- 00:04Vue d'ensemble
- 01:07Principles of Mössbauer Spectroscopy
- 03:43Mössbauer Spectroscopy of Ferrocene
- 06:04Representative Results: Zero-Field Mössbauer Spectrum of Ferrocene
- 06:58Applications
- 08:52Summary
뫼스바우어 분광법
English
Diviser
Vue d'ensemble
출처: 조슈아 워포드, 타마라 M. 파워스, 텍사스 A&M 대학교 화학학과
뫼스바우어 분광법은 고체 상태에서 감마선에 의한 원자의 핵 여기를 검사하는 대량 특성화 기술이다. 결과 Mössbauer 스펙트럼은 분자의 전자 구조 및 리간드 배열 (geometry)에 대한 증거를 제공하는 대상 원자 주위의 산화 상태, 스핀 상태 및 전자 환경에 대한 정보를 제공합니다. 이 비디오에서는 뫼스바우어 분광법의 기본 원리에 대해 배우고 페로센의 57페 뫼스바우어 스펙트럼을 제로 필드로 수집합니다.
Principles
Procédure
Résultats
Zero field 57Fe Mössbauer of ferrocene at 5 K.
δ = 0.54 mm/s
ΔEQ = 2.4 mm/s
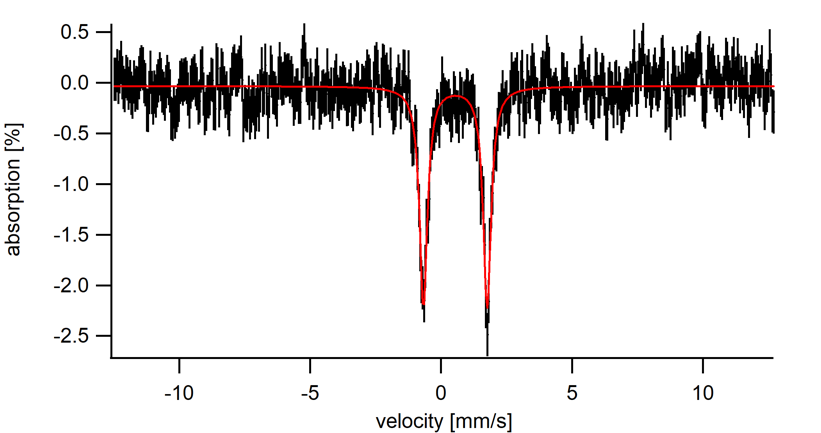
Referring to Table 1, we see that the isomer shift at 0.54 mm/s falls into several possible oxidation state/spin state ranges (Table 1). In cases such as this, it is not possible to determine the oxidation state and spin state based on δ-values alone. Chemists must use other characterization methods to gather evidence to support oxidation state and spin state assignments. Based on the proton NMR of ferrocene, we know that ferrocene is diamagnetic, and therefore must have a spin state of S = 0. The structure of ferrocene allows us to determine that the Fe center is in the 2+ oxidation state. The isomer shift value of 0.54 mm/s is close to the typical range of Fe(II), S = 0 compounds and therefore the Mössbauer spectrum is consistent with other characterization data.
Applications and Summary
Here, we learned about the basic principles of Mössbauer spectroscopy, including details on the experimental setup, the gamma ray source, and the information that can be gathered from a Mössbauer spectrum. We collected the zero field 57Fe Mössbauer spectrum of ferrocene.
Mössbauer spectroscopy is a powerful technique that provides information about the electronic field gradient around an atom. While there are numerous Mössbauer active atoms, only elements with a suitable gamma ray source (long-lived and low-lying excited nuclear energy state) can take advantage of this technique. The most commonly studied atom is 57Fe, which is used to characterize inorganic/organometallic molecular species, bioinorganic molecules, and minerals. For example, Mössbauer spectroscopy has been used extensively to study iron-sulfur (Fe/S) clusters found in metalloproteins.2 Fe/S clusters are involved in a variety of functions, ranging from electron transport to catalysis. 57Fe Mössbauer spectroscopy has helped elucidate valuable information about Fe/S clusters in proteins, including, but not limited to, the number of unique iron centers present in a Fe/S cluster as well as the oxidation state and spin state of those ions.
References
- Fultz, B. “Mössbauer Spectrometry”, in Characterization of Materials. John Wiley. New York. (2011).
- Pandelia, M.-E., Lanz, N., Booker, S., Krebs, C. Mössbauer spectroscopy of Fe/S proteins Biochim Biophys Acta. 1853, 1395–1405 (2015).
Transcription
Mössbauer spectroscopy is a method for evaluating the oxidation state, electronic spin state, and electronic environment of an atom.
The nuclear spin angular momentum of an atom, or nuclear spin for short, describes the discrete energetic states available to a nucleus. The energy levels are affected by the oxidation state, electronic spin state, and ligand environment.
Differences in nuclear energy levels are reflected in the nuclear excitation energy. Mössbauer spectroscopy takes advantage of this relationship by irradiating a solid sample with gamma rays over a narrow range of energies and comparing the energies absorbed by the sample to known values.
This video will discuss the underlying principles of Mössbauer spectroscopy, illustrate the procedure for determining the spin state and oxidation state of ferrocene, and introduce a few applications in chemistry.
When a nucleus absorbs or emits a gamma ray, some energy is lost to recoil. Thus, the gamma ray emitted by a relaxing nucleus cannot excite an identical nucleus.
However, a percentage of emission and absorption events in crystal structures have negligible recoil, allowing resonance to occur between identical nuclei in solids. This is called the Mössbauer effect.
A standard Mössbauer spectrometer consists of a moving gamma ray source and a sensitive radiation detector. Iron Mössbauer spectroscopy is performed with a 57Co source, which decays by electron capture to excited 57Fe.
The different chemical environments of the source and sample nuclei result in slightly different energy gaps between the ground and excited states. The source is therefore moved back and forth at various speeds to induce a Doppler shift in the gamma rays.
The radiation detector measures the gamma rays transmitted through the sample. When the received gamma rays are the precise energy needed to excite the sample, resonant absorption can occur between the source and the sample.
A Mössbauer spectrum typically plots % transmission vs. energy in terms of source velocity.
The isomer shift is the shift in resonance energy relative to the source, and is related to the oxidation state of the atom.
Nuclear energy levels split when the surrounding electric field gradient is non-spherical, resulting in two distinct absorption energies. This interaction, called quadrupole splitting, occurs in asymmetric ligand environments, and at nuclear spins greater than ½.
Quadrupole splitting results in a quadrupole doublet in the Mössbauer spectrum. In these cases, the isomer shift is halfway between the two peaks and the quadrupole splitting value is the difference between the peaks.
Hyperfine splitting occurs in an internal or external magnetic field. Each nuclear energy level splits into sub-states based on its nuclear spin state. 57Fe has six allowed transitions between those states, resulting in six peaks.
Now that you understand the principles of Mössbauer spectroscopy, let’s go through a procedure for determining the oxidation state and electronic spin state of ferrocene with Mössbauer spectroscopy.
To begin the procedure, measure 100 mg of ferrocene into a polyoxymethylene Mössbauer sample cup.
Add to the sample several drops of a cryoprotectant oil composed of a blend of polyisobutylenes. Use a spatula to mix the sample and oil into a uniform paste. Using tweezers, place the filled Mössbauer cup into a 20 mL scintillation vial and cap it for transportation to the Mössbauer instrument room.
Once in the instrumentation room, freeze the sample in liquid N2.
Next, remove the temperature probe from the sample rod. Unscrew the sample rod and fill the Mössbauer chamber with He gas. Then, with the He gas flowing, withdraw the sample rod.
Close the sample chamber with a cap, and close the He valve.
Transfer the Mössbauer sample into a secondary container filled with liquid N2. Then, carefully load the Mössbauer sample cup into the rod-mounted sample holder, and tighten the set screw to secure the cup in the holder.
Brush away any ice on the sample holder and the rod. Then, immerse the sample holder in liquid N2, and open the He valve.
Insert the sample rod into the chamber and fix the rod in place with screws.
Then, stop the He flow and evacuate the sample chamber. Once the sample chamber is at the minimum pressure, stop the vacuum pump and allow a small amount of He gas into the sample chamber. Finally, re-connect the temperature probe to the sample rod.
Open the gamma ray spectrometer interface to see a plot of the detector readings. Select the 14.4-keV peak and the 2-keV escape peak and hit the “Send to Windows” button.
Open the data collection software and set the source velocity range to 0 to 12 mm/s. Acquire data until the spectrum has achieved the desired resolution. Save the acquired data. Use appropriate software to fit the data and apply it to determine the isomer shift and the quadrupole splitting.
The Mössbauer spectrum of ferrocene has a single quadrupole doublet with an isomer shift of 0.54 mm/s. When compared to typical ranges of isomer shifts for iron containing compounds, the isomer shift suggests either an Fe(II), S = 0 complex or an Fe(III), S = 5/2 complex.
From the proton NMR of ferrocene, it is known that the compound is a diamagnetic, neutral complex. Furthermore, its two cyclopentadienyl ligands each bear a charge of 1-, indicating that the iron center in ferrocene is in the 2+ oxidation state. Finally, based on the Mössbauer result, it is evident that ferrocene has a spin state of 0.
Mössbauer spectroscopy is widely used in inorganic chemistry. Let’s look at a few examples.
Iron-sulfur proteins contain Fe/S clusters of two or more iron atoms bridged by S atoms. In a ferredoxin iron-sulfur protein, the diiron 2+ cluster contains two high-spin Fe(III) centers. Exchange coupling between these Fe centers results in an overall diamagnetic state with a spin of 0. The individual Mössbauer spectra of each Fe center are indistinguishable from each other, so the spectrum of the ferredoxin shows only one quadrupole doublet.
Ferredoxins participate in electron transport by redox reactions at their Fe atoms. For example, a ferredoxin can accept an electron by a single-electron reduction at one of the Fe centers, resulting in a cluster with one high-spin Fe(III) center and one high-spin Fe(II) center. This appears as two superposed quadrupole doublets in the Mössbauer spectrum.
Lipoyl synthase, which contains two 4-Fe/4-S clusters, performs the final step of lipoyl cofactor synthesis. The proposed mechanism involves an intermediate with the substrate cross-linked to a degraded Fe/S cluster.
To investigate the properties of the reaction intermediate, Mössbauer spectra were acquired in the presence and absence of a weak magnetic field. The resulting difference spectrum showed only the effects of an external magnetic field on the chemical shifts. The difference spectrum was combined with a simulated spectrum, revealing a 2:1 ratio from a mixed-valent Fe pair and an Fe(III) site.
You’ve just watched JoVE’s introduction to Mössbauer spectroscopy. You should now be familiar with the underlying principles of the Mössbauer effect, the procedure for performing 57Fe Mössbauer spectroscopy, and a few examples of how Mössbauer spectroscopy is used in inorganic chemistry. Thanks for watching!
