Fat Preference: A Novel Model of Eating Behavior in Rats
Summary
Dietary fat content influences both energy intake and body fat composition in mammals. By examining rats’ preference for high fat food in a series of choice experiments, it is possible to test genetic differences and pharmacological interventions on their preference for high fat food.
Abstract
Obesity is a growing problem in the United States of America, with more than a third of the population classified as obese. One factor contributing to this multifactorial disorder is the consumption of a high fat diet, a behavior that has been shown to increase both caloric intake and body fat content. However, the elements regulating preference for high fat food over other foods remain understudied.
To overcome this deficit, a model to quickly and easily test changes in the preference for dietary fat was developed. The Fat Preference model presents rats with a series of choices between foods with differing fat content. Like humans, rats have a natural bias toward consuming high fat food, making the rat model ideal for translational studies. Changes in preference can be ascribed to the effect of either genetic differences or pharmacological interventions. This model allows for the exploration of determinates of fat preference and screening pharmacotherapeutic agents that influence acquisition of obesity.
Introduction
Obesity is a prevalent problem in the United States1, with the Centers for Disease Control and Prevention estimating that over a third of American adults are obese. Obesity has also been identified as a risk factor for numerous health concerns, including type 2 diabetes, high blood pressure, and high cholesterol2. While many factors have been shown to influence the increase in obesity rates, there is ongoing interest and controversy in the role macronutrients play in obesity3,4.
One contributing factor to obesity is high dietary fat intake5. Increased dietary fat is correlated with increased human energy consumption6 and significant increases in body fat content7,8. Additionally, dietary fat has reward value both during and after consumption7,9. Therefore, determining what factors influence preference for high fat foods can both guide pharmacotherapeutic design and promote understanding of the underlying dietary choices that can lead to obesity. The Fat Preference model described here tests for rats’ preference between foods of differing fat content, but similar nutritional value. Specifically, this model presents the rats with a choice of two different foods simultaneously thus enabling the quantification of preference based on grams consumed of the lower fat food verses the higher fat food. Pharmacological and genetic effects can be measured as a change in preference for the food with higher fat content.
The Fat Preference model serves to complement the widely used palatable food intake models10 but also offers several advantages. This model allows the experimenter to specifically assess feeding behavior in a controlled environment in which two food options are available. Traditional high fat feeding models only offer one food which eliminates the capacity to study food choice, an important aspect of human food intake. Some assays do offer multiple food types and are often referred to as "cafeteria" type feeding studies11. These studies suffer from reproducibility because human food is often used in the assay and is not well suited to the laboratory environment due to nutrient variability. We use defined diets which contain individual purified ingredients thus greatly improving reproducibility and flexibility to change macronutrient content such as dietary fat. With higher dietary fat intake associated with obesity in humans5 and the natural human preference for higher fat foods12, treatments that alter rat preference for high fat foods can provide valuable insight into obesity.
Protocol
All experimental procedures are in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources (U.S.), 1996) and with the approval of the Institutional Animal Care and Use Committee at the University of Texas Medical Branch.
1. Subjects
- Single house male Sprague-Dawley rats weighing 225-250 g at 21 °C and 30-50% relative humidity with a 12 hr light-dark cycle (lights on 6:00 am-6:00 pm).
- Maintain rats on a low fat food (10% fat by energy). Habituate rats to food and colony room for at least 7 days prior to experimentation. Animals are habituated to handling and dosing of vehicle for three days prior to starting the studies. The first day after habituation is experimental Day 1.
2. Baseline Preference
- Day 1, fill two clean food hoppers, one marked with an A and the other with B, with 10% fat food for each cage. Weigh the food hoppers (with food) and place them in the cage, ensuring the rat has access to both.
- Day 2, at approximately the same time food hoppers were placed in the cage the day before, record the food hopper weights and switch the positions of hopper A and hopper B. This mitigates position bias. Always examine the home cage for any food removed from the hopper, weigh it, and account for it. Food intake is measured by change in hopper weight between days.
- Day 3, record the food hopper weights as before. Examine the amount of food consumed in each food hopper position and food hopper identification (A and B). This data gives a baseline understanding of position bias and specific hopper preference, and should have a preference score of approximately 50% indicating no preference. Throw out the remaining food.
3. Fat Preference
- Day 3, obtain two clean food hoppers for each cage. It is important to use clean food hoppers whenever switching between the different foods to prevent confounding scents from previous experiments. Fill hopper A with 12.5% fat food and hopper B with 15% fat food (Table 1). Record the starting weights of each hopper and place both in the cage.
- Day 4, record the weights of each hopper, switching the positions of hopper A and hopper B upon placing them back in the cage.
- Day 5, record the weights of each hopper. After determining the grams of each food consumed on both days, the rat’s food preference can be determined.
- Repeat steps 3.1 through 3.3 multiple times with the same animals using 12.5% fat food as compared to foods with increasing fat content (e.g. first 15%, next 17.5%, then 20%, and lastly 45% fat).
4. Data Calculations and Analysis
- Calculate the daily food intake for each animal by subtracting the final mass of the food+hopper from the starting mass of the food+hopper. Because each food is measured over 2 days, the 2 day total can be added together for each % fat content.
- Calculate the fat preference score by dividing the intake of the higher fat food from the total food consumed over each two day period. For example, one rat ate 8.9 g of the 12.5% fat diet and 27.5 g of the 17.5% diet over 2 days. Therefore the preference score for that individual animal is 76% (27.5 divided by 36.4).
- Average and graph the preference score for each diet (15%, 17.5%, 20%, and 45% fat) (Figure 3). The total food intake is also informative and can readily be plotted as grams (Figure 4).
- Express the preference score (Figure 5A) and total food intake (Figure 5B) as a function of total energy intake (kilocalories) using the energy density in Table 1.
- If using more than one group of animals, perform a two-way repeated measures ANOVA followed by a Bonferroni post-test. Power analysis indicates that n = 8 animals per group is appropriate for detecting a difference of 7% between treatment groups.
Representative Results
Rats had physical access to both food hoppers simultaneously (Figure 1) in order to demonstrate preference for available food choices with differing fat content (Table 1). Rat food was weighed daily and any food spillage was easily found and accounted for (Figure 2). Spillage of fine food particles was found to be minimal and does not substantially influence the data. Using the Fat Preference model, a clear preference between foods containing 12.5% fat and higher fat foods can be observed (p<0.05 preference over 12.5% compared to 17.5%, 20%, and 45%, by ANOVA followed post hoc test) (Figure 3A) which was dependent on the fat content (Figure 3B). This data can also be expressed as grams of food (Figure 4) or in energy intake (Figure 5), allowing for a choice in data presentation for publication. In these short term feeding studies using high fat foods, an increase in total energy consumed is typically observed when animals are given access to increasing dietary fat content (Figure 5B).
Statistical analysis of data was performed using SPSS 20 software. A repeated measures ANOVA with post hoc t test (t test was used since there were no experimental groups) was used to determine significant main effects, interaction of main effects, and differences in preference for individual foods.

Figure 1. A rat in its homecage with two food choices. The rat has physical access to the two food hoppers and to water. Food hoppers are filled to ensure ad libitum access to both foods.

Figure 2. Food spillage is highly visible and can be accounted for. While weighing food daily, any food that is not contained in the hopper is found and weighed.
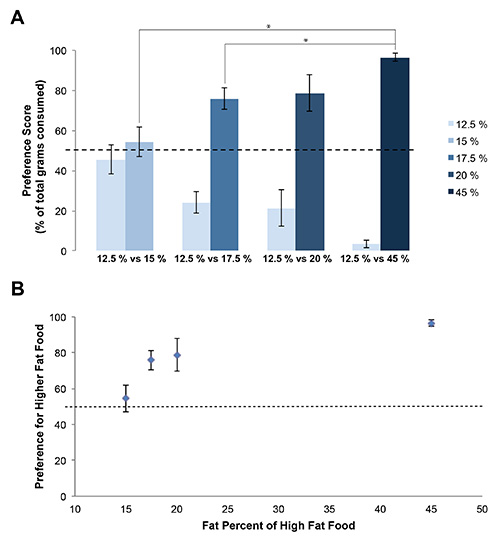
Figure 3. Rats demonstrate an increasing preference for foods with higher fat content. A) Rats demonstrate a significant preference for 15%, 17.5%, 20 %, and 45% fat foods compared to 12.5%. (* p<0.05, by ANOVA followed post hoc test). B) Fat Preference method results in a “dose-effect” curve where preference changes with fat content as compared to 12.5% fat food. Error bars represent the standard error of the mean.
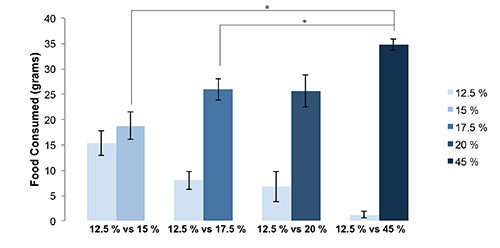
Figure 4. Representation in grams of rats’ preference for higher fat food. Preference expressed as grams of food consumed can provide additional information about changes in total food intake over the course of the experiment. (* p<0.05, by ANOVA followed post hoc test). Error bars represent the standard error of the mean.
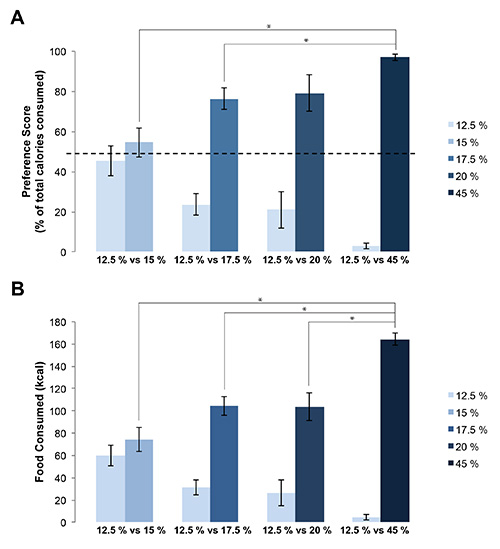
Figure 5. Representation in calories of rats’ preference for higher fat food. A. Rats consume more of their calories from higher fat foods during preference testing. This is dependent on the fat percentage of the foods offered. B. Preference for higher fat food expressed as total kcal per food pairing. (* p<0.05, by ANOVA followed post hoc test). Error bars represent the standard error of the mean.
| Fat | Carbohydrate | Protein | Energy Density | |||||||
| Diet | %gram | %kcal | %gram | %kcal | %gram | %kcal | kcal/gram | |||
| 10% fat | 4.3 | 10 | 67.3 | 70 | 19.2 | 20 | 3.85 | |||
| 12.5% fat | 5.4 | 12.5 | 65.7 | 67 | 19.5 | 20 | 3.9 | |||
| 15% fat | 6.6 | 15 | 64.2 | 65 | 19.8 | 20 | 3.95 | |||
| 17.5% fat | 7.8 | 17.5 | 62.5 | 62 | 20.1 | 20 | 4.01 | |||
| 20% fat | 9 | 20 | 61 | 60 | 20.3 | 20 | 4.06 | |||
| 45% fat | 24 | 45 | 41 | 35 | 24 | 20 | 4.73 | |||
Table 1. Macronutrient contents of experimental foods.
Discussion
The Fat Preference model is an informative and easy to perform assay of feeding behavior. This assay offers an opportunity to identify neural and molecular mechanisms which underlie food preference, which is an important yet understudied area of obesity research. Changes in fat preference from genetic differences or pharmacological manipulations could be visualized as rightward or leftward shifts in the preference curve (Figure 3B). It is important to include the middle fat foods (15%, 17.5%, and 20%) to visualize any shifts in the preference curve. The shifts in the fat preference curve can be interpreted similarly to how the various two bottle choice preference tests are interpreted13,14. As the rats have a bias toward eating high fat food, a significantly decreased preference high fat food represents a possible pharmacologically relevant change in food composition choices. Indeed, neuromedin U receptor 2 expression has recently been shown to alter fat preference using this model15. The colored food (Table 1) makes it easy to determine any spillage before daily food weighing (Figure 2). This helps ensure accurate measurement of daily food intake. The method is also quick to perform and can be repeated with increasing concentrations of fat in the food.
There are some special considerations for pharmacological and genetic manipulations of dietary fat preference. In pharmacological studies, compound exposure is an important consideration because this assay takes up to 8 days. Therefore, pharmacological compounds may need to be administered one or more times per day depending on the half-life and total dose of each compound being tested. In genetic studies, treatments such as viral knockdown of a gene may require 14 days for stable expression which will lengthen the experimental timeline.
The food composition in Table 1 was chosen to maintain constant protein with varying fat and carbohydrates. Protein was kept constant due to its high satiety per unit energy4 This method could easily be adapted to hold constant any one of three macronutrients in order to study the other two. However, this would require many additional foods and rats. The foods in Table 1 focus around the 17.5% fat food preference because this is approximately where a significant preference over 12.5% fat food can first be observed. Furthermore, preference testing was limited to two diet choices as opposed to a cafeteria style option. If all diets were presented simultaneously as in cafeteria style studies, the large preference for the 45% fat diet would reduce the assay sensitivity by minimizing the preference for the 17.5% and 20% fat diets.
One limitation for this method is the large number of food hoppers that require daily weighing and cleaning. Fortunately, these high labor and equipment periods are short (two days per preference test), require minimal training and can be interspersed with periods of only 10% fat food access as needed.
This model does have some limitations as well. First, the foods are presented in an increasing order of fat content which can produce an order effect. However, this order is necessary to minimize the long term effects of high fat food consumption on food choice. Lucas and colleagues have shown that rat consumption of pure or emulsified fat, in addition to rat chow, will decrease or increase over days, respectively16. This would make it difficult to interpret results from decreasing fat content experiments or following access to the 45% fat food in a latin square type experiment. A second limitation is the repeated use of the 12.5% fat food as a baseline for comparison. A decreased preference for the 12.5% fat food would cause an apparent increase in the preference for the higher fat food. However, failing to have a constant baseline food could influence fat intake16 and make comparing the preferences impossible. Lastly, our results could be interpreted as a preference for lower carbohydrates and not for higher fat. However, rats prefer increasing (not decreasing) carbohydrate content14,15 reducing the validity of this interpretation.
In conclusion, the Fat Preference model can be used to determine a rat’s preference between two foods of differing fat content. This test allows the experimenter to determine if genetic background or drug treatments can manipulate the fat content preference of a rat. This preference for a high fat diet offers excellent face validity and represents a translationally relevant model of eating behavior in humans.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors wish to acknowledge funding from UL1TR000071 (NCATS), P30DK079638 (NIDDK), P30DA028821 (NIDA) and T32DA07287 (NIDA).
Materials
| Rodent diet with 10 kcal% fat | Research Diets | D12450B | 10 % fat rat food |
| Rodent diet with 12.5 kcal% fat | Research Diets | D07040501 | 12.5 % fat rat food |
| Rodent diet with 15 kcal% fat | Research Diets | D07040502 | 15 % fat rat food |
| Rodent diet with 17.5 kcal% fat | Research Diets | D07040503 | 17.5 % fat rat food |
| Rodent diet with 20 kcal% fat | Research Diets | D07040504 | 20 % fat rat food |
| Rodent diet with 45 kcal% fat | Research Diets | D12451 | 45 % fat rat food |
| Rat feeders (3.75"W x 2.875"D x 5.25"H) | Labex of MA | 2528 | Food hoppers |
References
- Flegal, K. M., Carroll, M. D., Ogden, C. L., Curtin, L. R. Prevalence and trends in obesity among US adults. JAMA. 303 (3), 235-241 (1999).
- Mokdad, A. H., et al. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA. 289 (1), 76-79 (2003).
- Abete, I., Astrup, A., Martínez, J. A., Thorsdottir, I., Zulet, M. A. Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr. Rev. 68 (4), 214-231 (2010).
- Berthoud, H. R., Münzberg, H., Richards, B. K., Morrison, C. D. Neural and metabolic regulation of macronutrient intake and selection. Proc. Nutr. Soc. 71 (3), 390-400 (2012).
- Warwick, Z. S., Schiffman, S. S. Role of dietary fat in calorie intake and weight gain. Neurosci. Biobehav. Rev. 16 (4), 585-596 (1992).
- Kendall, A., Levitsky, D. A., Strupp, B. J., Lissner, L. Weight loss on a low-fat diet: consequence of the imprecision of the control of food intake in humans. Am. J. Clin. Nutr. 53 (5), 1124-1129 (1991).
- Ackroff, K., Sclafani, A., Montmayeur, J. P., leCoutre, J. Oral and Postoral Determinants of Dietary Fat Appetite. Fat Detection: Taste, Texture, and Post Ingestive Effects. Chapter 12. , (2010).
- West, D. B., York, B. Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am. J. Clin. Nutr. 67, 505-512 (1998).
- Sclafani, A., Ackroff, K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302 (10), (2012).
- Velloso, L. A. The brain is the conductor: diet-induced inflammation overlapping physiological control of body mass and metabolism. Arq. Bras. Endocrinol. Metabol. 53 (2), 151-158 (2009).
- Vanzela, E. C., et al. Pregnancy restores insulin secretion from pancreatic islets in cafeteria diet-induced obese rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298 (2), (2010).
- Johnson, S. L., McPhee, L., Birch, L. L. Conditioned preferences: young children prefer flavors associated with high dietary fat. Physiol. Behav. 50 (6), 1245-1251 (1991).
- Bolanos, C. A., Barrot, M., Berton, O., Wallace-Black, D., Nestler, E. J. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol. Psychiatry. 54, 1317-1329 (2003).
- Ignar, D. M., et al. Regulation of Ingestive Behaviors in the Rat by GSK1521498, a Novel {micro}-Opioid Receptor-Selective Inverse Agonist. J. Pharmacol. Exp. Ther. 339, 24-34 (2011).
- Benzon, C. R., Johnson, S. B., McCue, D. L., Li, D., Green, T. A., Hommel, J. D. Neuromedin U receptor 2 knockdown in the paraventricular nucleus modifies behavioral responses to obesogenic high-fat food and leads to increased body weight. Neurosciences. , (2013).
- Lucas, F., Ackroff, K., Sclafani, A. Dietary fat-induced hyperphagia in rats as a function of fat type and physical form. Physiol Behav. 45 (5), 937-946 (1989).

