Longitudinal Two-Photon Imaging of Dorsal Hippocampal CA1 in Live Mice
Summary
This method describes a chronic preparation that allows optical access to the hippocampus of living mice. This preparation can be used to perform longitudinal optical imaging of neuronal structural plasticity and activity-evoked cellular plasticity over a period of several weeks.
Abstract
Two-photon microscopy is a fundamental tool for neuroscience as it permits investigation of the brain of live animals at spatial scales ranging from subcellular to network levels and at temporal scales from milliseconds to weeks. In addition, two-photon imaging can be combined with a variety of behavioral tasks to explore the causal relationships between brain function and behavior. However, in mammals, limited penetration and scattering of light have limited two-photon intravital imaging mostly to superficial brain regions, thus precluding longitudinal investigation of deep-brain areas such as the hippocampus. The hippocampus is involved in spatial navigation and episodic memory and is a long-standing model used to study cellular as well as cognitive processes important for learning and recall, both in health and disease. Here, a preparation that enables chronic optical access to the dorsal hippocampus in living mice is detailed. This preparation can be combined with two-photon optical imaging at cellular and subcellular resolution in head fixed, anesthetized live mice over several weeks. These techniques enable repeated imaging of neuronal structure or activity-evoked plasticity in tens to hundreds of neurons in the dorsal hippocampal CA1. Furthermore, this chronic preparation can be used in combination with other techniques such as micro-endoscopy, head-mounted wide field microscopy or three-photon microscopy, thus greatly expanding the toolbox to study cellular and network processes involved in learning and memory.
Introduction
In mammals, the hippocampus is a key brain region for the encoding and recall of episodic memories as well as for spatial navigation1,2,3,4. For this reason, the hippocampus has been – and still is – a very important model to study the basic mechanisms that allow the brain to encode and recall memories5,6,7 or to navigate in an environment8,9 collecting rewards and avoiding dangers. In addition, the hippocampal formation is one of the brain regions where new neurons are generated throughout the life of rodents10,11 and, possibly, of humans12,13. Finally, degeneration or impairment of the hippocampal formation are associated with neurological and psychiatric disorders, including Alzheimer’s disease14.
In mice, the hippocampus is located approximately 1 mm below the brain surface15. Its position has prevented optic access in the intact brain and consequently, longitudinal studies of hippocampal dynamics have relied mostly on magnetic resonance (MR) imaging, electrophysiology, and ex vivo imaging analyses. MR imaging methods allow tracking of biological processes (e.g., gene expression changes16) in the same animal over multiple days, but lack the spatial resolution to discriminate single neurons. Classic in vivo electrophysiological techniques offer very high temporal resolution and exquisite sensitivity to changes in membrane potential. However, they have a limited spatial resolution and they lack the ability to reliably track the same cells over longer time periods. Optical imaging allows more diverse processes to be studied by virtue of its high temporal and spatial resolutions. However, ex vivo imaging only provides snapshots of ongoing processes, and thus it is not suitable for longitudinal studies during which the animals learn and recall information.
In vivo optical imaging combines some advantages of MR imaging and electrophysiology with those of optical imaging. Therefore, it is very well suited for longitudinal and correlative analyses of mouse brain dynamics and behavior. This is relevant in studies of biological processes with very fast (milliseconds to seconds) or very slow (days to weeks) time scales. Examples for such processes that are relevant for neuroscience are membrane voltage dynamics, Ca2+ transients, cellular plasticity and structural changes, which are all believed to be very important for memory formation and recall. Different methods have extended in vivo imaging to the dorsal hippocampus18,19,20,21,22. Acute preparations have allowed the tracking of pyramidal neuron (PN) activity as well as their dendrites and dendritic spines for several hours20,22. This temporal timeframe, however, does not allow long-term structural changes, which might underlie incremental learning, to be studied. Chronic preparations – in combination with micro-endoscopes23,24 or with long working distance (WD) standard microscope objectives21 – have enabled repeated imaging of the dorsal hippocampus over several weeks.
Here, we describe a chronic preparation that provides recurrent optic access to the CA1 sub-field of the dorsal hippocampus of living mice using a permanently inserted imaging cannula. This preparation allows repeated access to the CA1 without functional disturbance and is suitable for intravital two-photon (2P) or wide-field epifluorescence imaging. Two examples of 2P deep brain chronic imaging in the dorsal CA1 of live mice are detailed: longitudinal imaging of dendritic structure and dendritic spine dynamics and longitudinal imaging of activity-evoked plasticity. The salient advantages and limitations of the technique are discussed.
Protocol
All of the methods described have been approved by the Government of Upper Bavaria (licence 2016_ROB-55.2Vet-2532.Vet_02-16-48) and by the Stanford and Max Planck Florida Institute for Neuroscience Administrative Panels on Laboratory Animal Care.
1. Preparation of the imaging cannula
- Hold a precision drill on a drill stand supplied with a movable ruler table.
- Clamp a 3.0 mm diameter stainless steel tube onto the movable ruler table.
- Cut the tube to a 1.6 mm-long metal ring. If the edges of the ring are not blunt after cutting, file out the irregularities.
- Rinse the metal ring and a circular 4 mm-diameter glass coverslip in 100% acetone and leave to dry for ≈5 min.
- Place the metal ring and the glass coverslip onto a smooth, even and clean work place, under a stereoscope.
- Place a drop of UV-curing optical adhesive on a smooth surface, such as a Petri dish. Use a needle or a spatula to spread out the adhesive to form a thin (<0.5 mm) layer.
- Use forceps to dip one side of the metal ring into the adhesive. Pay particular attention not to seal the inside of the ring. If this happens, use a needle to break the optical adhesive in the ring.
- Using the stereoscope, position the metal ring at the center of the glass coverslip with the side of the ring covered by adhesive touching the coverslip. A thin ring of adhesive should form at the interface between the metal ring and the coverslip. Avoid any adhesive spreading to the center of the coverslip.
- Turn on the UV-curing LED driver unit and shine light (365 nm) for 1 min.
CAUTION: UV light provokes skin burns and is a mutagenic agent. Wear UV-protecting glasses and cover hands and arms with gloves and a lab-coat to avoid skin exposure. - To cure the adhesive evenly, make sure all sides of the ring are equally illuminated by changing the direction of the light source.
- Let the adhesive harden for at least 2 h, preferably, overnight.
- Firmly hold the cannula from the open end of the metal ring by means of a hemostat. Using a dental drill fitted with a rotating file and working under the stereoscope, file off the excess glass coverslip until flush with the sides of the ring (Figure 1A).
2. Implantation of the imaging cannula over the dorsal hippocampus
- Preparation of equipment and setup
- Make sure all surgical instruments are clean and sterile. If using a glass bead sterilizer for sterilization, clean the instruments and place them in the sterilizer (set to 250 ˚C) for 10 min, prior to use. Alternatively, autoclave the instruments before use.
- Prepare all materials required for the surgery, as well as surgical instruments, so that they can be reached readily.
- Make sure there is enough freshly prepared medications such as pain killers or anti-inflammatory drugs.
- Make sure there is enough isoflurane for the whole duration of the surgery (approximately 1 h per surgery).
- Turn on the heating carpet and set it to 37 ˚C to keep animal temperature stable while under anesthesia.
- Make sure the scavenging system for isoflurane is functioning.
- Open the valve of oxygen flow.
- Anesthesia induction, and animal fixation
- Place the mouse in the anesthesia induction chamber at 3% isoflurane in 1 L/min O2 and wait until it loses consciousness. Assess anesthesia using the toe pinch reflex test.
- Weigh the animal.
- Apply anti-inflammatory (Meloxicam, 1mg/Kg) and pain killer (Vetalgin, 200mg/kg) drugs subcutaneously.
- Position the mouse on the heating carpet. Make sure that the animal is not in direct contact with the heating carpet to avoid thermal burns.
- Secure the head to the stereotactic apparatus and position the nose cone to cover the snout. To maintain anesthesia, decrease isoflurane to 1.5-2%. Throughout the surgery, monitor mouse state by visually monitoring breathing and by testing toe pinch reflex. Regulate isoflurane percentage if necessary.
- Apply ophthalmic ointment to the eyes to prevent dehydration and potential blindness.
NOTE: For anesthesia and animal medication, alternative methods and drugs are possible. Please, refer to your animal license and the relative literature.
- Optic cannula implantation
- Turn on fiber optic light source.
- Remove the hair and disinfect the skin over the mouse head.
- Using scissors and forceps, remove the mouse scalp. Start by making a small cut in the scalp in a position close to lambda. Continue by opening the scalp on the two sides. First move laterally in the direction of ears, then rostrally, in the direction of eye orbits, to form a triangle on the sagittal axis, approximately 4 mm rostral to bregma. Pay attention not to cut too close to ears and eyes, but expose bregma and lambda, as well as parietal bones and the posterior half of frontal bones.
- Apply one drop (≈10 mg) of lidocaine (28.9% v/v in alcohol) to the skull.
- Clear the periosteum and dry the skull using a cotton swab.
- Position the ear bars, to fix the mouse head.
- Make a small craniotomy, using a micro-drill with a 0.5 mm width burr. Position the hole into the frontal bone opposite to the imaged hippocampus, approximately 1.5 mm from the sagittal suture and 2 mm distant from the coronal suture.
- Screw a 0.86 mm-width stainless steel bone screw into the skull hole.
- If necessary, clean the debris and dry the skull.
- Preparation of a mixture of quick adhesive cement
- Using a small spoon (≈ 4.5 mm diameter), dispense 1-1.5 level scoops of L-powder into a mixing well.
- Dispense 3-4 drops of Quick Base into the well.
- Dispense 1 drop of Universal Catalyst into the well.
- Stir the mix for 5-10 s using a precision applicator.
NOTE: Alternative cements are available. Please refer to their manufacturer’s instructions.
- Use precision applicators to apply quick adhesive cement over the skull, screw and surrounding skin. Let it dry for 30 s to 1 min.
- Use a 3.0 mm diameter trephine drill to make a craniotomy in the parietal bone. Position the hole approximately 1.5 mm distant from the sagittal suture and 2 mm distant from the lambdoid suture.
- Carefully remove the bone flap.
- Check the size of the craniotomy using the cannula to make sure that it fits. Use a 0.5 mm or 0.9 mm width micro-drill to enlarge the craniotomy if necessary.
- Remove the meninges using Dumont forceps.
- Ablate cortical matter, to reach the external capsule. Use a 0.9 mm diameter (19 gauge) blunt needle connected to a vacuum pump. Irrigate with saline to avoid dehydration of the exposed tissue and to wash away residual blood after bleeding is resolved. Suck cortical tissue slowly, ≈50-100 µm at a time, until cortex detaches from the capsule, exposing the fibers of the cingulum or the corpus callosum. Change the needle frequently, to prevent clogging.
- Fibers extend mainly in three directions (Figure 1B). Carefully peel the dorsal fibers until the deepest fibers (alveus of the hippocampus) are exposed.
- Rinse the tissue with saline. Use thin forceps to dip the bottom cannula into saline and position it over the skull hole. Cannula and tissue have to be sealed by saline to avoid trapping air bubbles between the cannula and the tissue.
- Push the cannula into the skull (Figure 1C) until the glass coverslip is in contact with the fibers.
- Dry the skull well using absorption triangles, cotton swabs and/or the vacuum pump.
- Preparation of a mixture of quick adhesive cement (refer to step 2.3.10).
- Use precision applicators to apply quick adhesive cement over the skull. Apply adhesive also on the rim of the cannula. Be careful not to let the adhesive run into the cannula. Let it dry for 30 s to 1 min.
- Use a stereotaxic arm to position a head holder plate over the cannula, in contact with the skull.
- Preparation of a mixture of dental acrylic
- Using a spoon (≈9 mm diameter), dispense 1 level scoop (1 part) of powder into a mixing well.
- Dispense 2-3 parts of liquid in the same well. Cover the whole powder with the liquid.
- Stir for ≈1 min using a spatula.
NOTE: Alternative acrylics are available. Please refer to their manufacturer’s instructions.
- Use a spatula or a precision applicator to apply acrylic across the cranium. Cover the entire exposed skull, the screw and the open skin with dental acrylic. This makes the preparation stable. Do not let acrylic run down the neck, ears and eyes.
- Let the acrylic dry and harden for about 15 min.
- Apply a removable adhesive film onto the head holder plate, to prevent debris from entering the cannula. It is recommended that the film size matches that of the plate.
- Turn off isoflurane flow, remove the animal from stereotactic apparatus and position it on a heating plate to maintain physiological body temperature while it wakes up from anesthesia.
- Monitor the animal until it has regained sufficient consciousness to maintain sternal recumbency.
- Return the animal which has undergone surgery to the company of other animals only when fully recovered.
3. Postoperative care
- Check the status of the mouse for 2 days following surgery, by monitoring the weight and general behavior.
- Apply anti-inflammatory (Meloxicam, 1mg/Kg) and pain killer (Vetalgin, 200mg/kg) drugs subcutaneously for 2 days following surgery
NOTE: Alternative monitoring procedures, drugs and dosages are possible for postoperative care. Please, refer to your animal license and the relative literature.
4. Preparation of the imaging session
- Turn on the imaging setup in advance and let the laser to warm up and stabilize, if necessary.
- Anesthetize the mouse (refer to step 2.2.1).
- Use forceps to carefully remove the adhesive film from the head holder plate. Hold the mouse in a hand and the head holder plate with the fingers. Remove the film gently, to avoid damaging the preparation.
- Position the mouse under the microscope, over the heating carpet and secure the head plate to the holder (Figure 1D).
- Position the nose cone to cover the snout and decrease isoflurane to 1.5-2%.
- Apply ophthalmic ointment to the mouse eyes.
- Clean the imaging cannula by rinsing with deionized water. Use a syringe and a thin needle to drop water into the cannula and a vacuum pump to remove it.
5. Imaging session
- Use low magnification, long working distance objectives to visually check the cannula for residual water, dirt, integrity and the presence of fluorescence.
- Align the cannula to the optic axis by adjusting the angles of the head holder arms.
- Switch to the 25X 1.0 NA, 4 mm WD or the 40X 0.8 NA, 3 mm WD, water immersion objectives. Add enough deionized water to fill the cannula and maintain excess water on top of the cannula. Avoid the formation of air bubbles.
- Use 2P excitation and image fluorescent signals.
NOTE: The imaging protocol is highly dependent on the time and spatial scales to be imaged. Please refer to the sections representative results and discussion for details about the imaging settings we have used to produce the images shown in this article.
Representative Results
Since the cannula is placed just dorsal to CA1, the dorsal aspect of the CA1 is more proximal to the microscope if compared to the ventral one. The alveus is the most proximal structure followed in order by stratum oriens (SO), stratum pyramidale (SP), stratum radiatum (SR) and stratum lacunosum moleculare (SLM), the most distal layer (Figures 1B, C). Longitudinal 2P imaging of neuronal structure with subcellular resolution and of activity-evoked plasticity with cellular resolution are presented as representative results. To excite the fluorophores green fluorescent protein (GFP), d2Venus and TurboFP635, a femtosecond pulsed Ti:Sapphire laser tuned to 920 nm is used and the average laser power is adjusted to 5-25 mW at the sample. The different emission wavelengths are separated using emission filters and different photomultiplier tubes.
Longitudinal imaging of dendritic structure and dendritic spines dynamics.
To sparsely label hippocampal PNs and visualize their structure, a Thy1-GFP transgenic mouse line (M line) is used. Thy1-GFP mice express cytoplasmic enhanced GFP under the control of the Thy1 promoter in a sparse, random population of PNs25. Generally, the major axis of CA1 PNs is roughly perpendicular to the XY-imaging plane (Figures 1B, Figure 2A and Supplementary Movie 1). Basal dendrites extend in the SO, from the soma towards the cannula, whereas apical and apical tuft dendrites extend distally to the cannula, in the opposite direction (Supplementary Movie 1). Since the preparation leaves the deepest fibers of the alveus intact, a few fluorescent fibers traversing the field of view, just beneath the cannula, should be visible (Figure 2A and Movie 1). The preparation allows imaging of PN dendrites with spine resolution (Figure 2 A-C). To image dendrites and dendritic spines, a 25X 1.0 NA, 4 mm WD, water immersion commercial objective lens is used.
For longitudinal tracking, several brain regions within the field of view of the cannula are defined during the first imaging session. Each region corresponds to an area of approximately 240 x 240 µm and contains between 1 and 7 dendritic segments (Figure 2B). These regions are manually mapped to a low magnification three-dimensional stack showing the pattern of GFP expression in the volume below the imaging cannula (Figure 2A). Then, 1 µm z-step image stacks of CA1 PN basal dendrites are acquired at different time intervals (from 24 h to 3 days) for up to about 14 days (Figure 2C). Longer imaging durations and intervals are possible26. Each imaging session lasts approximately 60 to 90 min. Although most images are of dendritic spines in the SO, it is also possible to image dendritic spines in the oblique dendrites of SR (Figures 2D-F). In addition to spine density, this method enables the study of spine dynamics by quantifying their survival, gain and loss rates26,27,28,29,30,31,32. To score and track dendritic spines over time (Figure 2C), a custom MATLAB interface is used. This enables the alignment of the image stacks acquired at different time points and supports manual labeling of dendrites and spines while tracking dendritic lengths and spine positions over time26. Importantly, this method can be used to distinguish (per each time point, excluding the first one) between pre-existing and newborn dendritic spines. This is important as the different classes of dendritic spines are thought to have different roles in memory acquisition and retention33.
Longitudinal imaging of activity-evoked plasticity.
To image activity-evoked plasticity in CA1 PNs, the dorsal CA1 hippocampal area is injected with a viral vector expressing green fluorescent destabilized d2Venus via an enhanced form of the synaptic activity-responsive element (E-SARE) within the Arc enhancer/promoter and red fluorescent TurboFP635 via the ePGK promoter34. This allows for imaging levels of activity-evoked plasticity of hundreds of CA1 PNs in each animal35. Given the very dense labeling of PNs, it is generally not possible to resolve the dendrites of CA1 PNs (Supplementary Movie 2).
To image the somata of CA1 PNs, a 40X 0.8 NA, 3 mm WD, water immersion objective lens is used. For longitudinal tracking, 1 to 9 brain regions are defined per mouse during the first imaging session. Each region corresponds to an area of approximately 300 x 300 µm and contains between 50 and 150 cells (Figure 3A). These regions are manually mapped to local tissue landmarks visible at a lower magnification. Then, 3 µm z-step image stacks are acquired, which encompass the SP of CA1 PNs (Figure 3A and Supplementary Movie 2) at different time intervals (from 24 h to 6 days) for up to about 30 days. Each imaging session lasts approximately 60 to 90 min. E-SARE activation peaks 6 to 8 h after an exposure to a new or enriched environment (EE, Figure 3B) and decays over the course of a few days. Thus, we generally image 6 to 8 h after experience and allow for 5 days between imaging sessions35.
To quantify d2Venus and TurboFP635 fluorescence values, a circular region-of-interest 4.64 µm in diameter is drawn, which is smaller than a neural cell body, centered to the cell soma. We then progress to the next time point, score the soma of the same cell in the same way, and iterate this procedure for all time points and all visible cells in the longitudinal dataset. The mean value of each neuron’s (activity-dependent) d2Venus emission is normalized by its mean (activity-independent) TurboFP635 emission. This method enables the investigation of long-term dynamics of ensemble plasticity of CA1 PNs35 (Figure 3C).
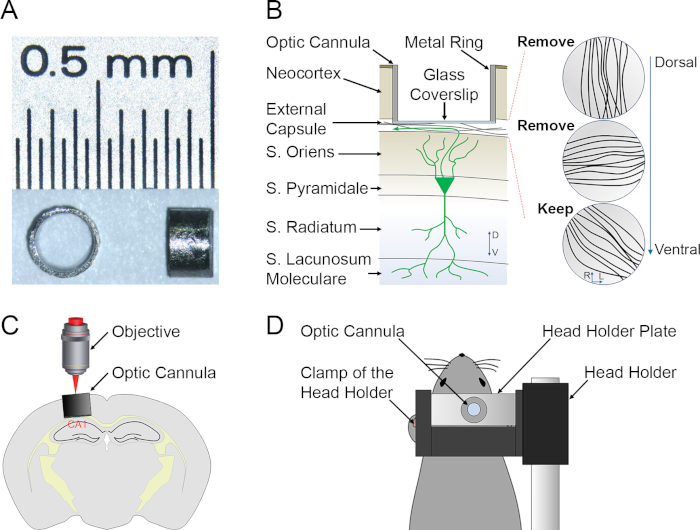
Figure 1: Preparation for in vivo deep brain optical imaging. (A). Top (left) and side (right) views of example imaging cannuals. Imaging cannulas have a transparent glass bottom to allow optical access to the hippocampus. (B). Schematic description of the preparation highlighting the relative position of the imaging cannula, a CA1 PN and the three layers of fibers from dorsal to ventral. (C, D). Schematic description of the imaging setup (C) and of the animal fixation (D) during an imaging session. Please click here to view a larger version of this figure.
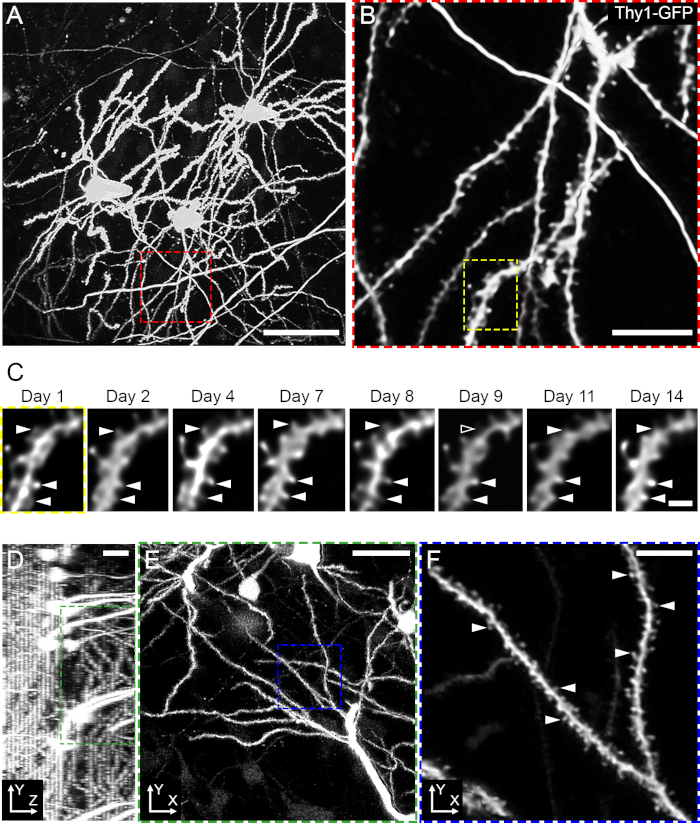
Figure 2: Longitudinal imaging of dendritic structure and dendritic spines dynamics. (A). 2P image stack (Maximum Intensity Projection (MIP) of 59 image planes, 2 µm z-spacing) of neurons and dendrites labelled by GFP in a live Thy1-GFP mouse. (B). Higher magnification (MIP of 53 image planes, 1 µm z-spacing) detailing basal dendrites located in SO. (C). Time-lapse image sequence of a dendritic segment imaged over 14 days. Arrowheads indicate dendritic spines tracked over 14 days. (D, E). 2P image stack of neurons and dendrites labelled by GFP in a live Thy1-GFP mouse; (D) ZY projection (31 image planes, 3 µm z-spacing) and (E) XY projections (17 image planes, 3 µm z-spacing). (F). Higher magnification (single image plane) detailing apical dendrites and dendritic spines located in SR. Arrowheads indicate dendritic spines. Excitation: 920 nm; emission peak: 510 nm. Scale bars: A, 50 µm; B, 10 µm; C, 2 µm; D and E, 15 µm; F, 4 µm. Please click here to view a larger version of this figure.
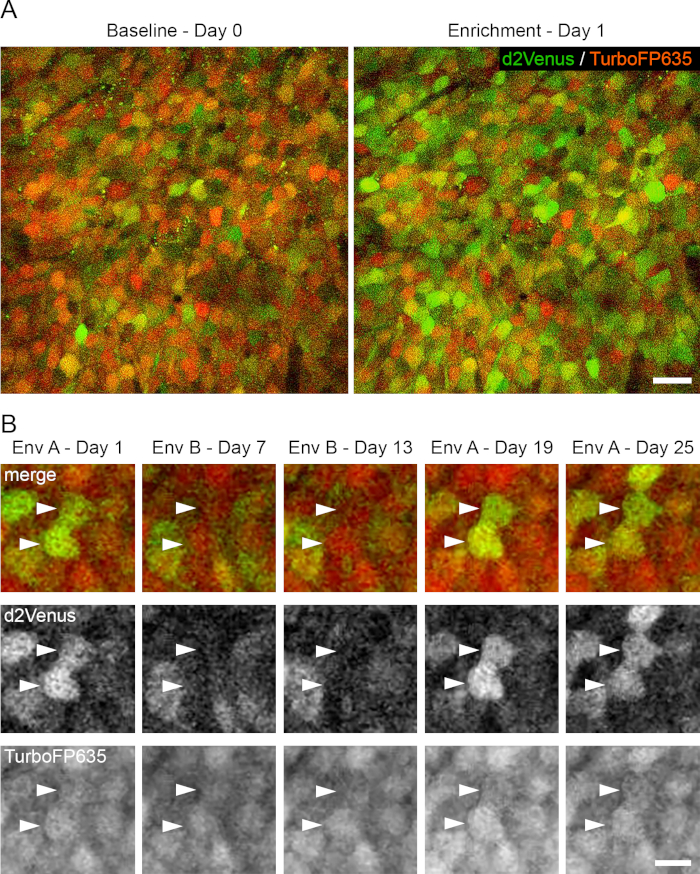
Figure 3: Longitudinal imaging of activity-evoked plasticity. (A). 2P images (single image planes) from a live mouse, showing the same cells on Baseline Day 0 and after EE on Day 1. (B). 2P image stacks (MIPs of 4-6 image planes, 3 µm z-spacing) showing E-SARE activation patterns specific for environment A (Days 1, 19 and 25) and environment B (Days 7 and 13). Green: d2Venus fluorescence. Red: TurboFP635 fluorescence. Excitation: 920 nm; d2Venus emission peak: 530 nm; TurboFP635 emission peak: 635nm. Scale bars: A, 20 µm; B, 10 µm. This figure has been modified from Attardo et al., 201835. Please click here to view a larger version of this figure.
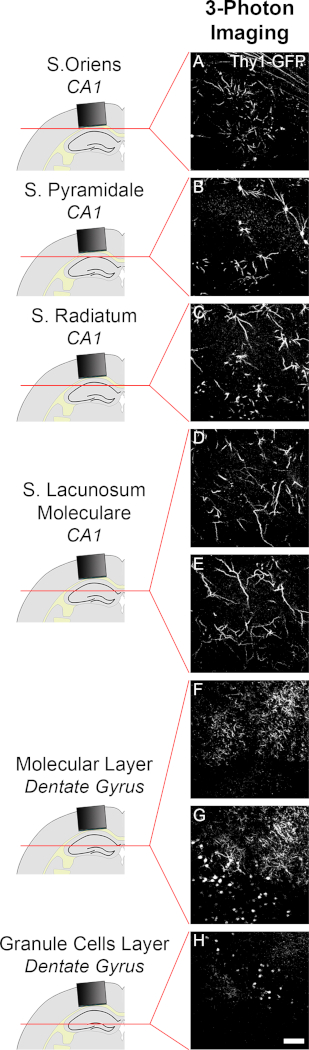
Figure 4: Imaging of neuronal structure in hippocampal DG using three-photon (3P) microscopy. (A-H). 3P images (single image planes) of neurons and dendrites labelled by GFP in a live Thy1-GFP mouse detailing (A-E) PNs in the CA1 and (F-H) granule cells in the DG. Excitation: 1400 nm; emission peak: 510 nm. Scale bar: 40 µm. Please click here to view a larger version of this figure.
Supplementary Movie 1: Imaging field of view in a live Thy1-GFP mouse. 2P image stack (83 image planes, 7 µm z-spacing) of neurons and dendrites labelled by GFP (white) in a live Thy1-GFP mouse extending from the bottom of the cannula to SLM. To account for the decay of fluorescence signal with increasing depth, we used a non-linear gradient of photomultiplier tubes’ gain. Please click here to download this file.
Supplementary Movie 2: Imaging of activity-evoked plasticity. 2P image stack (28 image planes, 3 µm z-spacing) of neurons expressing E-SARE reporter of IEG expression in a live mouse encompassing SP. Green: d2Venus fluorescence. Red: TurboFP635 fluorescence. Please click here to download this file.
Discussion
Here, a procedure for repeated 2P imaging of the dorsal CA1 in live mice is described. After the surgery, the mouse usually recovers within 2 days. The procedure induces minimal astrogliosis26,43. Hemorrhage and edema which might follow the surgery are usually re-adsorbed within 10 to 14 days. Generally, from 14 days post-implantation onwards the preparation is sufficiently clear to perform intravital imaging. The success of the surgery does not depend on working in a sterile environment. However, it is crucial to maintain a high level of hygiene, to avoid complications due to surgery-associated infections. This is obtained by meticulously cleaning the surgical instruments before and after the surgery and by heat-sterilizing them immediately prior to each usage (step 2.1.1). The optic cannula is kept into a clean, sterilized container and rinsed with sterile saline just before the implantation. Performing common surgical practices of hands disinfection and cleaning of the surgical station is also very important. The preparation remains stable and allows cellular and subcellular resolution imaging for several weeks26,35.
Critical steps, modifications and troubleshooting.
It is important to peel the external capsule until the deepest fibers are exposed. Failure to expose the alveus might result in the inability to focus on the soma of PNs, or in reduced resolution imaging dendritic spines, when using commercial objectives with 3- or 4-mm WD. To this aim, it is useful to ablate the neocortex very slowly using a 0.9 mm diameter needle and then switch to a 0.3-0.5 mm diameter (24-29 gauge) needle for a finer control of suction when removing the most dorsal fibers. Alternatively, fine forceps may be used to remove the remaining cortex after fiber exposure36.
Bleeding during surgery can be problematic, as blood obstructs the view. Waiting for the clot to form and then rinsing with saline to wash away residual blood is recommended. Repeat as necessary.
A snug fit between the cannula and the craniotomy helps increase the stability of the preparation by keeping the cannula in place before application of the cement, especially if the outer rim of the cannula is flush with the skull. Since the sizes of the trephine drill and the cannula are matched, a loose fit can arise because of irregularities on the side of the cannula – which require slightly larger craniotomies to fit (see step 2.3.14) – or from an irregular craniotomy. Any cannula irregularities must be filed off (steps 1.3 and 1.12) and the trephine must be held perpendicular to the skull until the craniotomy is completed (step 2.3.12). Removing the trephine from the skull before the craniotomy is completed may result in irregular craniotomies.
Limitations-Invasiveness and stability of the preparation.
It is difficult to evaluate the effect of cortical ablation as it is arduous to precisely define the areas affected directly and indirectly. In general, the surgery removes part of the parietal cortex and part of the visual and hindlimb sensory cortex21. The ablated cortex does not directly project to the hippocampus and hippocampal tissue is neither touched nor injured. Importantly, it has been shown that implantation of an imaging cannula does not grossly alter hippocampal function and specifically hippocampal-dependent learning21,36,37,38,39. Still, it would be important to quantify to what extent both the cannula and the external part of the implant (head holder plate and dental acrylic cap) are chronic stressors by assessing corticosterone blood levels and adrenal gland weight in comparison to unimplanted mice.
The preparation generally remains stable from weeks to months26. In the long term, skin and bone growth tend to displace the acrylic cap and to increase the instability of the imaging preparation.
Optical limitations.
Conventional 2P microscopy allows imaging up to about 1 mm deep into neocortical tissue40,41. Consistent with this, it is possible to image dendrites and dendritic spines located in the SR (Figure 2D-F) or SLM36. However, imaging through a cannula poses limitations to the effective NA. To achieve the maximum resolution, the diameter and depth of the imaging cannulas should be matched to the imaging NA, as smaller diameters and longer depths will clip light of high NA objectives. For instance, when imaging with a 1.0 NA water immersion objective through a 1.6 mm long cannula, a 3.65 mm inner diameter is needed to keep the full NA. However, using a cannula of this diameter will increase the compression on the hippocampus and might affect the health of the tissue, for this reason, we use a cannula with a smaller diameter. When imaging with a 0.8 NA water immersion objective through a 1.6 mm long cannula, an inner diameter of 2.5 mm would be sufficient to keep the full NA. However, 0.8 NA water immersion objectives have a shorter WD (3 mm in our case), which can prevent from focusing at the SP.
These calculations apply to the center of the field of view at the bottom of the cannula. However, moving the imaging field of view sidewise – closer to the edges of the cannula – or focusing deeper into the tissue – farther from the glass surface of the cannula – further decreases the effective NA at the focal plane and thus reduces resolution. This will lead to non-homogenous resolution across the different volumes of imaged tissue and can be a concern for quantitative imaging at subcellular resolution, especially when using super-resolution techniques such as 2P-STED microscopy42. These issues are less important when imaging at cellular resolution.
Tissue motion.
Motion within the tissue – originating from breathing and heartbeat in anesthetized animals – tends to become more severe with increased distance from the imaging cannula. This is possibly because the imaging cannula applies mechanical pressure to the brain thus counteracting some of the motion in the vicinity of the cannula (similarly to neocortical preparations). Thus, although imaging of dendritic spines is possible in SR and SLM, in our hands, it is most robust dorsal to the SO up to ≈200 µm from the surface of the cannula. To compensate for motion, we use resonant scanners and offline averaging. Several images (4 to 6 repetitions) are acquired per image plane of a z-stack at the maximum available speed (30 frames/s). All the repetitions for each z-plane are then deconvolved (using the commercial software, AutoQuant), registered (using ImageJ) and averaged into a single image26. For imaging of somata, motion is often negligible upon anesthesia35 and two averages are often sufficient to compensate for motion artifacts.
Future applications or directions of the method.
The preparation can be combined with micro-endoscopes26,43. Micro-endoscopes are rigid optic probes which use gradient refractive index (GRIN) microlenses to guide light to and from deep tissue18. The use of micro-endoscopes allows cannulas of smaller diameters or even no cannulas at all. However, commercial micro-endoscopes are less well corrected for optical aberrations and have lower NA than commercial objectives. Current probes reach lateral and axial resolutions of ≈0.6-1 µm, ≈10-12 µm, respectively17,18,44. The use of micro-endoscopes also enables the combination of this preparation with head-mounted integrated widefield microscopes45,46,47.
The method lends itself also to use in non-anesthetized mice, and it has been used to investigate cellular activity using Ca2+ sensors in awake head-fixed mice21,37,48,49. In these cases, due to the fast time scales of the fluorescence changes, it is advisable to implement line registration50. It is also possible to adapt the preparation for imaging of other hippocampal sub-regions such as the dentate gyrus (DG)39,51,52. Combining this preparation with 3P excitation53,54 with 1 MHz frequency pulsed laser tuned to 1400 nm, we were able to image deeper into the hippocampal formation reaching the molecular layer, granule cell layer and the hilus of the DG (Figure 4) without removing the overlaying CA1.
In conclusion, we present a method that provides optical access to the dorsal hippocampus and allows longitudinal and correlative studies of the dynamics of hippocampal structure and activity. This technique extends the possibilities of analysis of hippocampal function under physiological and pathological conditions.
Divulgations
The authors have nothing to disclose.
Acknowledgements
U. A. F. is supported by the Schram foundation; C.-W. T. P. and W. G. are supported by the Max Planck Society; L.Y. and R.Y. are supported by the Max Planck Society and National Institute of Health (R01MH080047, 1DP1NS096787); A. C. is supported by an FP7 Grant from the European Research Council, the ERANET and I-CORE programs, the Chief Scientist Office of the Israeli Ministry of Health, the Federal Ministry of Education and Research, Roberto and Renata Ruhman, Bruno and Simone Lich, the Nella and Leon Benoziyo Center for Neurological Diseases, the Henry Chanoch Krenter Institute for Biomedical Imaging and Genomics, The Israel Science Foundation the Perlman Family, Adelis, Marc Besen, Pratt and Irving I. Moskowitz foundations; A. A. is supported by the Max Planck Society, the Schram foundation and the Deutsche Forschungsgemeinschaft (DFG). The 3P images were acquired during the Advanced Course on Neuroimaging Techniques at the Max Planck Florida Institute for Neuroscience. The Advanced Course on Neuroimaging Techniques is supported by the Max Planck Society, the Florida State Max Planck Scientific Fellowship program and by the Max Planck Florida Institute Corporation Partnership program. We would like to thank Thorlabs, Coherent and SpectraPhysics for providing support and equipment for the 2P / 3P imaging system during the course. We are also grateful to Henry Haeberle and Melissa Eberle for assistance with the system during the course.
Materials
| Professional drill/grinder IBS/E | Proxxon GmbH | 28481 | Pecision drill |
| MICROMOT drill stand MB 200 | Proxxon GmbH | 28600 | Movable ruler table |
| MICRO compound table KT 70 | Proxxon GmbH | 27100 | Movable ruler table |
| Machine vice MS 4 | Proxxon GmbH | 28132 | Movable ruler table |
| Stainless steel tube Ø 3,0 x 0,25 mm (Inner Ø 2,5 mm ) L = 500 mm | Sawade | R00303 | Stainless steel tube for the cannula metal ring |
| Microscope Cover glass (4 mm round) | Engelbrecht Medizin and Labortechnik | Glass coverslips for the cannula glass | |
| Schlusselfeilensatz 6-tgl. Im Blechetui | Hoffmann Group | 713750 160 | Manual files |
| Präzisions-Nadelfeile Gesamtlänge 140 mm 4 | Hoffmann Group | 527230 4 | Manual files |
| UV-Curing Optical Adhesives | Thorlabs | NOA81 | UV-curing adhesive |
| UV Curing LED System, 365 nm | Thorlabs | CS2010 | UV-curing LED driver unit |
| Stemi 305 | Zeiss | Stereoscope | |
| Presto II | NSK-Nakanishi Germany | Z307015 | Dental drill |
| Diamantbohrer FG (5 St.), Zylinder flach, 837-014 fein | MF Dental | F837.014.FG | Files for the dental drill |
| Diamantbohrer FG (5 St.), Zylinder flach, 837-014 grob | MF Dental | G837.014.FG | Files for the dental drill |
| Graefe Forceps – Straight / Serrated | Fine Science Tools | 11050-10 | Forceps for the surgery |
| Burrs for Micro Drill | Fine Science Tools | 19008-05 | 0.5 mm width burr for the micro-drill |
| Burrs for Micro Drill | Fine Science Tools | 19008-09 | 0.9 mm width burr for the micro-drill |
| MicroMotor mit Handstück | DentaTec | MM11 | Micro-drill for the craniotomy |
| Dumont #3 Forceps | Fine Science Tools | 11231-30 | Dumont forceps for the surgery |
| Fine Scissors – ToughCut | Fine Science Tools | 14058-09 | Scissors for the surgery |
| Trephine | MW Dental | 229-020 | Trephine drill – 3.0 mm diameter; for the micro-drill |
| Stainless Steel Self-Tapping Bone Screws | Fine Science Tools | 19010-10 | 0.86 mm width bone screws |
| Stereotaxic apparatus | Kopf | Stereotaxic apparatus | |
| 3-D-Gelenkarm | Hoffmann Group | 442114 | Stereotaxic arm and plate holder |
| Aufnahme 2SM | Hoffmann Group | 442100 2SM | Stereotaxic arm and plate holder |
| Hot Bead Sterilizers | Fine Science Tools | 18000-45 | Glass beads sterilizer |
| Isofluran CP, Flasche 250 ml | Henry Schein VET GmbH | 798932 | Liquid isoflurane for anesthesia |
| Harvard Apparatus Isoflurane Funnel-Fill Vaporizer | Harvard Apparatus GmbH | 34-1040 | Isoflurane vaporizer |
| Lab Active Scavenger | Gropper Medizintechnik | UV17014 | Isoflurane scavenger system |
| Metacam 0,5% Injektionslsg. (Hund / Katze), Flasche 20 ml | Henry Schein VET GmbH | 798566 | Meloxicam, anti-inflammatory |
| Vetalgin 500 mg/ml | MSD Tiergesundheit | Vetalgin, pain killer | |
| CMA 450 Temperature Controller | Hugo Sachs Elektronik – Harvard Apparatus GmbH | 8003770 | Heating blanket |
| Bepanthen Augen- und Nasensalbe | Bayer AG | Ophtalmic ointment | |
| KL 1500 LCD | Schott | Fiber optic light source | |
| Xylocain Pumpspray | AstraZeneca GmbH | Lidocain, local anesthetic | |
| Absorption Triangles – Unmounted | Fine Science Tools | 18105-03 | Absorption triangles for the surgery |
| Parkell C&B Metabond clear powder L | Hofmeester dental | 013622 | Quick adhesive cement |
| Parkell C&B Metabond Quick Base B | Hofmeester dental | 013621 | Quick adhesive cement |
| Parkell C&B Metabond Universal Catalyst C | Hofmeester dental | 013620 | Quick adhesive cement |
| Adjustable Precision Applicator Brushes | Parkell | S379 | Precision applicators for the surgery |
| Blunt needles 0.9×23 mm | Dentina | 0441324 | Blunt needles |
| Blunt needles 0.5×42 mm | Dentina | 0452155 | Blunt needles |
| Blunt needles 0.3×23 mm | Dentina | 0553532 | Blunt needles |
| Kallocryl A/C | Speiko | 1615 | Acrylic liquid component |
| Kallocryl | Speiko | 1609 | Acrylic powder |
| Hydrofilm transparent roll | Hartmann | Adhesive film | |
| Head plates | Custom made | 30 mm x 10 mm size; 8 mm diameter hole, titanium | |
| Head plate clamp | Custom made | Head plate holder | |
| Pedestal post holders | Thorlabs | PH20E/M | Head plate holder |
| Stainless steel post | Thorlabs | TR30/M | Head plate holder |
| Stainless steel post | Thorlabs | TR75/M | Head plate holder |
| Stainless steel post | Thorlabs | TR150/M | Head plate holder |
| Post connector clamps | Custom made | Head plate holder | |
| Aluminum Breadboard, 300 mm x 450 mm x 12.7 mm, M6 Taps | Thorlabs | MB3045/M | Microscope stage |
| 7" x 4" Lab Jack | Thorlabs | L490/M | Microscope stage |
| Low profile face mask small mice | Emka Technologies | VetFlo-0801 | Anesthesia facemask holder |
| RS4000 Tuned Damped Top Performance Optical Table | Newport | Floating table | |
| S-2000A Top Performance Pneumatic Vibration Isolators with Automatic Re-Leveling | Newport | Floating table | |
| Power Meter Model 1918-R | Newport | Power meter | |
| X-Cite 120Q | Excelitas Technologies | Fluorescence lamp | |
| Two-photon microscope | Bruker | Ultima IV | Two-photon microscopes |
| Two-photon microscope | Thorlabs | Bergamo | Two-photon microscopes |
| Plan N 4x/0.10 ∞/-/FN22 | Olympus | Objectives | |
| Plan N 10x/0.25 ∞/-/FN22 | Olympus | Objectives | |
| LMPlan FLN 20x/0.40 ∞/-/FN26.5 | Olympus | Objectives | |
| XLPlan N 25x/1.00 SVMP ∞/0-0.23/FN18 | Olympus | Objectives | |
| Ultafast tunable laser for 2P excitation | Spectraphysics | Mai Tai Deep See | Excitaiton lasers |
| Ultafast tunable laser for 2P excitation | Spectraphysics | InSight DS+ Dual beam | Excitaiton lasers |
| Ultafast tunable laser for 3P excitation | Coherent | Monaco | Excitaiton lasers |
References
- O’Keefe, J., Nadel, L. . The hippocampus as a cognitive map. , (1978).
- Zola-Morgan, S., Squire, L. R. Memory Impairment in Monkeys Following Lesions Limited to the Hippocampus. Behavioral Neuroscience. 100 (2), 155-160 (1986).
- Squire, L., Zola-Morgan, S. The medial temporal lobe memory system. Science. 253 (5026), 1380-1386 (1991).
- Leutgeb, S., Leutgeb, J. K., Barnes, C. A., Moser, E. I., McNaughton, B. L., Moser, M. B. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 309 (5734), 619-623 (2005).
- Silva, A. J., Zhou, Y., Rogerson, T., Shobe, J., Balaji, J. Molecular and cellular approaches to memory allocation in neural circuits. Science. 326 (5951), 391-395 (2009).
- Tonegawa, S., Pignatelli, M., Roy, D. S., Ryan, T. J. Memory engram storage and retrieval. Current Opinion in Neurobiology. 35, 101-109 (2015).
- Poo, M., et al. What is memory? The present state of the engram. BMC Biology. 14, (2016).
- O’Keefe, J., Dostrovsky, J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 34 (1), 171-175 (1971).
- Moser, M. B., Moser, E. I. Functional differentiation in the hippocampus. Hippocampus. 8 (6), 608-619 (1998).
- Altman, J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anatomical Record. 145, 573-591 (1963).
- Kuhn, H., Dickinson-Anson, H., Gage, F. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. The Journal of Neuroscience. 16 (6), 2027-2033 (1996).
- Sorrells, S. F., et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 555 (7696), 377-381 (2018).
- Boldrini, M., et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 22 (4), 589-599 (2018).
- Polanco, J. C., et al. Amyloid-β and tau complexity — towards improved biomarkers and targeted therapies. Nature Reviews Neurology. 14 (1), 22-39 (2017).
- Rosene, D. L., Van Hoesen, G. W., Jones, E. G. The hippocampal formation of the primate brain. in Cerebral Cortex. Chapter 9, 345-456 (1987).
- Klohs, J., Rudin, M. Unveiling molecular events in the brain by noninvasive imaging. The Neuroscientist. 17 (5), 539-559 (2011).
- Wilt, B. A., et al. Advances in light microscopy for neuroscience. Annual Reviewof Neuroscience. 32, 435-506 (2009).
- Jung, J. C., Schnitzer, M. J. Multiphoton endoscopy. Optics Letters. 28 (11), 902 (2003).
- Levene, M. J., Dombeck, D. A., Kasischke, K. A., Molloy, R. P., Webb, W. W. In vivo multiphoton microscopy of deep brain tissue. Journal of Neurophysiology. 91 (4), 1908-1912 (2004).
- Mizrahi, A., Crowley, J. C., Shtoyerman, E., Katz, L. C. High-Resolution in vivo imaging of hippocampal dendrites and spines. The Journal of Neuroscience. 24 (13), 3147-3151 (2004).
- Dombeck, D. A., Harvey, C. D., Tian, L., Looger, L. L., Tank, D. W. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nature Neuroscience. 13 (11), 1433-1440 (2010).
- Busche, M. A., et al. Critical role of soluble amyloid- for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Science. 109 (22), 8740-8745 (2012).
- Jung, J. C., Mehta, A. D., Aksay, E., Stepnoski, R., Schnitzer, M. J. In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy. Journal of Neurophysiology. 92 (5), 3121-3133 (2004).
- Deisseroth, K., et al. Next-generation optical technologies for illuminating genetically targeted brain circuits. The Journal of Neuroscience. 26 (41), 10380-10386 (2006).
- Feng, G., et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 28 (1), 41-51 (2000).
- Attardo, A., Fitzgerald, J. E., Schnitzer, M. J. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. 523 (7562), 592-596 (2015).
- Trachtenberg, J. T., et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 420 (6917), 788-794 (2002).
- Zuo, Y., Yang, G., Kwon, E., Gan, W. B. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 436 (7048), 261-265 (2005).
- Holtmaat, A. J. G. D., et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 45 (2), 279-291 (2005).
- Xu, T., et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 462 (7275), 915-919 (2009).
- Yang, G., Pan, F., Gan, W. B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 462 (7275), 920-924 (2009).
- Gu, L., et al. Long-term in vivo imaging of dendritic spines in the hippocampus reveals structural plasticity. The Journal of Neuroscience. 34, 13948-13953 (2014).
- Fu, M., Zuo, Y. Experience-dependent structural plasticity in the cortex. Trends in Neurosciences. 34 (42), 177-187 (2011).
- Kawashima, T., et al. Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nature Methods. 10 (9), 889-895 (2013).
- Attardo, A., et al. Long-term consolidation of ensemble neural plasticity patterns in hippocampal area CA1. Cell Reports. 25 (3), 640-650 (2018).
- Schmid, L. C., et al. Dysfunction of somatostatin-positive interneurons associated with memory deficits in an Alzheimer’s disease model. Neuron. 92 (1), 114-125 (2016).
- Kaifosh, P., Lovett-Barron, M., Turi, G. F., Reardon, T. R., Losonczy, A. Septo-hippocampal GABAergic signaling across multiple modalities in awake mice. Nature Neuroscience. 16 (9), 1182-1184 (2013).
- Lovett-Barron, M., et al. Dendritic inhibition in the hippocampus supports fear learning. Science. 343 (6173), 857-863 (2014).
- Hainmueller, T., Bartos, M. Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature. 558 (7709), 292-296 (2018).
- Beaurepaire, E., Oheim, M., Mertz, J. Ultra-deep two-photon fluorescence excitation in turbid media. Optics Communications. 188, 25-29 (2001).
- Theer, P., Hasan, M. T., Denk, W. Two-photon imaging to a depth of 1000 mm in living brains by use of a Ti:Al2O3 regenerative amplifier. Optics Letters. 28 (12), 1022-1024 (2003).
- Pfeiffer, T., et al. Chronic 2P-STED imaging reveals high turnover of dendritic spines in the hippocampus in vivo. eLife. 7, e34700 (2018).
- Barretto, R. P. J., et al. Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Nature Medicine. 17 (2), 223-228 (2011).
- Barretto, R. P. J., Messerschmidt, B., Schnitzer, M. J. In vivo fluorescence imaging with high-resolution microlenses. Nature Methods. 6 (7), 511-512 (2009).
- Ghosh, K. K., et al. Miniaturized integration of a fluorescence microscope. Nature Methods. 8 (10), 871-878 (2011).
- Ziv, Y., et al. Long-term dynamics of CA1 hippocampal place codes. Nature Neuroscience. 16 (3), 264-266 (2013).
- Cai, D. J., et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 534 (7605), 115-118 (2016).
- Sheffield, M. E. J., Dombeck, D. A. Calcium transient prevalence across the dendritic arbour predicts place field properties. Nature. 517 (7533), 200-204 (2015).
- Basu, J., et al. Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science. 351 (6269), aaa5694 (2016).
- Kaifosh, P., Zaremba, J. D., Danielson, N. B., Losonczy, A. SIMA: Python software for analysis of dynamic fluorescence imaging data. Frontiers in Neuroinformatics. 8, (2014).
- Gonçalves, J. T., et al. In vivo imaging of dendritic pruning in dentate granule cells. Nature Neuroscience. 19 (6), 788-791 (2016).
- Danielson, N. B., et al. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron. 90 (1), 101-112 (2016).
- Hell, S. W., et al. Three-photon excitation in fluorescence microscopy. Journal of Biomedical Optics. 1 (1), 71 (1996).
- Horton, N. G., et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nature Photonics. 7 (3), 205-209 (2013).

