the existing literature that specifically investigate this issue7.
The multiresidue determination of pesticides in matrices containing an appreciable percentage of fat and pigments constitutes a challenging task. The considerable amount of coextracted material can significantly affect the extraction efficiency and interfere with the subsequent chromatographic determination, potentially damaging the column, source, and detector, and resulting in significant MEs8,9,10. Consequently, the analysis of pesticides at trace levels in such matrices necessitates a significant reduction of matrix components before analysis while ensuring high recovery values7. Obtaining high recovery values is crucial to ensure that pesticide analyses remain reliable, accurate, and compliant with regulatory standards. This is vital for ensuring food safety, environmental protection, and informed decision-making in agriculture and related fields.
Avocado is a fruit of high commercial value cultivated in tropical and Mediterranean climates worldwide and widely consumed both in its regions of origin and in the numerous export markets. From the analytical point of view, avocado is a complex matrix containing a significant number of fatty acids (i.e., oleic, palmitic, and linoleic), similar to nuts, a significant pigment content, as in green leaves, as well as sugars and organic acids, similar to those found in other fruits11. Due to its fatty nature, special attention must be given when employing any analytical method for analysis. While pesticide residue analysis has been conducted on avocados using GC-MS in some instances8,12,13,14,15,16,17,18,19,20, it has been relatively less frequent compared to other matrices. In most cases, a version of the Quick-Easy-Cheap-Effective-Rugged-Safe (QuEChERS) method has been applied8,12,13,14,15,16,17,18. None of these studies have investigated the consistency of MEs among different avocado varieties.
Therefore, the aim of this work was to study the consistency of MEs and recovery values for 45 representative pesticides across different varieties of avocado (i.e., Criollo, Hass, and Lorena) using the QuEChERS method with ammonium formate and GC-MS/MS. To the best of our knowledge, this is the first time that this type of study has been conducted on such fatty matrix samples.
Protocol
1. Preparation of the stock and working solutions
NOTE: For safety reasons, it is advisable to wear nitrile gloves, a laboratory coat, and safety glasses throughout the protocol.
- Prepare individual stock solutions of each of the 45 commercial pesticide standards (see Table of Materials) at approximately 1,000 mg/L in acetonitrile in 10 mL volumetric flasks.
- Combine the above individual stock solutions to prepare a 400 mg/L stock solution in acetonitrile in a 25 mL volumetric flask.
NOTE: This mixed solution will be utilized to prepare the working solutions for recovery and calibration experiments. - Prepare stock solutions of atrazine-d5 and triphenyl phosphate (TPP) at concentrations of 750 mg/L and 1,050 mg/L, respectively, in acetonitrile in 10 mL volumetric flasks. Utilize atrazine-d5 as a procedural internal standard (P-IS) and TPP as an injection internal standard (I-IS).
NOTE: The ideal scenario would involve the utilization of an isotopically labeled internal standard for each specific target analyte. - Prepare stock recovery solutions in acetonitrile containing 0.05% (v/v) of formic acid (to prevent degradation) in 10 mL volumetric flasks to yield 10, 100, and 400 µg/kg sample equivalents for the pesticides and 200 µg/kg for the P-IS separately. Store these solutions in amber glass vials in the dark at −20 °C.
- Prepare calibration solutions of the pesticides and P-IS together in acetonitrile with 0.05% (v/v) of formic acid in 10 mL volumetric flasks to yield 5, 10, 25, 75, 200, 400, and 600 µg/kg, and 200 ng/ng, respectively, and store them in amber glass vials in the darkness at −20 °C.
NOTE: The same solutions may be utilized throughout the experimental work but storing them under the specified conditions immediately after each use is essential. - Prepare a mixture of analyte protectants containing 100 g/L of 3-ethoxy-1,2-propanediol, 10 g/L of L-gulonic acid γ-lactone, 10 g/L of D-sorbitol, and 5 g/L of shikimic acid in a 4/1 (v/v) ratio of acetonitrile to water with 0.5% (v/v) of formic acid.
NOTE: This mixture of analyte protectants is to be added just before the injection to mitigate ME.
2. Sample collection
- Collect samples from three avocado species (e.g., Criollo, Hass, and Lorena) available at supermarkets. Ensure that each sample weighs approximately 1 kg, which is enough for conducting all the subsequent studies and aligns with Directive 2002/63/CE21.
NOTE: Organic samples were preferentially selected to minimize the likelihood of the presence of pesticide residues. - Transport the collected avocado samples to the laboratory, and individually homogenize them without the pipe using a chopper (see Table of Materials). Store the homogenized samples in amber glass containers at 4 °C until analysis.
NOTE: The same avocado samples will be used throughout the entire study. Therefore, it is crucial to store them under the specified conditions immediately after each use.
3. Sample preparation utilizing the QuEChERS method with ammonium formate
NOTE: Figure 1 illustrates a schematic representation of the QuEChERS method with ammonium formate.
- Weigh 5 g of each avocado sample in a 50 mL centrifuge tube (see Table of Materials).
- Add 50 µL of the P-IS solution to yield a concentration of 200 µg/kg. For recovery assessment, also add the pesticide solutions prepared in step 1.4 to yield concentrations of 10, 100, and 400 µg/kg (n = 5 each).
- Vortex the tube for 30 s to ensure thorough integration of the spike into the sample.
- Add 10 mL of acetonitrile to the centrifuge tube. Shake the tube at 70 rpm for 5 min.
- Add 2.5 g of ammonium formate, shake the tube again at 70 rpm for 5 min, and subsequently centrifuge it at 1,800 × g for 5 min.
- To a 2 mL centrifuge tube containing 150 mg of anhydrous MgSO4, 50 mg of primary-secondary amine (PSA), 50 mg of octadecylsilane (C18), 10 mg of graphitized carbon black (GCB), and 60 mg of a zirconium oxide-based sorbent Z-Sep+, add 1 mL of the extract for purification utilizing dispersive-solid phase extraction (d-SPE). Vortex the tube for 30 s and centrifuge it at 1,800 × g for 5 min.
- Transfer 200 µL of the extract to an autosampler vial, add 20 µL of the analyte protectant solution prepared in step 1.6, and include 50 µL of the TPP solution.
- Perform instrumental analysis using a GC-MS/MS system (see section 4).
- Perform matrix-matched calibration following the same procedure as described above, using blank extracts, except, during the d-SPE step (step 3.6), clean 5 mL of the supernatant in 15 mL tubes. Add the spike and P-IS solutions at step 3.7. Add the calibration standard solutions to the autosampler vials to yield 5, 10, 25, 50, 100, 200, 400, and 600 µg/kg, along with the TPP, resulting in a final volume of 270 µL.
NOTE: Overall, be sure to construct matrix-matched calibration curves for each avocado variety plus the acetonitrile-only calibrations.
4. Instrumental analysis using GC-MS/MS
- Conduct the analyses employing a GC-MS/MS system with a triple quadrupole (TQ) equipped with an electron ionization interface (−70 eV) and an autosampler (see Table of Materials).
- Employ an MS GC column (silica Bond of 30 m length, 0.25 mm inner diameter, 0.25 µm film thickness) along with ultrahigh purity Helium as the carrier gas at a constant flow rate of 1.2 mL/min.
- Verify the following parameters before proceeding with the equipment operation:
- Ensure that the gas pressures are correct: Helium at 140 psi and Argon at 65 psi.
- Check the condition of the rotary pump oil to ensure that it is clear and at the appropriate level.
- Ensure that the injection syringe does not have any obstructions from previous injections.
- Confirm that the wash vials contain a sufficient volume of each solvent.
- Check that the consumables counter (septum, liner) has not reached its limit.
- Turn on the main GC switch located on the front panel and turn on the MS switch located at the back.
- Open the GCMS Real Time Analysis software that controls all the parameters of the GC-MS/MS system.
NOTE: The instrument system includes the GCMS Real Time Analysis software by default. - Click on Vacuum Control | Advanced | Rotary Pump 1 to initiate the vacuum system.
NOTE: In this window, monitor the pressure to determine the optimal vacuum values, which should be lower than 9.0 Pa. It will take approximately 12 h. - Click on Start to turn on turbo molecular pump 1 and turbo molecular pump 2.
- Click on Start for the Ion Source Heater option.
NOTE: After a recommended time of 1 h, check the system's vacuum to confirm that the recommended value is lower than 1.6e-3 Pa. - Set the MS interface temperature à 250 °C and the ion source temperature à 300 °C.
- Maintain the GC oven at an initial temperature of 50 °C for 1 min, then ramp it up to 180 °C at a rate of 25 °C/min. Subsequently, increase the temperature to 230 °C at 5 °C/min and then to 290 °C at 25 °C/min. Finally, keep the temperature constant at 290 °C for 6 min. The total analysis time is 24.6 min.
- Click on Close once all these systems are turned on.
- Click on the Tuning option from the analysis software and click on Peak Monitor View to perform an initial verification of the mass spectrometer conditions.
NOTE: If necessary, perform autotuning. - Click on Acquisition, and from the displayed window, click on Download Initial Parameters. Verify that the equipment is ready GC and ready MS.
5. Data acquisition
- Click on New Batch File from the software and create a sequence containing information such as sample name, sample ID, method file, data file, injection volume, and tuning file. Add rows as necessary and click on Save.
- Click on Batch Start and let the injection process commence.
- Perform the injection at 250 °C in the splitless mode, maintaining an injection volume of 1 µL. After 1 min following the injection, open the split.
NOTE: Between injections, be sure to clean the 10 µL syringe with methanol, ethyl acetate, and acetonitrile, using a single rinse with each solvent. All the injections are performed in triplicate. - Analyze the analytes using the multiple reaction monitoring (MRM) mode, which is the standard mode employed in MS/MS systems with a TQ.
NOTE: Table 1 provides the retention times (in min) and the quantifier and qualifier transitions for the multiclass pesticides, P-IS, and I-IS. The quantitative analysis relies on the ratio of the peak area of the quantitation ion to the P-IS ion. The I-IS is employed for quality control during injection. Supplementary File 1 contains chromatograms for all the 45 analyzed pesticides. - Open the Postrun Analysis software for data analysis.
NOTE: The instrument system includes the GCMS Postrun Analysis software by default. - Click on the injection to be analyzed, navigate through the table containing the analytes, and select the peak of interest.
- Click on the peak or the region of interest to visualize the chromatogram. Review the peak integrations, and if necessary, perform manual integration. Verify the areas of all analytes to perform the necessary calculations and method evaluation.
Representative Results
Comprehensive validation of the analytical method was conducted according to SANTE/11312/2021 guidelines6, encompassing assessments of linearity, ME, recovery, and repeatability.
For the linearity assessment, matrix-matched calibration curves were constructed using spiked blank samples at multiple concentration levels (ranging from 5 to 600 µg/kg). The determination coefficients (R2) for most of the selected pesticides were found to be higher than or equal to 0.99, indicating a highly linear relationship between concentration and response. The lowest calibration level (LCL) of 5 µg/kg was chosen, adhering to the established maximum residue limit (MRL) established of 10 µg/kg for food monitoring purposes22.
To evaluate the ME, the slopes of the multiclass pesticides´ calibration curves were compared between pure solvent and matrix-matched calibration conditions. As an illustrative example, Figure 2 shows the comparison of the curves in the solvent and each of the three matrices for carbofuran. The ME was calculated using equation (1)7, yielding percentages that signify signal enhancement (positive percentages) or signal suppression (negative percentages).
Matrix effect (%) = 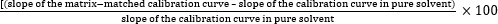 (1)
(1)
The presented ME classification system, based on percentage ranges, provides insights into the impact of the matrix on the pesticide signals, aiding in the interpretation of analytical findings. In all cases for carbofuran, a positive ME greater than 20% was obtained. However, the findings from the generation of matrix-matched calibration curves revealed a relatively consistent ME of less than 20% (classified as a soft ME) for most pesticide/variety combinations (see Table 2 and Figure 3).
To evaluate the accuracy and repeatability of the analysis, blank samples were spiked with pesticides at three different concentration levels (10, 100, and 400 µg/kg; n = 5 for each concentration). The results in Figure 4 demonstrate the count of pesticides whose average recovery percentages were within the acceptable range of 70-120% for each type of avocado. Furthermore, Table 3 presents detailed data for all the specific values obtained. A significant proportion of the tested pesticides exhibited recovery percentages falling within the specific range, with relative standard deviation (RSD) values below 20%.
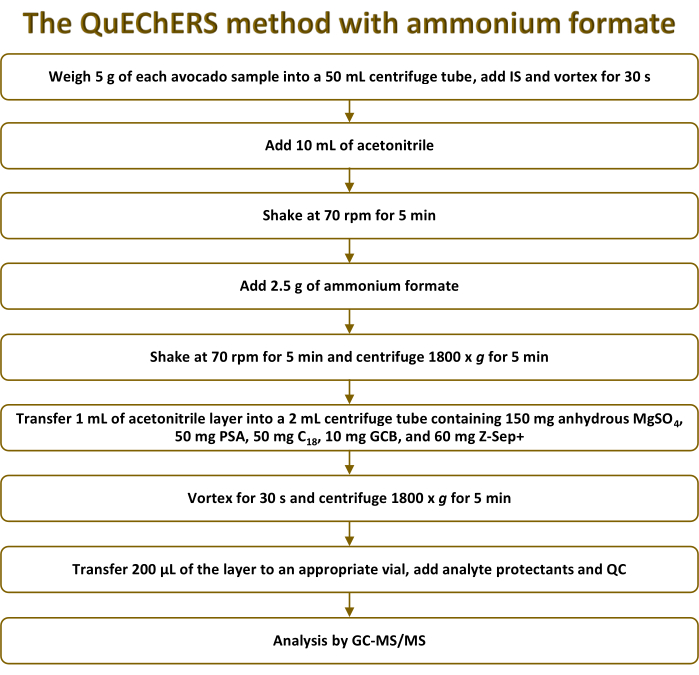
Figure 1: Schematic representation of the QuEChERS method with ammonium formate employed for the extraction of pesticide residues from avocado samples. Abbreviations: QuEChERS = Quick-Easy-Cheap-Effective-Rugged-Safe; IS = internal standard; PSA = primary-secondary amine; GCB = graphitized carbon black; QC = quality control; GC-MS/MS = gas chromatography-tandem mass spectrometry. Please click here to view a larger version of this figure.
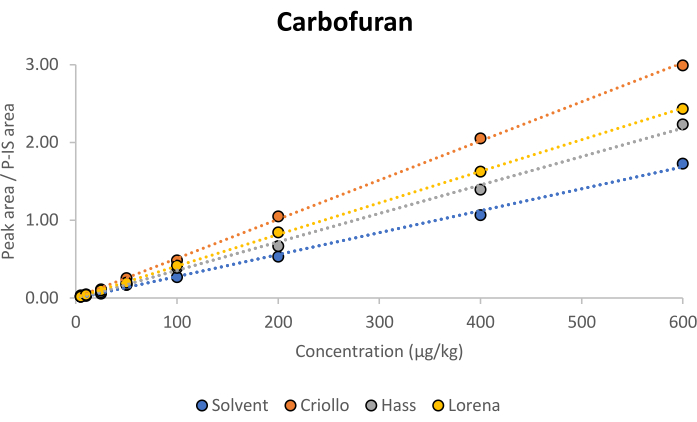
Figure 2: Comparison of the calibration curves in the solvent and matrices for carbofuran. Solvent: y = 0.0028x – 0.0054 and R2 = 0.9974; Criollo: y = 0.0050x + 0.0050, R2 = 0.9994, and ME = 80%; Hass: y = 0.0037x – 0.0109, R2 = 0.9977, and ME = 30%; Lorena: y = 0.0041x + 0.0053, R2 = 0.9998, and ME = 42%. Abbreviations: ME = matrix effect; P-IS = procedural internal standard. Please click here to view a larger version of this figure.
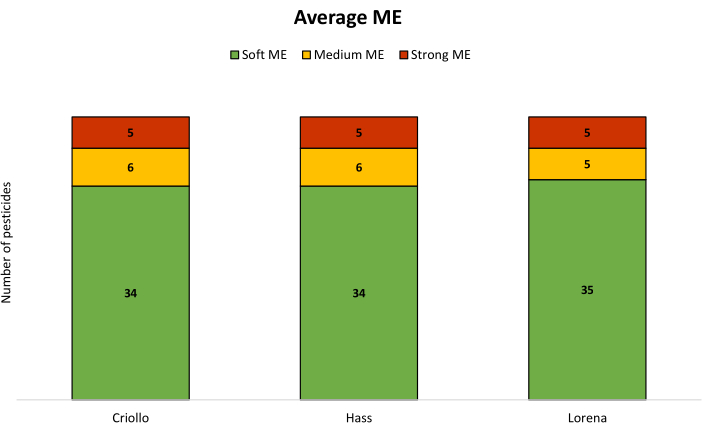
Figure 3: Number of selected pesticides categorized by their respective ranges of ME for avocado varieties. The classification of ME is based on three categories: soft (values between −20% and 20%), medium (values ranging between −20% and −50% or between 20% and 50%), and strong (values exceeding 50% or falling below −50%). Abbreviation: ME = matrix effect. Please click here to view a larger version of this figure.
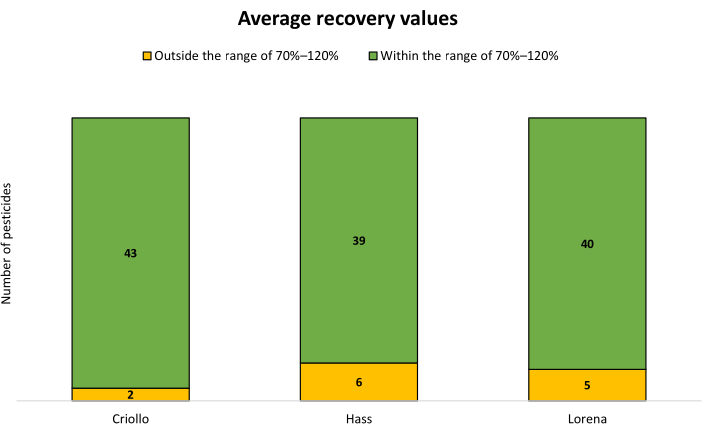
Figure 4: Number of pesticides that fall outside and within the acceptable recovery range spiked at 10, 100, and 400 µg/kg (n = 15) in the three avocado varieties. Please click here to view a larger version of this figure.
Table 1: Retention times, quantifier, and qualifier transitions utilized in GC-MS/MS analyses of the selected pesticides, along with the P-IS and I-IS. Abbreviations: P-IS = procedural internal standard; I-IS = injection internal standard; GC-MS/MS = gas chromatography-tandem mass spectrometry; HCB = hexachlorobenzene; α-HCH = alpha-hexachlorocyclohexane; β-HCH = beta-hexachlorocyclohexane; 4,4´-DDD = 4,4´-dichlorodiphenyldichloroethane; 4,4´-DDE = 4,4´-dichlorodiphenyldichloroethylene; 4,4´-DDT = 4,4´-dichlorodiphenyltrichloroethane; TPP = triphenyl phosphate; EPN = ethyl nitrophenyl phenylphosphonothioate. Please click here to download this Table.
Table 2: Matrix effect values (%) for the selected pesticides in different avocado varieties during the validation of the final analytical method. Abbreviations: HCB = hexachlorobenzene; α-HCH = alpha-hexachlorocyclohexane; β-HCH = beta-hexachlorocyclohexane; 4,4´-DDD = 4,4´-dichlorodiphenyldichloroethane; 4,4´-DDE = 4,4´-dichlorodiphenyldichloroethylene; 4,4´-DDT = 4,4´-dichlorodiphenyltrichloroethane; TPP = triphenyl phosphate; EPN = ethyl nitrophenyl phenylphosphonothioate. Please click here to download this Table.
Table 3: Recovery values and their corresponding RSDs in parentheses (n = 5 at each spiking level), both in %, for the selected pesticides in different avocado varieties during the validation of the final analytical method. Abbreviations: RSDs = relative standard deviations; HCB = hexachlorobenzene; α-HCH = alpha-hexachlorocyclohexane; β-HCH = beta-hexachlorocyclohexane; 4,4´-DDD = 4,4´-dichlorodiphenyldichloroethane; 4,4´-DDE = 4,4´-dichlorodiphenyldichloroethylene; 4,4´-DDT = 4,4´-dichlorodiphenyltrichloroethane; TPP = triphenyl phosphate; EPN = ethyl nitrophenyl phenylphosphonothioate. Please click here to download this Table.
Supplementary File 1: Mass spectrometric spectra of all pesticides. Abbreviations: HCB = hexachlorobenzene; α-HCH = alpha-hexachlorocyclohexane; β-HCH = beta-hexachlorocyclohexane; 4,4´-DDD = 4,4´-dichlorodiphenyldichloroethane; 4,4´-DDE = 4,4´-dichlorodiphenyldichloroethylene; 4,4´-DDT = 4,4´-dichlorodiphenyltrichloroethane; EPN = ethyl nitrophenyl phenylphosphonothioate. Please click here to download this File.
Discussion
The primary limitation associated with matrix-matched calibration arises from the use of blank samples as calibration standards. This leads to an augmented number of samples to be processed for analysis and an increased injection of matrix components in each analytical sequence, potentially leading to higher instrument maintenance demands. Nonetheless, this strategy is more suitable than standard addition, which would generate a much larger number of samples to be injected due to the need to perform a calibration curve for each sample. Consequently, in both cases, the use of sample preparation techniques that minimize such co-extraction while remaining cost-effective, fast, and reliable is required. In this context, the QuEChERS method has demonstrated its usefulness in analyzing pesticide residues in avocado samples8,12,13,14,15,16,17,18. However, none of those approaches have explored the application of the QuEChERS method employing ammonium formate. This choice aims to mitigate the drawbacks of using magnesium and sodium salts in MS analysis23,24,25,26,27. Both magnesium and sodium salts have low vapor pressures that have the propensity to form solid deposits on surfaces within the MS source, which may potentially affect instrument performance. While this phenomenon occurs in liquid chromatography (LC) systems, it also poses challenges in the context of GC, where these can accumulate in the inlet liner, necessitating more frequent replacements of the liner27. To overcome these limitations and enhance compatibility with MS detection, the substitution of these salts with highly volatile alternatives has been implemented. Ammonium salts are preferred as they can be easily evaporated and/or decomposed, thereby overcoming the disadvantages. The current investigation represents the first instance of utilizing the QuEChERS method employing ammonium formate for the analysis of pesticide residues in avocados. In particular, the extraction process comprised subjecting the avocado sample to an extraction step using acetonitrile, with the addition of 0.5 g of ammonium formate per gram of sample to facilitate salting out (Figure 1).
As the second step of the QuEChERS method, the dSPE step is crucial because it serves to remove undesired matrix components that could potentially lead to analytical interferences26. However, achieving an effective d-SPE step often requires a combination of various sorbents to address the diverse co-extractives originating from the sample matrix. When dealing with avocados, this step may include anhydrous MgSO4 to remove excess water and improve pesticide partitioning, PSA to eliminate fatty acids, organic acids, and sugars, C18 to enhance the removal of nonpolar components, GCB for chlorophyll removal, and zirconia materials such as Z-Sep+ to eliminate high amounts of fat15,26,28. Consequently, the avocado extracts were transferred to centrifuge tubes containing specific amounts of each sorbent: 150 mg of anhydrous MgSO4, 50 mg of PSA, 50 mg of C18, 10 mg of GCB, and 60 mg of Z-Sep+ (Figure 1).
To initiate the validation process involving the extraction and cleaning steps, the calibration curves were rigorously examined. This involved assessing matrix-matched calibration curves for each analyte/avocado variety combination, in addition to acetonitrile-only calibrations (Figure 2). In both scenarios, a previously proposed analyte protectants mixture29, consisting of 3-ethoxy-1,2-propanediol, L-gulonic acid γ-lactone, D-sorbitol, and shikimic acid, was employed. The evaluation encompassed linearity across a 5 to 600 µg/kg concentration range. The LCL of 5 µg/kg falls below the stringent MRL of 10 µg/kg as set by international regulations governing the analysis of pesticide residues in food commodities22. Furthermore, the LCL of 5 µg/kg yielded a signal-to-noise ratio higher than 10 for all the selected multiclass pesticides. Visual inspection of calibration plots was also performed to verify the precision of slope values employed for calculating ME. Results indicated that most of the selected pesticides exhibited R2 values higher than or equal to 0.99 across all four calibration curves for each of them. The overall assessment of calibration outcomes demonstrated the accuracy and suitability of these equations for precise ME calculations in each avocado variety.
The ME was determined to be soft (ME ≤ 20%) for most of the pesticides in each of the three avocado varieties under investigation (Table 2 and Figure 3). In this context, three key points are worth highlighting. Firstly, the final sample extracts were relatively clean due to the effectiveness of the implemented sample preparation protocol, thereby resulting in minimal interferences. Secondly, in GC systems, MEs are subject to the influences stemming from interactions transpiring within the matrix and the interactions occurring at active sites within the system29. The mixture of analyte protectants used comprehensively covered nearly the entire spectrum of pesticides. However, pesticides eluting early (propoxur, dichlorvos, carbofuran, and diphenylamine), as well as those eluting later (pyriproxyfen, fenvalerate, esfenvalerate, and deltamethrin), exhibited the highest and less consistent ME values. Thirdly, considering these differences, it was decided to utilize the matrix-matched calibration of each variety separately for conducting the recovery study. It is important to note that one variety can reasonably represent the other varieties for the remaining pesticides.
The recovery and reproducibility assessment were performed at three different concentration levels (10, 100, and 400 µg/kg) in quintuplicate (n = 15). To achieve this, avocado samples were spiked at the beginning of the application of the QuEChERS method. Recoveries were calculated by comparing the ratios of pesticide peak area to the peak of the P-IS (atrazine-d5) obtained from matrix-matched calibration. Each replicate was injected in triplicate within the same sequence to ensure consistency. The use of an isotopically labeled IS enables compensation for potential pesticide losses during the protocol, while also accounting for methodological errors and instrumental variability. The results showed that most pesticides met the acceptable criteria, with recoveries ranging from 70 to 120% and RSD below 20% at each spiking level6 (Figure 4), indicating the method's effectiveness and repeatability. However, certain pesticides exhibited recoveries beyond this acceptable range (Table 3). This is the hexachlorobenzene (HCB) case, showing recoveries in the range of 28-55% for all concentration levels and matrices. This can be attributed to HCB's planar molecular structure, which leads to a strong affinity with GCB, causing its retention and reducing extraction efficiency30. Despite the lower recoveries for HCB and a few other cases, the method still demonstrated consistent and reliable recovery for these pesticides, with RSD values remaining below the recommended limit.
In conclusion, the analysis of pesticide residues in food samples encounters ME, which can impact the accuracy of GC-MS/MS. Matrix-matched calibration proves to be a straightforward and effective strategy for mitigating these effects, even in matrices such as avocados, which are rich in fatty acids and other co-extractive materials such as pigments. Through the application of the QuEChERS method employing ammonium formate together with matrix-matched calibration and analyte protectants, highly accurate quantification is achieved. Consequently, this approach ensures reliable and enforceable pesticide residue analysis in avocado samples, making it suitable for regulatory applications.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We would like to thank EAN University and the University of La Laguna.
Materials
| 3-Ethoxy-1,2-propanediol | Sigma Aldrich | 260428-1G | |
| Acetonitrile | Merk | 1006652500 | |
| Ammonium formate | Sigma Aldrich | 156264-1KG | |
| AOAC 20i/s autosampler | Shimadzu | 221-723115-58 | |
| Automatic shaker MX-T6-PRO | SCILOGEX | 8.23222E+11 | |
| Balance | OHAUS | PA224 | |
| Centrifuge tubes, 15 mL | Nest | 601002 | |
| Centrifuge tubes, 2 mL | Eppendorf | 4610-1815 | |
| Centrifuge tubes, 50 mL | Nest | 602002 | |
| Centrifuge Z206A | MERMLE | 6019500118 | |
| Choper 2L | Oster | 2114111 | |
| Column SH-Rxi-5sil MS, 30 m x 0.25 mm, 0.25 µm | Shimadzu | 221-75954-30 | MS GC column |
| Dispensette 5-50 mL | BRAND | 4600361 | |
| DSC-18 | Sigma Aldrich | 52600-U | |
| D-Sorbitol | Sigma Aldrich | 240850-5G | |
| Ethyl acetate | Merk | 1313181212 | |
| GCMS-TQ8040 | Shimadzu | 211552 | |
| Graphitized carbon black | Sigma Aldrich | 57210-U | |
| Injection syringe | Shimadzu | LC2213461800 | |
| L-Gulonic acid γ-lactone | Sigma Aldrich | 310301-5G | |
| Linner splitless | Shimadzu | 221-4887-02 | |
| Magnesium sulfate anhydrus | Sigma Aldrich | M7506-2KG | |
| Methanol | Panreac | 131091.12.12 | |
| Milli-Q ultrapure (type 1) water | Millipore | F4H4783518 | |
| Pipette tips 10 – 100 µL | Biologix | 200010 | |
| Pipette tips 100 – 1000 µL | Brand | 541287 | |
| Pipette tips 20 – 200 µL | Brand | 732028 | |
| Pipettes Pasteur | NORMAX | 5426023 | |
| Pippette Transferpette S variabel 10 – 100 µL | BRAND | 704774 | |
| Pippette Transferpette S variabel 100 – 1000 µL | BRAND | 704780 | |
| Pippette Transferpette S variabel 20 – 200 µL | SCILOGEX | 7.12111E+11 | |
| Primary-secondary amine | Sigma Aldrich | 52738-U | |
| Shikimic acid | Sigma Aldrich | S5375-1G | |
| Syringe Filter PTFE/L 25 mm, 0.45 µm | NORMAX | FE2545I | |
| Triphenyl phosphate (QC) | Sigma Aldrich | 241288-50G | |
| Vials with fused-in insert | Sigma Aldrich | 29398-U | |
| Z-SEP+ | Sigma Aldrich | 55299-U | zirconium oxide-based sorbent |
| Pesticides | CAS registry number | ||
| 4,4´-DDD | Sigma Aldrich | 35486-250MG | 72-54-8 |
| 4,4´-DDE | Sigma Aldrich | 35487-100MG | 72-55-9 |
| 4,4´-DDT | Sigma Aldrich | 31041-100MG | 50-29-3 |
| Alachlor | Sigma Aldrich | 45316-250MG | 15972-60-8 |
| Aldrin | Sigma Aldrich | 36666-25MG | 309-00-2 |
| Atrazine | Sigma Aldrich | 45330-250MG-R | 1912-24-9 |
| Atrazine-d5 (IS) | Sigma Aldrich | 34053-10MG-R | 163165-75-1 |
| Buprofezin | Sigma Aldrich | 37886-100MG | 69327-76-0 |
| Carbofuran | Sigma Aldrich | 32056-250-MG | 1563-66-2 |
| Chlorpropham | Sigma Aldrich | 45393-250MG | 101-21-3 |
| Chlorpyrifos | Sigma Aldrich | 45395-100MG | 2921-88-2 |
| Chlorpyrifos-methyl | Sigma Aldrich | 45396-250MG | 5598-13-0 |
| Deltamethrin | Sigma Aldrich | 45423-250MG | 52918-63-5 |
| Dichloran | Sigma Aldrich | 45435-250MG | 99-30-9 |
| Dichlorvos | Sigma Aldrich | 45441-250MG | 62-73-7 |
| Dieldrin | Sigma Aldrich | 33491-100MG-R | 60-57-1 |
| Diphenylamine | Sigma Aldrich | 45456-250MG | 122-39–4 |
| Endosulfan A | Sigma Aldrich | 32015-250MG | 115-29-7 |
| Endrin | Sigma Aldrich | 32014-250MG | 72-20-8 |
| EPN | Sigma Aldrich | 36503-100MG | 2104-64-5 |
| Esfenvalerate | Sigma Aldrich | 46277-100MG | 66230-04-4 |
| Ethion | Sigma Aldrich | 45477-250MG | 563-12-2 |
| Fenamiphos | Sigma Aldrich | 45483-250MG | 22224-92-6 |
| Fenitrothion | Sigma Aldrich | 45487-250MG | 122-14-5 |
| Fenthion | Sigma Aldrich | 36552-250MG | 55-38-9 |
| Fenvalerate | Sigma Aldrich | 45495-250MG | 51630-58-1 |
| HCB | Sigma Aldrich | 45522-250MG | 118-74-1 |
| Iprodione | Sigma Aldrich | 36132-100MG | 36734-19-7 |
| Lindane | Sigma Aldrich | 45548-250MG | 58-89-9 |
| Malathion | Sigma Aldrich | 36143-100MG | 121-75-5 |
| Metalaxyl | Sigma Aldrich | 32012-100MG | 57837-19-1 |
| Methidathion | Sigma Aldrich | 36158-100MG | 950-37-8 |
| Myclobutanil | Sigma Aldrich | 34360-100MG | 88671-89-0 |
| Oxyfluorfen | Sigma Aldrich | 35031-100MG | 42874-03-3 |
| Parathion-methyl | Sigma Aldrich | 36187-100MG | 298-00-0 |
| Penconazol | Sigma Aldrich | 36189-100MG | 66246-88-6 |
| Pirimiphos-methyl | Sigma Aldrich | 32058-250MG | 29232-93-7 |
| Propiconazole | Sigma Aldrich | 45642-250MG | 60207-90-1 |
| Propoxur | Sigma Aldrich | 45644-250MG | 114-26-1 |
| Propyzamide | Sigma Aldrich | 45645-250MG | 23850-58-5 |
| Pyriproxifen | Sigma Aldrich | 34174-100MG | 95737-68-1 |
| Tolclofos-methyl | Sigma Aldrich | 31209-250MG | 5701804-9 |
| Triadimefon | Sigma Aldrich | 45693-250MG | 43121-43-3 |
| Triflumizole | Sigma Aldrich | 32611-100MG | 68694-11-1 |
| α-HCH | Sigma Aldrich | 33377-50MG | 319-86-8 |
| β-HCH | Sigma Aldrich | 33376-100MG | 319-85-7 |
References
- Raposo, F., Barceló, D. Challenges and strategies of matrix effects using chromatography-mass spectrometry: An overview from research versus regulatory viewpoints. Trends Analyt Chem. 134, 116068 (2021).
- Rahman, M. M., Abd El-Aty, A. M., Shim, J. H. Matrix enhancement effect: a blessing or a curse for gas chromatography?-A review. Anal Chim Acta. 801, 14-21 (2013).
- Poole, C. F. Matrix-induced response enhancement in pesticide residue analysis by gas chromatography. J Chromatogr A. 1158 (1-2), 241-250 (2007).
- Anastassiades, M., Maštovská, K., Lehotay, S. J. Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides. J Chromatogr A. 1015 (1-2), 163-184 (2003).
- Trufelli, H., Palma, P., Famiglini, G., Cappiello, A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom Reviews. 30 (3), 491-509 (2011).
- European Commission SANTE/11312/2021. Guidance document on analytical quality control and method validation procedures for pesticide residues analysis in food and feed. European Commission. , (2021).
- Kwon, H., Lehotay, S. J., Geis-Asteggiante, L. Variability of matrix effects in liquid and gas chromatography-mass spectrometry analysis of pesticide residues after QuEChERS sample preparation of different food crops. J Chromatogr A. 1270, 235-245 (2012).
- Lehotay, S. J., Maštovská, K., Yun, S. J. Evaluation of two fast and easy methods for pesticide residue analysis in fatty food matrixes. J AOAC Int. 88 (2), 630-638 (2005).
- González-Curbelo, M. &. #. 1. 9. 3. ;., González-Sálamo, J., Varela-Martínez, D. A., Hernández-Borges, J. Analysis of pesticide residues in pollen and dairy products. Sustainable Agriculture Reviews 47. 47, 47-89 (2020).
- Madej, K., Kalenik, T. K., Piekoszewski, W. Sample preparation and determination of pesticides in fat-containing foods. Food Chem. 269, 527-541 (2018).
- Yanty, N. A. M., Marikkar, J. M. N., Long, K. Effect of varietal differences on composition and thermal characteristics of avocado oil. J Am Oil Chem Soc. 88, 1997-2003 (2011).
- Pano-Farias, N. S., Ceballos-Magaña, S. G., Muniz-Valencia, R., Gonzalez, J. Validation and assessment of matrix effect and uncertainty of a gas chromatography coupled to mass spectrometry method for pesticides in papaya and avocado samples. J Food Drug Anal. 25 (3), 501-509 (2017).
- Pano-Farias, N. S., Ceballos-Magaña, S. G., Jurado, J. M., Aguayo-Villarreal, I. A., Muñiz-Valencia, R. Analytical method for pesticides in avocado and papaya by means of ultra-high performance liquid chromatography coupled to a triple quadrupole mass detector: Validation and uncertainty assessment. J Food Sci. 83 (8), 2265-2272 (2018).
- Pano-Farias, N. S., Ceballos-Magaña, S. G., Gonzalez, J., Jurado, J. M., Muñiz-Valencia, R. Supercritical fluid chromatography with photodiode array detection for pesticide analysis in papaya and avocado samples. J Sep Sci. 38 (7), 1240-1247 (2015).
- Lozano, A., Rajski, &. #. 3. 2. 1. ;., Uclés, S., Belmonte-Valles, N., Mezcua, M., Fernández-Alba, A. R. Evaluation of zirconium dioxide-based sorbents to decrease the matrix effect in avocado and almond multiresidue pesticide analysis followed by gas chromatography tandem mass spectrometry. Talanta. 118, 68-83 (2014).
- Han, L., Matarrita, J., Sapozhnikova, Y., Lehotay, S. J. Evaluation of a recent product to remove lipids and other matrix co-extractives in the analysis of pesticide residues and environmental contaminants in foods. J Chromatogr A. 1449, 17-29 (2016).
- Chamkasem, N., Ollis, L. W., Harmon, T., Lee, S., Mercer, G. Analysis of 136 pesticides in avocado using a modified QuEChERS method with LC-MS/MS and GC-MS/MS. J Agric Food Chem. 61 (10), 2315-2329 (2013).
- Rajski, &. #. 3. 2. 1. ;., Lozano, A., Uclés, A., Ferrer, C., Fernández-Alba, A. R. Determination of pesticide residues in high oil vegetal commodities by using various multi-residue methods and clean-ups followed by liquid chromatography tandem mass spectrometry. J Chromatogr A. 1304, 109-120 (2013).
- Hernández-Borges, J., Ravelo-Pérez, L. M., Hernández-Suárez, E. M., Carnero, A., Rodríguez-Delgado, M. &. #. 1. 9. 3. ;. Analysis of abamectin residues in avocados by high-performance liquid chromatography with fluorescence detection. J Chromatogr A. 1165 (1-2), 52-57 (2007).
- Moreno, J. F., Liébanas, F. A., Frenich, A. G., Vidal, J. M. Evaluation of different sample treatments for determining pesticide residues in fat vegetable matrices like avocado by low-pressure gas chromatography-tandem mass spectrometry. J Chromatogr A. 1111 (1), 97-105 (2006).
- . Commission Directive 2002/63/EC of 11 July 2002 establishing Community methods of sampling for the official control of pesticide residues in and on products of plant and animal origin and repealing Directive 79/700/EEC. Official Journal of the European Union. L187, 30-43 (2002).
- . European Regulation, 396/2005, Regulation (EC) NO 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Official Journal of the European Union. L70, 1-16 (2005).
- González-Curbelo, M. &. #. 1. 9. 3. ;., Lehotay, S. J., Hernández-Borges, J. Validation of a modified QuEChERS version for high-throughput analysis of a wide range of pesticides in foods. Abstracts of Papers of the American Chemical Society. 246, (2013).
- González-Curbelo, M. &. #. 1. 9. 3. ;., Lehotay, S. J., Hernández-Borges, J., Rodríguez Delgado, J. Ammonium formate buffer in QuEChERS for high throughput analysis of pesticides in food by fast, low-pressure GC-MS/MS and LC-MS/MS. Abstracts of Papers of the American Chemical Society. 248, (2014).
- González-Curbelo, M. &. #. 1. 9. 3. ;., Lehotay, S. J., Hernández-Borges, J., Rodríguez-Delgado, M. &. #. 1. 9. 3. ;. Use of ammonium formate in QuEChERS for high-throughput analysis of pesticides in food by fast, low-pressure gas chromatography and liquid chromatography tandem mass spectrometry. J Chromatogr A. 1358, 75-84 (2014).
- Varela-Martínez, D. A., González-Sálamo, J., González-Curbelo, M. &. #. 1. 9. 3. ;., Quick Hernández-Borges, J. Quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction. Handbooks in Separation Science. , 399-437 (2020).
- González-Curbelo, M. &. #. 1. 9. 3. ;. Analysis of organochlorine pesticides in a soil sample by a modified QuEChERS approach using ammonium formate. J Vis Exp. 191, e64901 (2023).
- González-Curbelo, M. &. #. 1. 9. 3. ;., Dionis-Delgado, S., Asensio-Ramos, M., Hernández-Borges, J. Pesticide analysis in toasted barley and chickpea flours. J Sep Sci. 35 (2), 299-307 (2012).
- Varela-Martínez, D. A., González-Curbelo, M. &. #. 1. 9. 3. ;., González-Sálamo, J., Hernández-Borges, J. High-throughput analysis of pesticides in minor tropical fruits from Colombia. Food Chem. 280, 221-230 (2019).
- Li, L., Li, W., Qin, D. M., Jiang, S. R., Liu, F. M. Application of graphitized carbon black to the QuEChERS method for pesticide multiresidue analysis in spinach. J AOAC Int. 92 (2), 538-547 (2009).

