Intracellular Staining and Flow Cytometry to Identify Lymphocyte Subsets within Murine Aorta, Kidney and Lymph Nodes in a Model of Hypertension
Summary
This article provides detailed methodology to identify and quantify functional T lymphocyte subsets present within murine kidney, aorta and lymph nodes by intracellular staining and flow cytometry. The model of angiotensin II induced hypertension was chosen to explain, step-by-step, the procedures and fundamental principles of flow cytometry and intracellular staining.
Abstract
It is now well known that T lymphocytes play a critical role in the development of several cardiovascular diseases1,2,3,4,5. For example, studies from our group have shown that hypertension is associated with an excessive accumulation of T cells in the vessels and kidney during the development of experimental hypertension6. Once in these tissues, T cells produce several cytokines that affect both vascular and renal function leading to vasoconstriction and sodium and water retention1,2. To fully understand how T cells cause cardiovascular and renal diseases, it is important to be able to identify and quantify the specific T cell subsets present in these tissues. T cell subsets are defined by a combination of surface markers, the cytokines they secrete, and the transcription factors they express. The complexity of the T cell population makes flow cytometry and intracellular staining an invaluable technique to dissect the phenotypes of the lymphocytes present in tissues. Here, we provide a detailed protocol to identify the surface and intracellular markers (cytokines and transcription factors) in T cells isolated from murine kidney, aorta and aortic draining lymph nodes in a model of angiotensin II induced hypertension. The following steps are described in detail: isolation of the tissues, generation of the single cell suspensions, ex vivo stimulation, fixation, permeabilization and staining. In addition, several fundamental principles of flow cytometric analyses including choosing the proper controls and appropriate gating strategies are discussed.
Introduction
Recent evidence demonstrates that the adaptive immune system, particularly T lymphocytes, play a critical role in the development of several cardiovascular diseases1,2,3,4,5. For example, in the model of angiotensin II induced hypertension, an accumulation of T cells in the vessels and kidneys of mice has been described6. The vascular accumulation is predominantly in the adventitia and the perivascular fat. In the kidney, T cells accumulate in both the medulla and renal cortex. Depending on which subset is involved, these T cells give rise to different cytokines that can affect vascular and renal function and lead to the development of pathology (reviewed by McMaster et al.6).
CD4+ T helper lymphocytes can be divided into several subsets: T helper 1 (Th1), Th2, Th9, Th17, Th22, T regulatory (Treg) cells, and T follicular helper (Tfh) cells based on their functions and signature cytokines7. Similarly, CD8+ cytotoxic T cells can be classified as Tc1, Tc2, Tc17 or Tc98. There are also double negative T cells (i.e. cells that do not express the CD4 or CD8 T cell markers). A subset of these cells possess an alternate gamma delta T cell receptor (instead of the classical alpha and beta receptors) and are therefore referred to as gamma delta T cells. The multi-parameter analysis by flow cytometry of surface marker, cytokine and transcription factor constitutes the best approach to identify these cells. Although this method is extensively used in the field of immunology, it is less well described in solid organs and in the setting of cardiovascular diseases.
Historically, the identification of lymphocytes in tissues was limited to immunohistochemistry or RT-PCR approaches. Although immunohistochemistry and immunofluorescence are powerful methods to determine the tissue distribution of an antigen of interest, they are inadequate to phenotypically identify the subsets involved. In addition, while RT-PCR analysis is useful to detect mRNA expression of antigens, cytokines or transcription factors, it doesn't allow the detection of multiple proteins simultaneously at the level of individual cells.
The advent of flow cytometry, especially when combined with intracellular staining to detect cytokines and transcription factors, provides investigators with a powerful technique that allows identification and quantification at the single cell level of immune cell subsets in solid organs. We have optimized an intracellular staining assay to identify by flow cytometry the major T cell subsets present within murine kidney, aorta and aortic draining lymph nodes in a model of angiotensin II induced hypertension. The optimization of each step: tissue digestion, ex vivo activation, permeabilization, and surface and intracellular staining results in a highly reproducible assay that can be applied to other cardiovascular and renal disease models.
Protocol
Vanderbilt University's Institutional Animal Care and Use Committee has approved the procedures described herein. Mice are housed and cared for in accordance with the Guide for the Care and Use of Laboratory Animals (National Academies Press. Revised 2010).
1. Isolation of the Aortic Draining Lymph Nodes, Kidney and Aorta from Mice
- Euthanize the mice by CO2 inhalation. Spray the chest with 70% ethanol and carefully open the skin and the chest wall with scissors to expose the heart.
- To perfuse the vasculature, perform a small incision in the right atrium and steadily inject at least 10 ml of cold PBS (approximately 1 ml/sec) into the apex of the left ventricle using a 21 or 23 G needle. Perfuse until all organs have blanched. The heart blanches first. Blanching of the liver indicates that the perfusion has been well performed.
- Using small forceps and fine scissors, gently cut away and pull out the intestines, stomach, spleen, pancreas and liver to better visualize the aorta.
NOTE: This step has to be done very precisely as damage to the gastrointestinal tract can induce contamination. - Cut out and remove each lung with scissors. Rinse the chest cavity with phosphate buffered saline (PBS) using a needleless syringe. Remove excess blood and fluid using sterile gauze.
- Remove the abdominal aortic draining lymph nodes using fine dissecting forceps. Make sure not to rupture the capsule. Remove the remaining fat on the surface of each lymph node with the dissecting forceps. Cut out the two kidneys with fine scissors.
- Dissect the entire aorta (thoracic and abdominal aorta) with fine, curved scissors. Start at the level of the heart and carefully dissect away from the esophagus and the vertebrae down to the level of the iliac bifurcation making sure to keep the surrounding perivascular fat attached to the aorta.
NOTE: Further dissection of the aorta to remove surrounding structures can be done under a microscope. Specifically, remove arterial branches (carotid arteries, subclavian arteries, celiac, mesenteric, and renal arteries) and lymph nodes. Do keep a consistent layer of perivascular fat around each aorta since this is a site where many inflammatory cells reside in the setting of hypertension. Place each tissue in separate tubes containing cold PBS.
2. Generation of Single Cell Suspensions from Each Tissue
- The kidneys
NOTE: The kidney can be dissociated into a single cell suspension by combining mechanical dissociation plus enzymatic digestion.- Prepare the kidney digestion solution by adding collagenase D (2 mg/ml) and DNase I (100 µg/ml) to RPMI 1640 medium (supplemented with 5% fetal bovine serum (FBS)). Prepare 10 ml per kidney sample.
- Use forceps to transfer the two kidneys into a dissociation tube containing 10 ml of kidney digestion solution.
- Perform the mechanical dissociation using a semi-automated dissociator device according to the manufacturer's instructions.
- After mechanical dissociation, detach the tube from the device and perform the enzymatic digestion. Incubate the samples for 20 min at 37 ºC under continuous rotation. Immediately stop the enzymatic digestion by adding cold RPMI 1640 medium supplemented with 5% FBS.
- Transfer the solution by pipetting into a 50 ml conical tube after filtering through a 40 µm strainer. Tease the remaining tissue apart by pressing with the plunger of a 1 ml syringe. Pellet the cells (500 x g, 8 min, 4 ºC).
- Resuspend the pellet into 3 ml of 36% density gradient centrifugation media. Transfer the suspension into a 15 ml conical tube. Gently add 3 ml of 72% density gradient centrifugation media beneath the 3 ml 36% cell suspension without any disruption. Centrifuge (1,000 x g without brake, 20 min, 4 °C).
- The immune cells will be located at the interface. Remove the topmost yellowish layer containing cellular debris and then bring the volume up to 15 ml using cold PBS. Shake well to breakdown the gradient. Spin the cells (300 x g, 8 min, 4 °C) and resuspend the pellet in 3 ml of RPMI with 5% FBS. Count the number of live cells on a hemocytometer using Trypan blue exclusion.
- The aorta
- Prepare the aorta digestion solution by adding collagenase A (1 mg/ml), collagenase B (1 mg/ml), and DNase I (100 µg/ml) to RPMI 1640 medium (supplemented with 5% FBS).
- Using fine forceps, transfer the aorta into a 2 ml tube containing 1 ml of aorta digestion solution. Mince the whole aorta into very small pieces with fine scissors in the 1 ml digestion solution. Keep on ice. Incubate the samples for 30 min at 37 °C under continuous rotation.
- Transfer the solution to a tissue culture dish and stop the enzymatic digestion by adding 5 times the volume of cold RPMI with 5% FBS.
- Transfer the solution by pipetting into a 50 ml conical tube after filtering through a 40 µm strainer. Tease the remaining tissue apart into a single cell suspension by pressing with the plunger of a 1 ml syringe. Flush the strainer and pellet the cells (300 x g, 8 min, 4 ºC).
- Wash the pellet by adding 10 ml of RPMI with 5% FBS. Spin the cells (300 x g, 8 min, 4 °C) and resuspend the pellet in 3 ml of RPMI with 5% FBS. Count the number of live cells on a hemocytometer using Trypan blue exclusion.
- The aortic draining lymph nodes
- Place a 40 µm strainer in a tissue culture dish (60 mm x 15 mm). Place the lymph nodes on the strainer and tease them apart into a single cell suspension by pressing with the plunger of a 1 ml syringe. Flush the strainer with 2 ml of RPMI with 5% FBS. Collect the cells in the dish.
- Transfer the cells into a 15 ml falcon tube and pellet the cells (300 x g, 8 min, 4 ºC). Resuspend the pellet in 500 µl of RPMI with 5% FBS. Count the number of live cells on a hemocytometer using Trypan blue exclusion.
3. Ex Vivo Stimulation for Cytokine Detection by Flow Cytometry
- Prepare the cell culture media: RPMI with 5% FBS, 0.1 mM of non-essential amino acids, 50 µM β-mercaptoethanol and 1% penicillin/streptomycin.
- Centrifuge each cell suspension obtained from the kidney, aorta and lymph nodes (300 x g, 8 min, 4 ºC). Resuspend each pellet with the cell culture media at a concentration of 1 x 106 cells per ml.
- Stimulate cells by adding 2 µl of the cell activation cocktail (containing Phorbol 12-Myristate 13-Acetate (PMA), the calcium ionophore ionomycin, and the Golgi inhibitor Brefeldin A) for every 1 ml of cell culture.
NOTE: The final concentrations of each component are: 81 nM of PMA, 1.33 µM of ionomycin and 5 µg/ml of Brefeldin A. Treatment with PMA and ionomycin activates cells to produce cytokines. Cytokines are secreted proteins that must be trapped in the cells to be detected. Brefeldin A causes the accumulation of normally secreted proteins in the endoplasmic reticulum and Golgi apparatus. Thus, the technique described here enhances the detectability of cytokine-producing lymphocytes by flow cytometry. - Plate 1 to 2 million cells in a 12-well (aorta or kidney) or 24-well (lymph node) low-adherence plates. Place the plate in a 37 °C humidified CO2 incubator for 3 hr.
NOTE: The number of plated cells may be lower in lymph nodes from control mice. Also, the time of stimulation has to be empirically determined for each cell population and cytokine studied. In the case of T cells isolated from aorta, kidney or lymph nodes, we recommend stimulating for 3 to 4 hr. A longer stimulation will not increase the cytokine secretion and will induce a further downregulation of several T cell surface markers like CD3, CD4 and CD8. (Note that even a 3-4 hr stimulation will induce some downregulation of these markers.)
4. Surface Staining
- Transfer the cells into a polystyrene FACS tube and centrifuge (300 x g, 8 min, 4 °C). Wash the cells twice with FACS buffer (Ca2+ and Mg+ free PBS containing 4% FBS and 2mM EDTA) and divide the cell suspension equally into different FACS tubes based on the number of antibody panels desired and the number of control tubes needed (see below).
- To perform compensation for each panel, prepare unstained control tubes and single stained tubes for each tissue type and antibody panel. In addition, prepare fluorescence minus one (FMO) control tubes for each antibody panel by adding all but one of the antibodies.
- Adjust the volume of each tube to 2 ml with FACS Buffer. Centrifuge the cell suspension (300 x g, 8 min, 4 °C), remove the supernatant and block Fc receptors by incubating the cells with 0.5 µg of anti-CD16/CD32 antibodies per million cells for 10 min on ice.
NOTE: CD16 is low affinity IgG Fc receptor III (FcR III) and CD32 is FcR II. CD16/CD32 are expressed on granulocytes, mast cells, monocytes/macrophages, natural killer cells, B cells, dendritic cells and some activated mature T cells. - Perform viability staining: Wash the cells with 1x PBS, centrifuge (300 x g, 8 min, 4 °C) and resuspend in 1,000 µl of 1x PBS. Add 1 µl of a cell-impermeant amine-reactive dye (viability stain). Incubate for 15 min at 4 °C protected from light.
- During the incubation, prepare the cocktail of antibodies (for surface staining) in an appropriate volume of FACS buffer. Wash the cells twice with 1-2 ml of FACS buffer, centrifuge (300 x g, 8 min, 4 °C) and resuspend each pellet with 100 µl of the antibody mixture. Incubate for 30 min at 4 °C protected from light.
NOTE: For each flow cytometry antibody, it is useful to determine the optimal amount of antibody needed by performing a dose titration curve. - Wash the cells twice with 2 ml of FACS buffer and pellet the cells (300 x g, 8 min, 4 °C).
5. Fixation, Permeabilization and Intracellular Staining
- Perform the intracellular staining with a fixation and permeabilization kit containing appropriate buffers by following the manufacturer's instructions. Fix and permeabilize the cells by resuspending them in the appropriate buffer. Incubate samples for 40 min at 4 °C protected from light.
- Add 1 ml of 1x permeabilization and wash buffer directly to the fixed and permeabilized cells. Centrifuge (500 x g, 8 min, 4 °C) and remove the supernatant. Resuspend the pellet with 2 ml of 1x wash buffer.
- Prepare the mixtures of antibodies (for intracellular staining only) in an appropriate volume of 1x wash buffer (usually 100 µl per tube).
NOTE: As described above, the optimal antibody concentration needs to be determined for each antibody in the panel. For example, for antibodies to IL-17A or IL-17F, we use a dilution factor of 1:30, but for antibodies to IFNγ and T-bet, we use a dilution factor of 1:100. - Centrifuge the cells (500 x g, 8 min, 4 °C) and resuspend each pellet with 100 µl of the antibody mixture. Incubate for 40 min at 4 °C protected from light.
- Wash the cells twice with 2 ml of 1x wash buffer and pellet the cells (500 x g, 8 min, 4 °C). Resuspend the pellet with 300 µl of 1x wash buffer and analyze on a flow cytometer with appropriate filters.
- For quantification of the total number of cells per tissue sample, use counting beads. Prior to acquisition, add the recommended volume/number of counting beads to each tube.
6. Compensation, Gating, Normalization, and Tips
- Compensation and gating
- Run unstained and single stained control tubes for compensation to adjust for spectral overlap.
NOTE: The use of compensation beads rather than cell-based compensation controls is necessary in cases where the antigen of interest or cell population in a sample is present at such low levels that compensation using cells will be difficult. Also of note, when using tandem dyes, it is advisable to prepare compensations for each experiment since tandem dyes vary lot-to-lot and with each day. - Run fluorescence minus one controls (FMOs) for all the fluorophores in the panel when starting a new multicolor experiment.
NOTE: The FMO controls are samples that contain all the antibodies in the panel except for one. These controls permit accurate discrimination of positive versus negative signals for the omitted antibody to properly adjust the gates. These controls are particularly important when the expression levels are low or when the separation of the target population is poor (for example, when the target is a cytokine or transcription factor). Although not always necessary, in some cases, isotype controls may be preferred in place of FMO controls as they provide proper compensation for the antibody isotype and fluorophore conjugate without the specificity of the antigen. - Set up the primary gate: adjust Forward Scatter Area (FSC-A) and Side Scatter Area (SSC-A) voltage in order to detect the leukocyte population.
- Exclude the dead cells.
NOTE: The viability stain reacts with free amines present on both the surface and the interior of cells. In contrast to live cells, dead cells (with compromised membranes) allow the dye to enter into the cytoplasm, increasing the amount of protein labeling. Thus, dead cells will be brighter than live cells allowing easy discrimination. Gate on the live cells.
NOTE: The viability of the cell suspensions from the aorta and the kidney may be lower than the viability of the cell suspensions from the lymph nodes due to the additional digestion step. - Plot Forward Scatter Area (FSC-A) [y-axis] and Forward Scatter Height (FSC-H) [x-axis]. Singlets appear as a diagonal on this dot plot. Gate on this diagonal to exclude doublets. Then plot SSC-A [y-axis] and CD45 [x-axis].
- The CD45+ leukocyte population can be easily identified. Gate on this population. Subsequent populations can be identified from this population.
- Run unstained and single stained control tubes for compensation to adjust for spectral overlap.
- Normalization of cell counts
- Counting beads have characteristics that result in low FSC and high SSC. For example, if 50,000 beads are added into 300 µl of the single cell suspension, the equation for the calculation of the absolute number of any give cell type per tube is as follows:
Number of cells per tube = (50,000 / number of beads counted by the flow cytometer) x number of cells counted.
Using this calculation, the absolute number of any given cell type per tube can be determined. Based on how many tubes were generated from each organ, the number of cells per organ can be calculated.
NOTE: Keep all cells and reagents at 4 °C during the protocol. Avoid vigorous vortexing because it can damage cells. Use minimal forces to pellet the cells. Avoid making bubbles in the FACS tube since it can precipitate cell death. Don't let the pellets dry out at any time.
- Counting beads have characteristics that result in low FSC and high SSC. For example, if 50,000 beads are added into 300 µl of the single cell suspension, the equation for the calculation of the absolute number of any give cell type per tube is as follows:
Representative Results
The protocol described permits the identification of surface and intracellular markers in T cells isolated from murine kidney, aorta and aortic draining lymph nodes in a model of angiotensin II induced hypertension. Representative results are presented below.
Figure 1 demonstrates the gating strategy used to identify the T cell population in a single cell suspension prepared from the aorta of a WT mouse infused with angiotensin II to induce hypertension. A similar strategy is used in the kidney and lymph nodes. The Forward Scatter Area (FSC-A) and Side Scatter Area (SSC-A) voltage was adjusted to detect the leukocyte population (G1) and to exclude debris. The live cells (G2) are negative for the viability marker conjugated with Pacific blue. Live cells were then gated on Forward Scatter Height (FSC-H) and Forward Scatter Area (FSC-A) to gate only on the singlet population (G3). Leukocytes were then selected by gating on SSC-A and CD45 (G4). CD45 is conjugated with AmCyan in this example. Finally, T cells are gated as the CD45+CD3+ population (G5). CD3 is conjugated with PerCP-Cy5.5 in this example.
To determine the presence of IL-17A and IL-17F producing T cells within murine kidney and aorta in this model, single cell suspensions were stained for IL-17A and IL-17F. Figure 2 illustrates fluorescence minus one controls (FMOs) for IL-17A and IL-17F staining in a kidney sample (conjugated with FITC and APC, respectively). FMO controls are samples that contain all the antibodies in a panel except for one. As expected, very few cells are positive for IL-17A (Figure 2A) or IL-17F (Figure 2B) in the FMO controls. These controls permit accurate discrimination of positive versus negative signals to properly adjust the gates to identify the cells positive for IL-17A or IL-17F in the experimental samples.
Figure 3 provides an example of intracellular staining of T cells isolated from kidney (Figure 3A) and aorta (Figure 3B) of WT mice infused with vehicle (Sham) or angiotensin II (Ang II). Indeed, a subset of T cells that express IL-17A or IL-17F can be detected in both tissues in this model.
Figure 4 illustrates intracellular staining of T cells within the aortic draining lymph nodes in this model (Figure 4A). CD8+ T cells were first gated (Figure 4B). Then, the expression of two Tc1 markers, the cytokine IFNγ or the transcription factor T-bet, were quantified from the CD8+ T cell subset. Figure 4C illustrates the FMOs for IFNγ and T-bet staining in the lymph node samples (conjugated with FITC and PE-Cy7, respectively). Figure 4D shows that in the aortic draining lymph nodes of WT mice infused with angiotensin II, a population of CD8+IFNγ+ and CD8+T-bet+ cells can be identified.
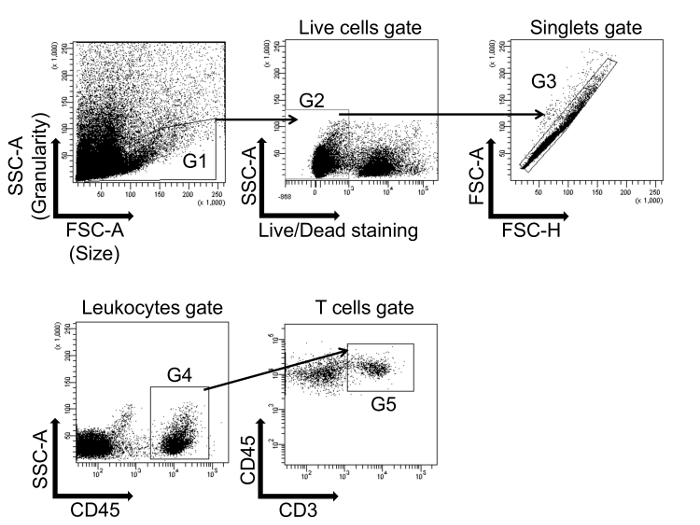
Figure 1: Flow cytometry gating strategy to identify T cells. Leukocytes are first gated on a forward scatter/side scatter (FSC-A/SSC-A) dot plot (G1), and live cells are selected (G2). The cells are then gated on singlets (FSC-A/FSC-H) (G3). Cells from G3 are further characterized by the expression of CD45 (G4). Finally, T cells are gated on CD45+CD3+ double positive cells (G5). This single cell suspension was prepared from the whole aorta isolated from a wild type (WT) mouse infused with angiotensin II to induce hypertension. Please click here to view a larger version of this figure.
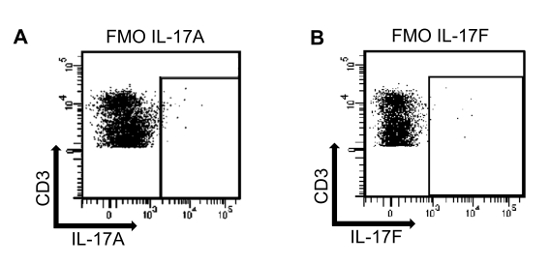
Figure 2: Fluorescence minus one controls (FMO) for IL-17A and IL-17F. (A) A single cell suspension isolated from an angiotensin II treated murine kidney sample was stained using a fixable viability marker (Pacific Blue), CD45 (AmCyan), CD3 (PerCP-Cy5.5) and IL-17F (APC). The antibody for IL-17A was omitted to determine the proper gating for IL-17A. (B) A single cell suspension isolated from an angiotensin II treated murine kidney sample was stained using a fixable viability marker (Pacific Blue), CD45 (AmCyan), CD3 (PerCP-Cy5.5) and IL-17A (FITC). In this case, the antibody for IL-17F was omitted. Please click here to view a larger version of this figure.
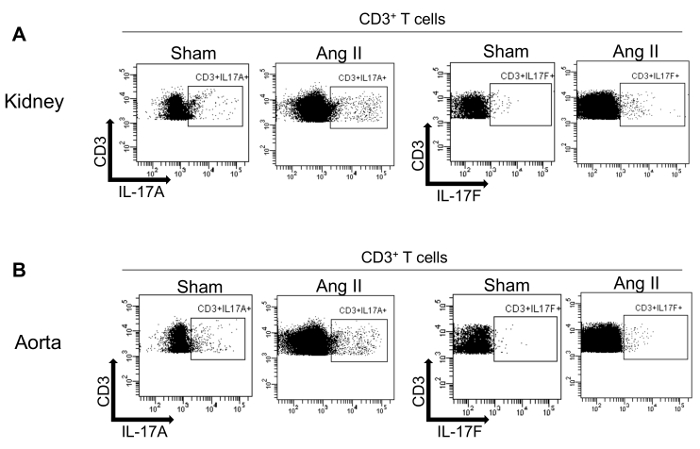
Figure 3: Intracellular staining of T cells isolated from murine kidney and aorta for IL-17A and IL-17F. Flow cytometry dot plots showing IL-17A and IL-17F expression in T cells from kidney (A) and aorta (B) from WT mice infused with vehicle (Sham) or angiotensin II (Ang II) and previously gated on CD45+CD3+ cells. Please click here to view a larger version of this figure.
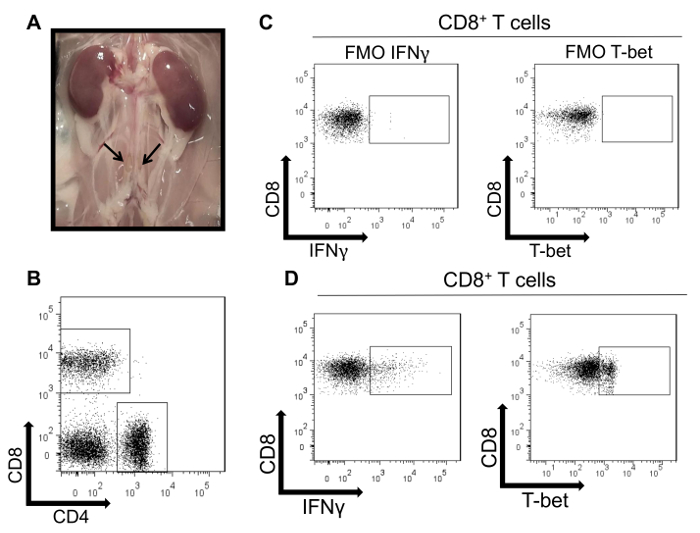
Figure 4: Intracellular staining of T cells isolated from murine aortic draining lymph nodes. (A) Macroscopic appearances of two lumbar aortic draining lymph nodes in a WT mouse. (B) Representative flow cytometry dot plots of CD4+ and CD8+ T cells isolated from four aortic draining lymph nodes isolated from a WT mouse infused with angiotensin II and previously gated on CD45+CD3+ cells. (C) A single cell suspension isolated from lymph nodes was stained using a viability marker (Pacific Blue), CD3 (PerCP-Cy5.5), CD4 (APC-Cy7), and CD8 (APC). FMO controls are shown in which the antibody for IFNγ (FITC) or T-bet (PE-Cy7) was omitted to determine the proper gating. (D) Flow cytometry dot plots demonstrating positive IFNγ and T-bet expression within CD8+ lymph node T cells using the gates determined by the FMO controls. Please click here to view a larger version of this figure.
Discussion
The protocol described herein has been optimized to properly identify T cell subsets present within murine kidneys, aorta and lymph nodes. This protocol can be easily adapted to examine other immune cell subsets such as B lymphocytes and innate immune cells and can be modified to include other tissue types. The digestion step is critical and has to be modified and optimized for each tissue9. A prolonged digestion step or the use of an inappropriate enzyme can affect the stability of antigen expression. Similarly, an incomplete digestion can affect the results leading to a lower number of positive cells. Furthermore, the time of ex vivo stimulation needs to be carefully adjusted for each cell type/cytokine to allow for optimal stimulation with minimal cell toxicity.
Although intracellular staining coupled with flow cytometry analysis is one of the most powerful methods to analyze immune processes at a cellular level in blood and tissues, this technique has its limitations. These limitations include the relatively low number of immune cells in cardiovascular and renal tissues. Thus, depending on the cell type examined, it may be necessary to pool organs from several mice to obtain one sample. Furthermore, the instability of markers is another limitation as the expression of markers can be affected by several steps during the process. For this reason, it is advisable to perform histology in parallel to confirm the results obtained by flow cytometry. There are also special considerations regarding the use of the flow cytometer and the interpretation of the results. The flow cytometer is a sophisticated instrument and requires highly trained operators. Importantly, despite the use of compensation controls, there is still an issue of spectral overlap that limits experiments to 8-12 markers (usually 8) per sample.
Moreover, this technique is very sensitive and subject to several experimental manipulations which can lead to slight day-to-day variation in the quantification of results. Thus, if performing this technique across multiple days for a given study, it is important to include control and experimental groups each day to determine relative changes. Finally, another limitation/source of variation is the choice of the markers used to identify a population10. Each study tends to use different combinations of markers and fluorochromes to define a population, which can lead to slight differences in quantification.
Despite these limitations, this is a powerful experimental technique that allows the identification and quantification of T cell subsets in solid organs. In fact, while other techniques measure either the expression of surface markers, cytokines or transcription factors separately, this method permits the measurement of several markers simultaneously at the single cell level. Furthermore, once the cells are identified, several other parameters can then be determined11,12,13,14. For example, one can evaluate for markers of apoptosis, protein phosphorylation or the proliferation of a specific T cell subset15. In addition, there are newer proprietary methods to detect mRNAs in cells using flow cytometry16,17.
In conclusion, we have outlined a detailed and reproducible protocol to identify T cell subsets using surface and intracellular markers (cytokines and transcription factors) in murine kidney, aorta and aortic draining lymph nodes in a model of angiotensin II induced hypertension. This protocol can be applied to other tissues (with slight modification) and to other models of disease.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by an American Heart Association Fellowship Award (16POST29950007) to FL, a training grant from the National Institutes of Health (NIH T32 HL069765) to BLD, an American Heart Association Fellowship Award (14POST20420025) to MA Saleh, and an NIH K08 award (HL121671) to MSM. MSM is also supported by a research grant from Gilead Sciences, Inc.
Materials
| Collagenase D | ROCHE | 11088882001 | |
| Collagenase A | ROCHE | 10103586001 | |
| Collagenase B | ROCHE | 11088815001 | |
| Dnase | ROCHE | 10104159001 | |
| 1X Red blood cell lysis buffer | eBioscience | 00-4333-57 | |
| RPMI Medium 1614 1X | Gibco | 11835-030 | |
| DPBS without calcium and magnesium | Gibco | 14190-144 | |
| Percoll | GE Healthcare | 17-5445-02 | For density gradient centrifugation |
| GentleMACS ™ C tube | Miltenyi Biotec | 130-096-334 | |
| GentleMACS dissociator device | Miltenyi Biotec | 130-093-235 | Use the program SPLEEN_04 |
| Cell activation cocktail (with Brefeldin A) | Biolegend | 423303 | |
| anti-CD16/32 | eBioscience | 14-0161-81 | dilute 1:100 |
| LIVE/DEAD fixable violet dead cell stain kit | Life Technologies | L34955 | |
| Transcription factor buffer set | BD Pharmingen | 562725 | |
| OneComp eBeads | eBioscience | 01-1111-42 | |
| 123 count eBeads | eBioscience | 01-1234-42 | |
| CD45 AmCyan (clone 30-F11) | BioLegend | 103138 | |
| CD3 PerCP-Cy5.5 (clone 17A2) | BioLegend | 100218 | |
| IL-17A FITC (clone TC11-18H10.1) | BioLegend | 506910 | |
| IL-17F APC (clone 9D3.1C8) | BioLegend | 517004 | |
| CD4 APC-Cy7 (clone GK1.5) | BD Biosciences | 560181 | |
| CD8 APC (clone 53-67) | eBioscience | 17-0081-82 | |
| T-bet PE-Cy7 (clone 4B10) | BioLegend | 644823 | |
| IFNγ FITC (clone XMG1.2) | BD Biosciences | 557724 |
References
- Saleh, M. A., McMaster, W. G., Wu, J., et al. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest. 125 (3), 1189-1202 (2015).
- Madhur, M. S., Lob, H. E., McCann, L. A., et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 55 (2), 500-507 (2010).
- Laroumanie, F., Douin-Echinard, V., Pozzo, J., et al. CD4+ T Cells Promote the Transition From Hypertrophy to Heart Failure During Chronic Pressure Overload. Circulation. 129 (21), 2111-2124 (2014).
- Guzik, T. J., Hoch, N. E., Brown, K. A., et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 204 (10), 2449-2460 (2007).
- Ketelhuth, D. F. J., Hansson, G. K. Adaptive Response of T and B Cells in Atherosclerosis. Circ Res. 118 (4), 668-678 (2016).
- McMaster, W. G., Kirabo, A., Madhur, M. S., Harrison, D. G. Inflammation, Immunity, and Hypertensive End-Organ Damage. Circ Res. (6), 1022-1033 (2015).
- Luckheeram, R. V., Zhou, R., Verma, A. D., et al. CD4+T Cells: Differentiation and Functions. Clin Dev Immunol. 2012, 1-12 (2012).
- Mittrücker, H. -. W., Visekruna, A., Huber, M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch Immunol Ther Exp (Warsz). 62 (6), 449-458 (2014).
- Autengruber, A., Gereke, M., Hansen, G., Hennig, C., Bruder, D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur J Microbiol Immunol (Bp). 2 (2), 112-120 (2012).
- Finak, G., Langweiler, M., Jaimes, M., et al. Standardizing Flow Cytometry Immunophenotyping Analysis from the Human ImmunoPhenotyping Consortium. Sci Rep. 6, 20686 (2016).
- Herzenberg, L. A., Parks, D., Sahaf, B., Perez, O., Roederer, M., Herzenberg, L. A. The history and future of the fluorescence activated cell sorter and flow cytometry: a view from Stanford. Clin Chem. 48 (10), 1819-1827 (2002).
- Hrvatin, S., Deng, F., O’Donnell, C. W., Gifford, D. K., Melton, D. A. MARIS: method for analyzing RNA following intracellular sorting. PLoS One. 9 (3), 89459 (2014).
- Kalisky, T., Quake, S. R. Single-cell genomics. Nat Methods. 8 (4), 311-314 (2011).
- Chattopadhyay, P. K., Roederer, M. Cytometry: Today’s technology and tomorrow’s horizons. Methods. 57 (3), 251-258 (2012).
- Jiang, L., Tixeira, R., Caruso, S., et al. Monitoring the progression of cell death and the disassembly of dying cells by flow cytometry. Nat Protoc. 11 (4), 655-663 (2016).
- Lemoine, S., Jaron, B., Tabka, S., et al. Dectin-1 activation unlocks IL12A expression and reveals the TH1 potency of neonatal dendritic cells. J Allergy Clin Immunol. 136 (5), 1355-1368 (2015).
- Gaublomme, J. T., Yosef, N., Lee, Y., et al. Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell. 163 (6), 1400-1412 (2015).

