Экс Ситу нормотермических машина перфузии Печени доноров
Summary
Here we present a protocol describing oxygenated ex situ machine perfusion of donor liver grafts. This article contains a step by step protocol to procure and prepare the liver graft for machine perfusion, prepare the perfusion fluid, prime the perfusion machine and perform oxygenated normothermic machine perfusion of the liver graft.
Abstract
In contrast to conventional static cold preservation (0-4 °C), ex situ machine perfusion may provide better preservation of donor livers. Continuous perfusion of organs provides the opportunity to improve organ quality and allows ex situ viability assessment of donor livers prior to transplantation. This video article provides a step by step protocol for ex situ normothermic machine perfusion (37 °C) of human donor livers using a device that provides a pressure and temperature controlled pulsatile perfusion of the hepatic artery and continuous perfusion of the portal vein. The perfusion fluid is oxygenated by two hollow fiber membrane oxygenators and the temperature can be regulated between 10 °C and 37 °C. During perfusion, the metabolic activity of the liver as well as the degree of injury can be assessed by biochemical analysis of samples taken from the perfusion fluid. Machine perfusion is a very promising tool to increase the number of livers that are suitable for transplantation.
Introduction
The current method of organ preservation in liver transplantation is flush out with and subsequent storage of donor livers in cold (0-4 °C) preservation fluid (such as University of Wisconsin solution or Histidine-Tryptophan-Ketoglutarate solution). This method is referred to as static cold storage (SCS). Although the metabolic rate of livers at 0-4 °C is very low, there is still demand for 0.27 µmol oxygen/min/g liver tissue, which cannot be provided during SCS1. The conventional method of SCS, therefore, results in some degree of (additional) injury of donor livers. While this amount of preservation injury is not a problem in donor livers of good quality, it can become a critical and limiting factor in suboptimal livers that have already suffered some degree of injury in the donor. For this reason, livers with suboptimal quality or so-called extended criteria donor (ECD) livers are frequently rejected for transplantation as the risk of early graft failure is considered to be too high. High rates of delayed graft function, primary non-function, and non-anastomotic biliary strictures (NAS) have been described in recipients of livers from donation after circulatory death (DCD), older donors or recipients of steatotic grafts2. NAS are a major cause of morbidity and mortality after liver transplantation. NAS may occur in both extra- and intrahepatic donor bile ducts and can be accompanied by intraductal biliary sludge and cast formation3,4. Although the etiology of NAS is thought to be multifactorial, ischemia/reperfusion injury of the bile ducts during graft preservation and transplantation has been identified as a major underlying mechanism2,5. Transplantation of a DCD graft has been identified as one of the strongest risk factors for the development of NAS. The combination of a period of warm ischemia in a DCD donor, cold ischemia during organ preservation, and subsequent reperfusion injury in the recipient is thought to be responsible for irreversible injury of the bile ducts, which, in combination with a poor regenerative capacity of the bile ducts, results in fibrotic scarring and narrowing of the bile ducts after liver transplantation2,5. NAS have been reported in up to 30% of patients receiving a DCD liver6-8 . It has become clear that the current method of SCS of liver grafts for transplantation is insufficient for preinjured ECD livers such as those from DCD donors. Alternative methods are needed to increase and optimize the use of ECD livers for transplantation.
Machine perfusion (MP) is a method of organ preservation that may provide better preservation of donor organs, compared to SCS. MP could be especially relevant for the preservation of ECD grafts. An important advantage of MP is the possibility to provide oxygen to the graft during the preservation period. MP can be performed at various temperatures, which have been classified as hypothermic (0-10 °C), subnormothermic (10-36 °C) and normothermic (36-37 °C) MP (NMP). Depending on the temperature used for MP, the type of perfusion fluid has to be adjusted and with increasing temperature more oxygen should be supplied. The first clinical application of MP in human liver transplantation was based on hypothermic perfusion without active oxygenation of the perfusion fluid9,10. In animal models, hypothermic oxygenated MP (0-10 °C) has been shown to have protective effects against ischemia/reperfusion injury of liver grafts11 and to provide better preservation of the peribiliary vascular plexus of the bile ducts12. Subnormothermic oxygenated MP at 20 °C or 30 °C has also been studied in animal models and was shown to provide earlier recovery of graft function of DCD livers, compared to SCS13,14. The feasibility of subnormothermic oxygenated MP of human livers was recently reported in a series of seven discarded human donor livers15. NMP (37 °C) allows for the assessment of graft viability and functionality prior to transplantation16,17. Additionally, MP allows for gradual rewarming of the liver graft before transplantation, which has been demonstrated to facilitate recovery and resuscitation of the graft18.
The perfusion device used in the current protocol for hepatic machine perfusion enables dual perfusion (via the portal vein and the hepatic artery) using two centrifugal pumps, that provide a continuous portal flow and a pulsatile arterial flow. The system is pressure-controlled, allowing auto-regulation of the flow through the liver, depending on the intrahepatic resistance. Two hollow fiber membrane oxygenators allow for the oxygenation of the liver graft, as well as for the removal of CO2. The temperature can be set based on the intended type of MP (minimum temperature of 10 °C). Flow, pressure and temperature are displayed on the device in real-time allowing a continuous control of the perfusion process. A new sterile disposable set of tubing, reservoir and oxygenators is available for the perfusion of each graft (Figure 1).
The aim of this video article is to provide a step by step protocol for ex situ normothermic machine perfusion of human donor livers using this newly developed liver perfusion machine.
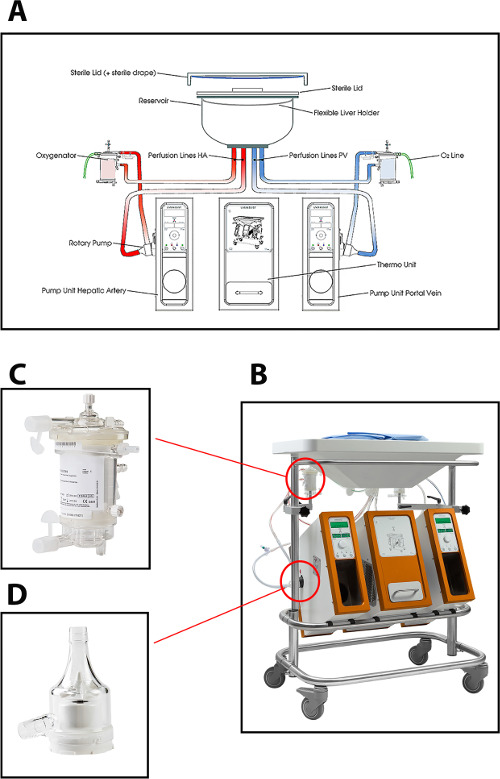
Figure 1: (A) A schematic drawing, (B) a photo of the perfusion machine, (C) a closer view of the oxygenator, and (D) centrifugal pump used for normothermic perfusion of human donor livers. Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
This video provides a step by step protocol for normothermic machine perfusion of human donor livers using a device that enables pressure controlled dual perfusion through the hepatic artery and portal vein. While following this protocol, technical failures of the perfusion machine did not occur and all grafts were well perfused and well oxygenated. The ex situ perfused livers had stable hemodynamics and were metabolically active, as defined by the production of bile16,17.
…
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research work was financially supported by grants provided by Innovatief Actieprogramma Groningen (IAG-3), Jan Kornelis de Cock Stichting and Tekke Huizingafonds, all in the Netherlands. We are appreciative to all the Dutch transplantation coordinators for identifying the potential discarded livers and obtaining informed consent.
Materials
| Liver Assist | Organ Assist | OA.Li.Li.140 | Perfusion device |
| Liver Assist disposable package | Organ Assist | OA.Li.DP.540 | Disposable set and cannulas |
| Meredith No.8 | Vygon Nederlands B.V. | 1362082 | Bile duct cannula |
| Human albumin 200g/l / ALBUMAN | Sanquin | 15522598 | 100 ml |
| Modified parenteral nutrition | Baxter Nederland B.V. | N14G30E | 7.35 ml |
| Multivitamins for infusion / CERNEVIT | Baxter International Inc. | 9800927 | 7 ul |
| Concentrated trace elements for infusion / NUTRITRACE | B. Braun Melsungen AG | 14811332 | 7.35 ml |
| Metronidazole 5mg/ml | Baxter Nederland B.V. | 98181882 | 40 ml |
| Cefazoline / SERVAZOLIN | Sandoz B.V. | 15611337 | 2 ml |
| Fast acting insulin | various vendors | 20 ml | |
| Calcium glubionate, intravenous solution 10%, 137.5 mg/mL | Sandoz | 97038695 | 40 ml |
| Sterile H2O | Fresenius Kabi Nederland B.V. | 98084453 | 51.3 ml |
| NaCl 0.9% | Baxter Nederland B.V. | 15262510 | 160 ml |
| Heparin 5000 IE/ml for i.v. administration | LEO Pharma B.V. | 98026178 | 4 ml |
| Sodium bicarbonate 8,4% | B. Braun Melsungen AG | 97973874 | The amount depends on the pH |
| Packed red blood cell (in SAGM) | Blood bank (Sanquin) | N0012000 | 750 ml |
| Fresh frozen plasma | Blood bank (Sanquin) | N04030A0/N04030B0 | 900 ml |
References
- Plaats, A., et al. Hypothermic machine preservation in liver transplantation revisited: Concepts and criteria in the new millennium. Ann. Biomed. Eng. 32 (4), 623-631 (2004).
- op den Dries, S., Sutton, M. E., Lisman, Y., Porte, R. J. Protection of bile ducts in liver transplantation: Looking beyond ischemia. Transplantation. 92 (4), 373-379 (2011).
- Seehofer, D., Eurich, D., Veltzke-Schlieker, W., Neuhaus, P. Biliary complications after liver transplantation: Old problems and new challenges. Am. J. Transplant. 13 (2), 253-265 (2013).
- Buis, C. I., et al. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver. Transpl. 13 (5), 708-718 (2007).
- Karimian, N., op den Dries, S., Porte, R. J. The origin of biliary strictures after liver transplantation: Is it the amount of epithelial injury or insufficient regeneration that counts. J. Hepatol. 58 (6), 1065-1067 (2013).
- Gastaca, M. Biliary complications after orthotopic liver transplantation: A review of incidence and risk factors. Transplant. Proc. 44 (6), 1545-1549 (2012).
- Sanchez-Urdazpal, L., et al. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 16 (1), 49-53 (1992).
- Dubbeld, J., et al. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br. J. Surg. 97 (5), 744-753 (2010).
- Guarrera, J. V., et al. Hypothermic machine preservation in human liver transplantation: The first clinical series. Am. J. Transplant. 10 (2), 372-381 (2010).
- Henry, S. D., et al. Hypothermic machine preservation reduces molecular markers of ischemia/reperfusion injury in human liver transplantation. Am. J. Transplant. 12 (9), 2477-2486 (2012).
- Schlegel, A., Rougemont, O., Graf, R., Clavien, P. A., Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 58 (2), 278-286 (2013).
- op den Dries, S., et al. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS. One. 9 (2), e88521 (2014).
- Tolboom, H., et al. Subnormothermic machine perfusion at both 20 degrees C and 30 degrees C recovers ischemic rat livers for successful transplantation. J. Surg. Res. 175 (1), 149-156 (2012).
- Gringeri, E., et al. Subnormothermic machine perfusion for non-heart-beating donor liver grafts preservation in a swine model: A new strategy to increase the donor pool? Transplant. Proc. 44 (7), 2026-2028 (2012).
- Bruinsma, B. G., et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am. J. Transplant. 14 (6), 1400-1409 (2014).
- op den Dries, S., et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am. J. Transplant. 13 (5), 1327-1335 (2013).
- Sutton, M. E., et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS. One. 9 (11), (2014).
- Minor, T., Efferz, P., Fox, M., Wohlschlaeger, J., Luer, B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am. J. Transplant. 13 (6), 1450-1460 (2013).
- Makowka, L., et al. Surgical technique of orthotopic liver transplantation. Gastroenterol. Clin. North. Am. 17 (1), 33-51 (1988).
- Hansen, T., et al. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation–a morphological clue to ischemic-type biliary lesion?. Virchows Arch. 461 (1), 41-48 (2012).
- Monbaliu, D., Brassil, J. Machine perfusion of the liver: Past, present and future. Curr. Opin. Organ. Transplant. 15 (2), 160-166 (2010).
- Oldani, G., et al. Pre-retrieval reperfusion decreases cancer recurrence after rat ischemic liver graft transplantation. J. Hepatol. 61 (2), 278-285 (2014).
- Tromans, D. Temperature and pressure dependent solubility of oxygen in water: A thermodynamic analysis. Hydrometallurgy. 48 (3), 327-342 (1998).

