Forward Genetic Approaches in Chlamydia trachomatis
Summary
We describe a methodology to perform genetic analysis in Chlamydia based on chemical mutagenesis and whole genome sequencing. In addition, a system for DNA exchange within infected cells is described that can be used for genetic mapping. This method may be broadly applicable to microbial systems lacking transformation systems and molecular genetic tools.
Abstract
Chlamydia trachomatis, the etiological agent of sexually transmitted diseases and ocular infections, remains poorly characterized due to its intractability to experimental transformation with recombinant DNA. We developed an approach to perform genetic analysis in C. trachomatis despite the lack of molecular genetic tools. Our method involves: i.) chemical mutagenesis to rapidly generate comprehensive libraries of genetically-defined mutants with distinct phenotypes; ii.) whole-genome sequencing (WGS) to map the underlying genetic lesions and to find associations between mutated gene(s) and a common phenotype; iii.) generation of recombinant strains through co-infection of mammalian cells with mutant and wild type bacteria. Accordingly, we were able to establish causal relationships between genotypes and phenotypes. The coupling of chemically-induced gene variation and WGS to establish correlative genotype–phenotype associations should be broadly applicable to the large list of medically and environmentally important microorganisms currently intractable to genetic analysis.
Introduction
The obligate intracellular bacterium Chlamydia trachomatis accounts for an estimated 2.8 million genital tract infections per year in the United States (Center for Disease Control) with associated sequelæ such as pelvic inflammatory disease, ectopic pregnancies, and infertility (1). Chlamydia spp have a unique physiology with a biphasic developmental cycle consisting of two forms: the infectious but non-replicating elementary body (EB) and the noninfectious but replicative reticulate body (RB). Infection begins with the attachment of EBs to epithelial cells followed by endoctyotosis (2). Within a membrane-bound vacuole termed an inclusion, EBs differentiate into the RB form, which then replicates by binary fission. At mid-cycle, RBs transitions back into EBs, which are then expelled into the extracellular space to initiate new rounds of infection when the host cell lyses (3).
C. trachomatis is refractory to routine manipulation with standard molecular genetic tools, such as targeted gene replacement, transposons, and transducing phages, which have been central to most studies in bacterial genetics, It is unclear the extent to which individual Chlamydia genes contribute to the evasion of innate immunity, nutrient acquisition, developmental transitions, or other processes important for the pathogen’s survival within a eukaryotic host (4). Consequently, this pathogen remains poorly characterized despite its clinical importance.
The genomes of Chlamydia spp. are relatively small (~1 Mb) (5) with multiple species and biovars sequenced using Next Generation sequencing technologies. Comparative genome analysis by WGS has provided unique insights into the evolution of chlamydial species and their adaptation to humans (6-8) and to some extent has provided some information as to the potential function of virulence factors (9, 10). The genetic diversity displayed by clinical isolates does not provide the resolution required to systematically map the function of most virulence factors, presumably because mutations in such genes would have been readily selected against. Without confounding effects from natural selection, mutagen-induced gene variation, coupled with defined assays that measure defects in virulence, can expand the spectrum of mutations that can be surveyed. Chemical mutagens, in particular, are useful as they can generate null, conditional, hypomorphic (reduced function), and hypermorphic (gain of function) alleles. With the arrival of robust next-generation genome-sequencing technologies, such mutations can be readily identified and mapped. In this manner, strong associations can be made between mutations in a gene or genetic pathway and a common phenotype, enabling the application of forward genetic approaches.
The genome sequences of clinical strains revealed mosaicism between serovars and loci of frequent recombination (11). Empirical evidence of recombination was demonstrated through the co-infection of two different antibiotic resistant strains and selection of dual resistant recombinant progeny, which was revealed to have genetic contributions from both strains (12, 13). Thus, genetic exchange between wild type and mutant strains in a co-infection setting allows segregation of chemically-induced mutations to pinpoint the affected gene that leads to the observed phenotype.
Here we describe a methodology to perform genetic analysis in Chlamydia based on chemical mutagenesis, WGS, and a system for DNA exchange within infected cells (14) (Figure 1).
Protocol
1. Chemical Mutagenesis
Note: We found that the replicative RB form is more amenable to chemical mutagenesis than the EB form. At mid-cycle (between 18 to 20 hpi), RBs are at the greatest numbers prior to RB-EB transition. Because Chlamydia trachomatis is an obligate intracellular pathogen, the effects of the mutagen on the host health can limit bacterial recovery. Vero cells were found to be more resistant to the adverse effects of high levels of EMS than other cell lines tested.
Segregation of mutations by recombination requires selection for antibiotic resistant recombinant progeny. We recommend using drug resistant strains (e.g. rifampin, spectinomycin, or trimethoprim) for generating mutants for the ability to perform recombinant analysis and to isolate isogenic strains. Antibiotic resistant strains were generated by a stepwise selection process (15, 16).
Note: Chlamydia trachomatis (strain L2/434/Bu / ATCC VR902B) is BSL2 pathogen. Refer to institutional standard operating procedures (SOP) for handling such pathogens.
- Preparation of cultured cells and infection:
- Seed approximately 1 x 106 Vero cells (ATCC CCL-81) per 25 cm2 (T25) flask in 3 ml of Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Incubate at 37 °C, 5% CO2. Cells should form a confluent monolayer within 24 h.
- Aspirate medium. Infect confluent T25 flasks of Vero cells with Chlamydia at a multiplicity of infection (moi) of 5 (~14 x 106) in a total volume of 3 ml of medium (DMEM, 200 ng/ml cyclohexamide, 10% FBS).
- Begin mutagenesis at 18 hr post infection (hpi):
- Prepare 20 mg/ml EMS (Ethyl methanesulfonate) in phosphate buffered saline (PBS) with 0.9 mM calcium chloride and 0.49 mM magnesium chloride in a chemical safety hood as EMS is a volatile liquid.
Note: EMS is a potent mutagen and carcinogen. Neutralize EMS waste and decontaminate spills, plastic, paper, and glassware with 1 M sodium hydroxide. - Aspirate medium and wash cells once with 3 ml of PBS/MgCl2/CaCl2. Incubate cells with 3 ml of 20 mg/ml EMS in PBS/MgCl2/CaCl2 for one hour in the fume hood at room temperature. Vero cells are able to survive this concentration of EMS and conditions outside the incubator for short periods. Remember to include a control sample that has not been mutagenized.
- Remove medium containing EMS and place it in a container of 1 M NaOH to inactivate the mutagen. Wash infected cells 3 times in 3 ml PBS + MgCl2/CaCl2 to remove residual mutagen. Add 6 ml of DMEM/10% FBS with 200 ng/ml cyclohexamide and 25 μg/ml gentamicin, and incubate at 5% CO2, 37 °C for 48-72 h. Inclusions appear devoid of bacteria about 36 hr after mutagenesis. At 72 h about 10% of inclusions are filled with bacteria while the rest remain empty.
- Extract EBs by hypotonic lysis: Completely aspirate medium. Add 1.0 ml H2O and rock to dislodge cells for 10 min. Lyse cells by pipetting up and down for at least 10 times. Collect lysate in a microfuge and add 0.250 ml 5x sucrose phosphate glutamate buffer (SPG) to bring it to 1x final concentration. Store at -80 °C.
Note: The level of mutagenesis can be assessed by the induction of rifampin resistance. To assay the frequency of rifampin resistance, plaque 1 x 108 and 7 x 10-fold serial dilutions of bacteria in the presence of 200 ng/ml of rifampin. The frequency of rifampin resistance is defined as the number of rifampin resistant plaques divided by total number of bacteria plated. See section below for plaque instructions.
- Prepare 20 mg/ml EMS (Ethyl methanesulfonate) in phosphate buffered saline (PBS) with 0.9 mM calcium chloride and 0.49 mM magnesium chloride in a chemical safety hood as EMS is a volatile liquid.
2. Clonal Isolation of Chlamydia trachomatis Mutant Strains by Plaque Purification
- Set confluent monolayers of Vero cells in 6 well plates: Seed ~0.4 x 106 cells in 3 ml DMEM/10% FBS per well. Allow cells to form a homogenous monolayer over the next 24 h.
- Thaw stock solution of mutagenized Chlamydia on ice. Perform 6 x 10-fold serial dilutions in a total volume of 200 μl per dilution in DMEM/10% FBS and set them on ice.
Note: Bacterial titer in inclusion forming units (IFU) is a good estimate of plaque forming units (pfu). Aim to plaque 10, 100, and 1000 pfu. - Wash Vero cells twice with 3 ml PBS per well.
- Add 3 ml DMEM to each well for 6-well plates and 100 μl of the bacterial dilutions in duplicate. Swirl the plate to ensure even mixture.
- Spin infected plates at 2,700 x g for 30 min at 15 °C then incubate at 37 °C in a humidified, 5% CO2 incubator for 1-2 hr.
- Prepare 0.54% agarose in DMEM (for 6 wells):
- Add the following ingredients: 18.9 ml cold 2x DMEM, 4.2 ml FBS, 0.42 ml 100x NEAA, 0.042 ml 200 μg/ml cyclohexamide, 0.021 ml 50mg/ml gentamicin. Mix well.
- Mix in 18.9 ml of hot sterile 1.2% agarose/H2O, stirring to prevent clumps. Mixture should be warm to touch. Keep in a warm water bath at 55 °C before adding to infected cells.
- Aspirate bacterial suspension from dishes and apply 6 ml 0.54% agarose/DMEM per well. Allow agarose to solidify completely at room temperature outside the hood for 15 min. Dry the plates with the lids removed, in the biosafety hood for 15 min.
Note: Vibration from the biological containment hood can distort the solidifying agarose. - Incubate at 37° C in CO2 incubator for 7-10 days. Plaques should be visible with the aid of a dissecting microscope. (Refer to Figure 3 for a typical image of plaques.)
- A day prior to isolating mutants, seed 1 x 104 Vero cells in 100 μl per well in a 96-well plate. Cells will be confluent within 24 h.
- Using a dissection microscope, mark plaques to be picked. Collect plugs of plaques using a sterile 1.0 ml barrier pipette tip.
- Resuspend plaques in 100 μl of DMEM supplemented with 10% FBS, 400 ng/ml cyclohexamide, 50 μg/ml gentamicin.
- To expand mutant strains, overlay the suspension onto confluent monolayers of Vero cells. Spin plates at 2,700 x g, 15° C for 30 min.
- Incubate at 37°C/5% CO2 in a humidified incubator. Harvest EBs when >50% of the cells are infected, which can occur as early as 48 hpi and as late as 14 days. This amount of infected cells allows enough viable bacteria to be stored. Mutant strains differ in growth rates and infectivity. Allow slow growing strains to naturally re-infect neighboring cells until enough cells are infected.
- Extract EBs by hypotonic lysis of infected cells:
- Completely aspirate medium.
- Add 160 μl of sterile water. Incubate at room temperature for 10 min.
- Disrupt cells by pipetting up and down several times to ensure complete lysis, use barrier tips to avoid cross contamination.
- Transfer 160 μl of lysates to microfuge tubes.
- Mix in 40 μl of 5x SPG. Store at -80 °C.
- Assay and screen mutants for phenotype(s) of interest.
3. Whole Genome Sequencing of Selected Chlamydia Mutants
- Extract genomic DNA from gradient-purified EBs, which are largely free of contaminating host DNA. Protocols for large scale culture of Chlamydia and purification of EBs can be found in ref. (17). Use commercial genomic DNA purification kits (e.g. Qiagen cat. 69504) to extract DNA from 2 x 109 bacteria following manufacturer instructions.
- Use a fluorometer (Qubit, Invitrogen cat. Q32866) to accurately measure the amount of DNA. 1-5 μg of purified DNA is required for the Illumina sequencing platform.
Note: Several platforms can be used to sequence bacterial genomes, including Illumina/Solexa- and SoliD-based systems. We chose to use HiSeq (Illumina) as it produces high density of short, but high quality sequencing reads. Smaller scale genome sequencers, such as IonTorrent (Life Technologies) and MySeq (Illumina) are suitable too and more than sufficient for multiplexing these quencing of up to 4 Chlamydia genomes at a time, which exceeds the minimal coverage of 25X for adequate genome assembly. Preparation of DNA sequencing libraries for the Illumina platform are described below. - Fragment DNA to appropriate size (300-400 base pair for Illumina sequencing) using an Adaptive Focused Acoustics S220 instrument (Covaris) according to manufacturer’s suggested settings. Note: DNA fragmentation by nebulization results in greater loss of sample due to aerosolization of sample.
- Prepare the genomic sequencing libraries using commercial construction kits (Illumina FC-102-1001), following the instructions of the manufacturer. Libraries can be indexed using barcoded primers (Illumina FC-121-1003) such that multiple samples can be pooled and sequenced simultaneously.
- Run sequencing samples. This step is usually performed by commercial services or core facilities operated by dedicated technical staff. Follow their procedures and recommendations.
- Illumina reads (FASTQ format) can be assembled to a reference genome using user friendly graphic user interface software, such as Geneious (Biomatters), which can be also used for SNP/mutation identification. To map mutations, set parameters to 90% for minimum variant frequency and 50X for minimum coverage. Open source genome assemblers, such as MAQ (18) and BWA (19), can also be used.
- Confirm mutations by Sanger sequencing: use genomic DNA as template for PCR amplification of 300-500 bp regions flanking identified mutations and sequence purified or diluted (1:20) PCR products by Sanger sequencing.
4. Generation of Chlamydia Recombinants
Note: Chlamydia can exchange DNA during infection, which allows for the generation of recombinant strains (12, 13, 20). After co-infection of cells with two unique antibiotic resistant strains, recombinant "progeny" can be selected for dual antibiotic resistance (12, 13). We took advantage of this phenomenon to segregate mutations and to generate co-isogenic strains. Hence, mutant strains were generated in antibiotic resistant background (e.g. rifampin resistance (rifR) H471Y in CTL0567 (rpoB)) such that they can be crossed to wild type strain bearing a different antibiotic resistant allele (e.g. spectinomycin resistance (spcR), G1197A in r01/r02 (16SRNA copies 1 and 2)). For details regarding DNA exchange and recombination in Chlamydia, refer to references (12, 13).
- Co-infect wild type and clonal mutant strains by centrifuging6 x 105 bacteria from each strain onto confluent monolayers of Vero cells grown on a 24-well plate. In parallel, infect monolayers with each strain alone.
- At 44 hpi, harvest EBs by hypotonic lysis of infected cells:
- Completely aspirate medium.
- Add 0.4 ml of sterile water and let stand for 10 min.
- Lyse cells by vigorous pipetting up & down for at least 10 times. Transfer lysates to a microfuge tube.
- Mix in 0.1 ml of 5x SPG for a final concentration of 1x SPG.
- Crude lysates can be used immediately or stored at -80 °C.
- Plaque 50 μl of the crude EB preps and 5 x 1:10 serial dilutions in agarose/DMEM supplemented with appropriate antibiotics to select for recombinants. (See table for concentrations of typical antibiotics for selection of recombinants.)
- Plaque purify and enrich recombinant strains as above. Score recombinants for presence or absence of the desired phenotype. Extract DNA from recombinant strains by DNAzol treatment (next section) for the purpose of genotyping for the presence or absence of mutations.
- Crosses can be repeated with strains bearing other antibiotic resistance markers to further segregate mutations, and generate isogenic strains. Co-infect selected recombinants to another wild type strain bearing a different antibiotic marker.
Table 1: Antibiotic concentrations for selection of recombinants:
| Final Concentration | Preparation instructions |
|---|---|
| 200 ng/ml rifampin | Make 25 mg/ml storage stock in dimethyl sulfoxide (DMSO). Make up 200 μg/ml working solution by diluting the storage stock with H2O. Store at -20 °C in the dark. |
| 200 μg/ml trimethoprim | Make a 100 mg/ml stock in DMSO. Store at -20 °C |
| 200 μg/ml spectinomycin | Make a 100 mg/ml stock in water in 100 μl aliquots. Store at -20 °C. Avoid repeated freeze thaw. |
5. Extraction of Genomic DNA from Recombinant Strains for Genotyping
Note: Column-based purification kits can also be used. An advantage of DNA extraction by DNAzol treatment is cost.
- Infect 1.2 x 105 chlamydiae onto monolayers of Vero cells grown on 12-well plates.
- At 36-48 hpi, aspirate medium and add 0.375 ml of DNAzol (Invitrogen 10503-027). Gently agitate the cells and pipet the lysates into a 1.5 ml microfuge tube.
- Precipitate DNA by the addition of 0.188 ml of 100% ethanol. Mix by inversion.
- Extract DNA by spooling with a pipette tip and place it in a clean tube. Alternatively, pellet the DNA by centrifugation at top speed for 2 min at room temperature or 4 °C, and decant supernatant.
- Wash DNA precipitate twice with 1.0 ml of 75% ethanol.
- Air-dry DNA after removing the ethanol for 15 sec.
- Solubilize DNA with 0.2 ml 8 mM NaOH then neutralize to pH 8.0 with 20.2 μl of 0.1 M HEPES. Bring up to a final volume of 0.5 ml with water or TE. Alternatively, resuspend DNA in TE buffer (DNA pellet will not solubilized completely.).
- Use DNAzol-extracted DNA as template for PCR amplification of 300-500 bp regions flanking identified mutations. Sequence purified or diluted (1:20) PCR products by Sanger sequencing.
Representative Results
Exposure to mutagen leads to inclusions that appear devoid of bacteria,presumably due to bacterial cell death. Typically,serovar LGV-L2 will completely lyse infected cells within 48 h post infection, but treatment with mutagens can extend this cycle to >90 h. Roughly 10% of inclusions are expected to recover. In our experiments, infected Vero cells treated with 20 mg/ml EMS led to a 99% decrease in the recovery of infectious progeny compared to untreated controls. Mutagen treatment also led to the emergence of plaques with altered morphologies, including small plaques (SPQ) (Figure 3). Other morphologies include plaques that appear granular, clumpy, or honeycomb shaped (Figure 3). These phenotypes may reflect defects in processes required for infection and survival within the host environment. Mutants with small plaque (SPQ) morphology generally produce significantly fewer infectious progeny and may take up to 2-3 weeks to amplify. Caution should be exercised when passaging these strains as reversions and suppressor mutations can accumulate at a high frequency.
The doses of EMS used in these studies can lead to between 3 to 30 mutations per Chlamydia genome, as assessed experimentally by WGS (Figure 4). Although gradient purified EBs are largely free of host cell material, host DNA is also detected and consists about 10-15% of genomic preps.
Co-infection of two strains can generate progeny with genetic contributions from both strains and occurs at frequencies of 10-4 to 10-3, approximately 104 times more frequently than spontaneous resistance (13) . Recombination break points typically occur between ~100 kb to 800 kb (12). An analysis of such recombinant strains can reveal mutations that are genetically linked to the phenotype under study.
Discussion
This methodology meets the basic requirements for genetic analysis as it establishes linkage between genotypes and phenotypes. Importantly, this is achieved without the aid of conventional molecular tools for recombinant DNA transformation and insertional inactivation of genes in bacteria, which is often a rate-limiting step in the analysis of gene function in non-model microbes.
One critical step is to ensure clonality of plaque-purified mutants. Cross contamination with wild type or "fitter" mutants can quickly lead to mutant strains being outcompeted. Similarly, mapping mutations by whole genome sequencing of non-clonal samples can lead to ambiguous results. Isolating mutants from heavily plaqued wells or plugging plaques that are too close to each other should be avoided. In addition, for slow growing mutants, revertants can emerge at relatively high frequencies. We recommend preserving original or low passage stocks. Replaque mutants if cross contamination or revertants are suspected.
The concentration of EMS can be lowered to decrease the frequency of mutations. The mutation rate, as assessed by the generation of rifampin resistant variants (due to point mutations in the gene encoding RNA polymerase, rpoB) was optimal in our hands at 20 mg/ml EMS. The induction of rifampin resistance can be assessed by the frequency of plaques formed in the presence of rifampin and should correlate to the frequency of chemically induced mutations.
EMS can be replaced with other mutagens to widen the spectrum of mutations that can be obtained. For instance, DNA intercalating agents like psoralen and its derivatives can be used to induce deletions and insertions (21).
Mutations that map close to each other in the genome (<100 kb apart) are difficult to unlink through recombination. When high mutagenesis rates are achieved, it may be difficult to unambiguously identify a causal mutation. However, since transformation of C. trachomatis is now possible with shuttle plasmids (22), albeit inefficiently, it may be possible to address these linkage problems by complementing mutations in trans with a wild type copy of the mutated gene on a plasmid.
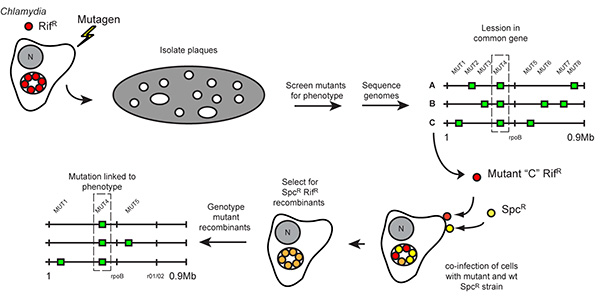
Figure 1. Strategy for forward genetic analysis and recombination-based mapping in Chlamydia. Rifampin resistant (RifR) C. trachomatis was mutagenized during its replicative stage and used to infect Vero cell monolayers until visible plaques formed. Individual clones of mutants were collected and assayed for specific phenotypes, such as altered plaque morphotypes. The genomes of mutants sharing a common phenotype were sequenced to identify common genetic lesions. To establish linkage between these gene lesions and specific plaque morphologies, Vero cells were co-infected with mutants generated in aRifR background and wild-type SpcR Chlamydia strains, and recombinant RifR SpcR strains among the resulting infectious progeny were selected in the presence of rifampin and spectinomycin ("crosses"). The segregation of individual mutations present in the parental RifR mutant strain among the recombinant bacteria displaying altered plaque morphology was determined by targeted DNA sequencing. (Reproduced with permission from PNAS (14)) Click here to view larger image.
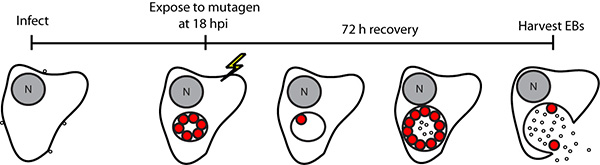
Figure 2. Schematic representation of EMS mutagenesis protocol. Chlamydia trachomatis LGV-L2-infected cells were exposed to EMS during the RB stage of the infectious cycle, and the infection was allowed to proceed for 72 h to allow for the generation of infectious elementary bodies (EB). Mutagenized EB pools were harvested and titered for inclusion forming units (IFU) and plaque-forming units on Vero cells. N, nucleus. (Reproduced with permission from PNAS (14)) Click here to view larger image.
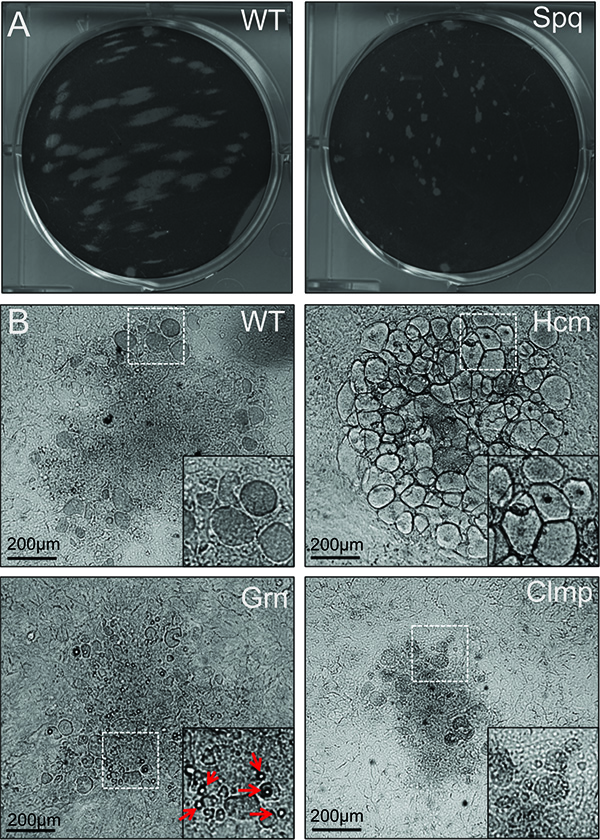
Figure 3. Examples of common plaque morphologies among EMS-mutagenized C. trachomatis. Mutagenized C. trachomatis LGV-L2 allowed to form plaques on Vero cell monolayers for 14 day. Plaques varied in size (A) and morphology (B), which can be isolated, amplified in Vero cells, and used in reinfections of Vero monolayers to confirm the stability of the plaque morphotypes. Examples of common phenotypes are shown including honeycomb (Hcm), clumped (Clmp), small plaque (Spq), and granular (Grn). Arrows indicate large granular deposits within a Grn plaque. (Reproduced with permission from PNAS (14)) Click here to view larger image.
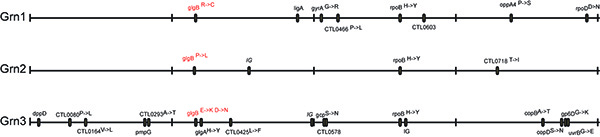
Figure 4. Identification of EMS-induced gene lesions. Chromosomal location of nucleotide variants in mutants that display the Grn plaque morphotype. Whole-genome sequencing of three Grn mutants identified mutations that lead to amino acid changes in glgB, coding for a glycogen-branching enzyme. (Reproduced with permission from PNAS (14)) Click here to view larger image.
Divulgations
The authors have nothing to disclose.
Materials
| Dulbecco’s Modified Eagle Medium (DMEM) | Life Technologies | 11995-073 | |
| Fetal Bovine Serum (FBS) | Cellgro | 35-010-CV | |
| Ethyl methanesulfonate (EMS) | Sigma | M0880 | |
| Cyclohexamide | Sigma | C4859-1ML | |
| Gentamicin | Life Technologies | 15750-060 | |
| Phosphate buffered saline (PBS) | Life Technologies | 14190-144 | |
| Phosphate buffered saline (PBS) solution with 0.493 mM MgCl2 and 0.901 mM CaCl2 (PBS+MgCl2/CaCl2) | Life Technologies | 14040-133 | |
| 1 M NaOH | |||
| 5x SPG buffer (1.25 M sucrose, 50 mM sodium phosphate, 25 mM glutamic acid) | |||
| SPG buffer (0.25 M sucrose, 10 mM sodium phosphate, 5 mM glutamic acid) | |||
| Water (sterile, tissue culture grade) | |||
| 2x DMEM (prepared from powder, buffered with 7.4 g/L Sodium Bicarbonate | Sigma | D7777 | |
| Nonessential amino acids (NEAA) | Life Technologies | 11140-050 | |
| 1.2% GTG Agarose , autoclaved | Lonzo | 50070 | |
| Genomic DNA purification kits | Qiagen | 69504 | |
| DNAzol | Life Technologies | 10503-027 | |
| Ethanol (molecular biology grade) | |||
| 8 mM NaOH | |||
| 0.1 M HEPES ( 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer | |||
| 25 cm2 tissue culture flasks (T25 flasks) | |||
| 6-well tissue culture plates | |||
| 12-well tissue culture plates | |||
| 96-well tissue culture plates | |||
| Chemical safety hood | |||
| Biological safety hood | |||
| >Centrifuge and adaptors for spinning tissue plates | |||
| >Dissection microscope | |||
| Fluorometer (Qubit) | Invitrogen | Q32866 | |
| Adaptive Focused Acoustics S220 instrument | Covaris |
References
- Haggerty, C. L., et al. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 201, S134-S155 (2010).
- Dautry-Varsat, A., Subtil, A., Hackstadt, T. Recent insights into the mechanisms of Chlamydia entry. Cell Microbiol. 7 (12), 1714-1722 (2005).
- Hybiske, K., Stephens, R. S. Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells. Infect Immun. 75 (8), 3925-3934 (2007).
- Heuer, D., Kneip, C., Maurer, A. P., Meyer, T. F. Tackling the intractable – approaching the genetics of Chlamydiales. Int J Med Microbiol. 297 (7-8), 569-576 (2007).
- Stephens, R. S., et al. Genome sequence of an obligate intracellular pathogen of humans, Chlamydia trachomatis. Science. 282 (5389), 754-759 (1998).
- Thomson, N. R., et al. Chlamydia trachomatis, genome sequence analysis of lymphogranuloma venereum isolates. Genome Res. 18 (1), 161-171 (2008).
- Harris, S. R., et al. Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing. Nat Genet. 44 (4), 413-419 (2012).
- Somboonna, N., et al. Hypervirulent Chlamydia trachomatis clinical strain is a recombinant between lymphogranuloma venereum (L(2)) and D lineages. MBio. 2 (3), e00045-00011 (2011).
- Voigt, A., Schofl, G., Saluz, H. P. The Chlamydia psittaci genome, a comparative analysis of intracellular pathogens. PLoS One. 7 (4), e35097 (2012).
- Jeffrey, B. M., et al. Genome sequencing of recent clinical Chlamydia trachomatis strains identifies loci associated with tissue tropism and regions of apparent recombination. Infect Immun. 78 (6), 2544-2553 (2010).
- Gomes, J. P., et al. Evolution of Chlamydia trachomatis diversity occurs by widespread interstrain recombination involving hotspots. Genome Res. 17 (1), 50-60 (2007).
- DeMars, R., Weinfurter, J. Interstrain gene transfer in Chlamydia trachomatis in vitro, mechanism and significance. J Bacteriol. 190 (5), 1605-1614 (2008).
- Demars, R., Weinfurter, J., Guex, E., Lin, J., Potucek, Y. Lateral gene transfer in vitro in the intracellular pathogen Chlamydia trachomatis. J Bacteriol. 189 (3), 991-1003 (2007).
- Nguyen, B. D., Valdivia, R. H. Virulence determinants in the obligate intracellular pathogen Chlamydia trachomatis revealed by forward genetic approaches. Proc Natl Acad Sci U S A. 109 (4), 1263-1268 (2012).
- Tipples, G., McClarty, G. Isolation and initial characterization of a series of Chlamydia trachomatis isolates selected for hydroxyurea resistance by a stepwise procedure. J Bacteriol. 173 (16), 4932-4940 (1991).
- Wang, L. L., Henson, E., McClarty, G. Characterization of trimethoprim- and sulphisoxazole-resistant Chlamydia trachomatis. Mol Microbiol. 14 (2), 271-281 (1994).
- Scidmore, M. A. Cultivation and Laboratory Maintenance of Chlamydia trachomatis. Curr Protoc Microbiol. Chapter 11, Unit 11A 11 (2005).
- Li, H., Ruan, J., Durbin, R. Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 18 (11), 1851-1858 (2008).
- Li, H., Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 25 (14), 1754-1760 (2009).
- Suchland, R. J., Sandoz, K. M., Jeffrey, B. M., Stamm, W. E., Rockey, D. D. Horizontal transfer of tetracycline resistance among Chlamydia spp. in vitro. Antimicrob Agents Chemother. 53 (11), 4604-4611 (2009).
- Sladek, F. M., Melian, A., Howard-Flanders, P. Incision by UvrABC excinuclease is a step in the path to mutagenesis by psoralen crosslinks in Escherichia coli. Proc Natl Acad Sci U S A. 86 (11), 3982-3986 (1989).
- Wang, Y., et al. Development of a transformation system for Chlamydia trachomatis, restoration of glycogen biosynthesis by acquisition of a plasmid shuttle vector. PLoS Pathog. 7 (9), e1002258 (2011).

