Proof-of-Concept for Gas-Entrapping Membranes Derived from Water-Loving SiO2/Si/SiO2 Wafers for Green Desalination
Summary
Presented here is a stepwise protocol for realizing gas-entrapping membranes (GEMs) from SiO2/Si wafers using integrated circuit microfabrication technology. When silica-GEMs are immersed in water, the intrusion of water is prevented, despite the water-loving composition of silica.
Abstract
Desalination through direct contact membrane distillation (DCMD) exploits water-repellent membranes to robustly separate counterflowing streams of hot and salty seawater from cold and pure water, thus allowing only pure water vapor to pass through. To achieve this feat, commercial DCMD membranes are derived from or coated with water-repellent perfluorocarbons such as polytetrafluoroethylene (PTFE) and polyvinylidene difluoride (PVDF). However, the use of perfluorocarbons is limiting due to their high cost, non-biodegradability, and vulnerability to harsh operational conditions. Unveiled here is a new class of membranes referred to as gas-entrapping membranes (GEMs) that can robustly entrap air upon immersion in water. GEMs achieve this function by their microstructure rather than their chemical make-up. This work demonstrates a proof-of-concept for GEMs using intrinsically wetting SiO2/Si/SiO2 wafers as the model system; the contact angle of water on SiO2 is θo ≈ 40°. Silica-GEMs had 300 μm-long cylindrical pores whose diameters at the (2 μm-long) inlet and outlet regions were significantly smaller; this geometrically discontinuous structure, with 90° turns at the inlets and outlets, is known as the "reentrant microtexture". The microfabrication protocol for silica-GEMs entails designing, photolithography, chrome sputtering, and isotropic and anisotropic etching. Despite the water loving nature of silica, water does not intrude silica-GEMs on submersion. In fact, they robustly entrap air underwater and keep it intact even after six weeks (>106 seconds). On the other hand, silica membranes with simple cylindrical pores spontaneously imbibe water (< 1 s). These findings highlight the potential of the GEMs architecture for separation processes. While the choice of SiO2/Si/SiO2 wafers for GEMs is limited to demonstrating the proof-of-concept, it is expected that the protocols and concepts presented here will advance the rational design of scalable GEMs using inexpensive common materials for desalination and beyond.
Introduction
As the stress on water/food/energy/environmental resources escalates, greener technologies and materials for desalination are needed1,2. In this context, direct contact membrane distillation (DCMD) process can utilize solar-thermal energy or waste industrial heat for water desalination3,4. DCMD exploits water-repellent membranes to separate counterflowing streams of hot seawater and cold deionized water, allowing only pure water vapor to transport across from the hot to cold side5,6,7,8,9. Commercial DCMD membranes almost exclusively exploit perfluorocarbons because of their water repellency, characterized by the intrinsic contact angle of water, θo ≈ 110°10. However, perfluorocarbons are expensive, and they get damaged at elevated temperatures11 and upon harsh chemical cleaning12,13. Their non-biodegradability also raises environmental concerns14. Thus, new materials for DCMD have been explored, e.g., polypropylene15, carbon nanotubes16, and organosilica17, along with variations of the process, e.g., interfacial heating18 and photovoltaic-MD19. Nevertheless, all materials investigated for DCMD membranes thus far have been intrinsically water-repellent, characterized by θo ≥ 90° for water).
Here, a protocol is described for exploiting water-loving (hydrophilic) materials towards achieving the function of water-repellent DCMD membranes i.e., separating water on either side by entrapping air robustly inside the membrane pores. Towards the proof-of-concept demonstration, double-sided polished silicon wafers with silica layers (2 µm thick) on both sides (SiO2/Si/SiO2; 2 µm/300 µm/2 µm, respectively) are used. Microfabrication processes are applied to achieve gas entrapping membranes (GEMs), which exploit a specific architecture to prevent liquids from entering the pores regardless of surface chemistry.
The inspiration for GEMs architecture originated from springtails (Collembola), soil-dwelling hexapods whose cuticles contain mushroom-shaped patterns20,21, and sea-skaters (Halobates germanus), insects living in the open ocean that have mushroom-shaped hair on their body22,23. The surface architecture, along with naturally secreted waxes, affords these insects with "super" water repellence, characterized by apparent contact angles for water (θr ≥ 150°)24. As a result, in their resting state, sea-skaters are essentially floating in air at the sea-air interface22,25. If submerged in water, they instantaneously trap a layer of air around their body (also known as plastron), which facilitates respiration and buoyancy20,23. Inspired by springtails, Kim and co-workers showed that silica surfaces with arrays of mushroom-shaped pillars can repel droplets of liquids with low surface tensions26. This was a remarkable discovery; albeit, it was found that the liquid repellence of these surfaces could be lost catastrophically through localized defects or boundaries27,28. To remedy this problem, researchers microfabricated silica surfaces with cavities whose diameters at the inlets were abruptly smaller (i.e., with a 90° turn) than the rest of the cavity27. These features are also known as "reentrant" edges, and the cavities are hereafter referred to as "reentrant cavities".
Reentrant cavities robustly entrap air on contact with liquid drops or upon submersion27. The performance of cavities of different shapes (circular, square, and hexagonal), profiles (reentrant and doubly reentrant), and sharpness of corners in relation to the stability of entrapped air over time has been compared29. It has been found that circular reentrant cavities are the most optimal in terms of their robustness for air entrapment under wetting liquids and the complexity associated with manufacturing. Also, it has been demonstrated that intrinsically wetting materials with reentrant cavities can entrap air upon immersion in wetting liquids, and thus, achieve the function of omniphobic surfaces. Based on this body of work27,28,29,30 and previous experience with DCMD31, we decided to create membranes that have pores with reentrant inlets and outlets. It was envisioned that such a membrane could entrap air upon immersion in wetting liquids due to its microtexture, giving rise to the idea of GEMs.
Consider a membrane made from a hydrophilic material comprising simple cylindrical pores: when immersed in water, this membrane will imbibe water spontaneously (Figure 1A,B) reaching the fully-filled, or the Wenzel state32. On the other hand, if the inlets and outlets of the pores have reentrant profiles (e.g., "T"-shaped), they may prevent the wetting liquid from penetrating the pore and entrap air inside, leading to Cassie states33 (Figure 1C,D). Once the air is trapped inside the pore, it will further prevent liquid intrusion due to its compressibility and low solubility in water over time34,35.
Such a system will slowly transition from Cassie to Wenzel state, and the kinetics of this process can be tuned by the pore's shape, size, and profile, vapor pressure of the liquid, and solubility of the trapped air in the liquid29,34,36. Researchers have been able to realize GEMs using silicon wafers and polymethylmethacrylate sheets as the test substrates, and proof-of-concept applications for DCMD in a cross-flow configuration have been demonstrated37. Here, a detailed microfabrication protocol for the generation of silica-GEMs is presented, starting with double-side polished silicon wafers with silica layers (2 μm thick) on both sides (SiO2/Si/SiO2; 2 μm/300 μm/2 μm, respectively). Also, the ability of the silica-GEMs to entrap air underwater is assessed using a custom-built pressure cell and confocal microscopy.
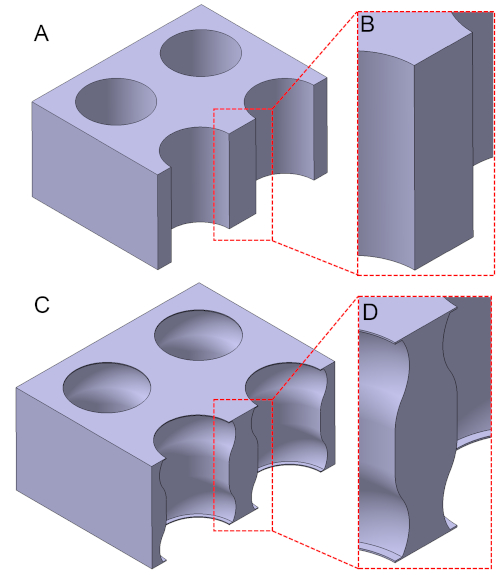
Figure 1: Schematic representation of a membrane with simple cylindrical pores (A,B) and one with reentrant pores (C,D). In contrast to the simple cylindrical pores, the reentrant pores become sharply broader after inlets/outlets, and it is this discontinuity (or the reentrant edges) that prevents liquids from intruding into the pores. Please click here to view a larger version of this figure.
In particular, this section describes the microfabrication protocol for carving arrays of pores with reentrant inlets and outlets using double-sided polished silicon wafers that are 300 µm thick (p-doped, <100> orientation, 4" diameter, 2 µm thick thermally grown oxide layers on both sides). This is referred to hereafter as SiO2(2 µm)/Si(300 µm)/SiO2(2 µm) (Figure 2).
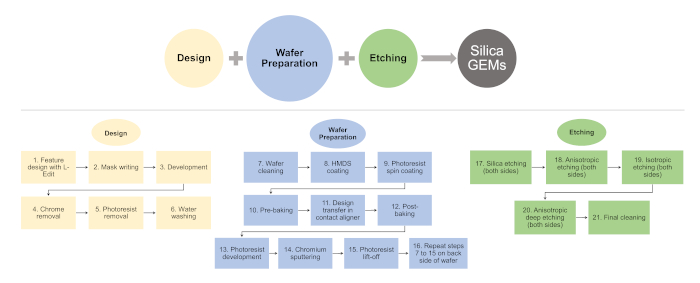
Figure 2: Flowchart listing key steps involved in the microfabrication of silica-GEMs. Please click here to view a larger version of this figure.
Protocol
1. Design
- Design 16 arrays, each comprising 625 circles (diameter, D = 100 μm; pitch, L = 400 μm), along with alignment marks to be translated onto 4" SiO2(2 µm)/Si(300 µm)/SiO2(2 µm) wafers using appropriate design software (see Table of Materials; Figure 3)38.
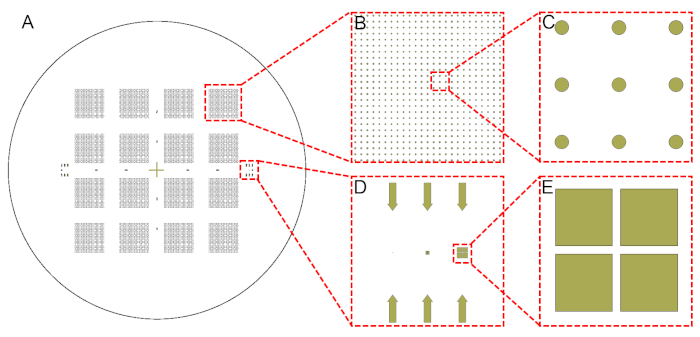
Figure 3: Designs of circular arrays. This design pattern was transferred onto SiO2(2 µm)/Si(300 µm)/SiO2(2 µm) wafers through photolithography. Shown are (A) the entire wafer, (B,C) zoomed-in views, and (D,E) alignment marks used for the manual back alignment. Please click here to view a larger version of this figure.
- Transfer features onto a 5" soda lime glass (CaxHyNazOn) mask with a 50 nm coating of chromium and thin film of photoresist (a positive photoresist; see Table of Materials) through UV exposure in a direct-writing system (exposure time = 25 ms, defocus = +10).
- Mask development
- Develop the photoresist by immersing the mask in 200 mL of developer (Table of Materials) for 60 s to expose the chrome underneath. Wash the mask with deionized (DI) water.
- Remove the exposed chromium by immersing the mask in a 200 mL bath of chrome etchant for 90 s. Wash the mask with DI water.
- Carry out a UV flood exposure (i.e., without mask) for 15 s.
- Completely remove photoresist from the mask by immersing in a 200 mL bath of developer until the photoresist completely disappears (60–120 s). Clean the mask surface with DI water and dry using a nitrogen (N2) gun.
2. Wafer cleaning
- Immerse the silicon wafer in a freshly prepared piranha solution (H2SO4:H2O2 = 3:1 by volume) maintained at a temperature of 388 K for 10 min.
NOTE: Wear appropriate personalized protection equipment (PPE) while working with piranha solution on the wet bench. - Rinse the wafer with DI water, two cycles in a wet bench, and dry it under a N2 environment in spin drier.
3. HMDS deposition
- Expose the wafer to the vapor of hexamethyldisilane (HMDS) to improve adhesion of the photoresist with the silica surface (details in Table 1).
| Stage 1: Dehydration and purging oxygen from chamber | ||
| Step | Function | Time (min) |
| 1 | Vacuum (10 Torr) | 1 |
| 2 | Nitrogen (760 Torr) | 3 |
| 3 | Vacuum (10 Torr) | 1 |
| 4 | Nitrogen (760 Torr) | 3 |
| 5 | Vacuum (10 Torr) | 1 |
| 6 | Nitrogen (760 Torr) | 3 |
| Stage 2: Priming | ||
| Step | Function | Time (min) |
| 1 | Vacuum (1 Torr) | 2 |
| 2 | HMDS (6 Torr) | 5 |
| Stage 3: Purging Prime Exhaust and Return to Atmosphere (Backfill) | ||
| Step | Function | Time (min) |
| 1 | Vacuum | 1 |
| 2 | Nitrogen | 2 |
| 3 | Vacuum | 2 |
| 4 | Nitrogen | 3 |
Table 1: HMDS priming process details.
4. Lithography
- Transfer the wafer onto a vacuum-chuck of a spin coater to spin coat the photoresist. Use AZ 5214 photoresist as a negative tone to achieve a 1.6 µm thick uniform film of the photoresist (the spin coating parameters are listed in Table 2).
NOTE: AZ 5214 can be used as a positive or negative tone photoresist based on the heat treatment (i.e., pre-baking and post-baking). If prebaked at 110 °C for 2 min, the photoresist behaves as a positive tone, such that exposed areas become dissolved during development. For the negative tone, the photoresist is prebaked at 105 ° C for 2 min followed by UV exposure and post-baking at 120 °C for 2 min.- Bake the photoresist-coated wafer at 105 °C on a hotplate for 2 min. This dries and hardens the photoresist film, which otherwise sticks to the glass mask and causes contamination issues during UV exposure, and it also improves adhesion of the photoresist to the silica surface.
NOTE: The pre-bake temperature should not be too high, as this may cause the partial destruction of light-sensitive components of the photoresist, reducing its sensitivity.
- Bake the photoresist-coated wafer at 105 °C on a hotplate for 2 min. This dries and hardens the photoresist film, which otherwise sticks to the glass mask and causes contamination issues during UV exposure, and it also improves adhesion of the photoresist to the silica surface.
| Step | Speed (rpm) | Ramp (rpm/s) | Time (s) |
| 1 | 800 | 1000 | 3 |
| 2 | 1500 | 1500 | 3 |
| 3 | 3000 | 3000 | 30 |
Table 2: Parameters for spin coating recipe to obtain a 1.6 µm layer of photoresist.
- Expose the wafer under UV exposure (80 mJ/cm2) for 15 s through the chrome mask using a mask alignment system (EVG 6200) to achieve the desired design on the photoresist.
- Bake the realized wafer at 120 °C on a hotplate for 2 min. During this step, the exposed negative photoresist film further cross-links. As a result, the UV-exposed parts of the photoresist are no longer soluble in the developer solution, while the unexposed areas are soluble.
- Further expose the wafer under UV light (200 mJ/ cm2) for 15 s in a UV cure system (PRX-2000-20).
NOTE: During this step, the photoresist areas that were not previously exposed (step 4.3) are exposed and can later be dissolved in the developer, leaving behind the desired structures on the wafer. This step is tolerant of overexposure because the desired features (in the negative tone) are no longer photosensitive after the post-baking step. - Immerse the wafer in a 50 mL bath of the AZ-726 photoresist-developer (in glassware) for 60 s to achieve the desired photoresist pattern on the silicon wafer.
- Subsequently clean the wafer using DI water and further blow-dry it with N2.
5. Sputter
- Sputter chromium on the wafer for 200 s to obtain a 50 nm thick chromium layer. The deposition is performed using a magnetron-type DC reactive sputter with a standard 2" round target source in an argon environment with the following parameters: 400 V, current = 1 A, and pressure = 5 mTorr.
NOTE: The chrome layer protects silica from dry etching under octafluorocyclobutane (C4F8).
6. Photoresist lift-off
- Sonicate the sputtered wafer in an acetone bath for 5 min to lift off the remaining photoresist (and chromium deposited on the photoresist) from the wafer, leaving behind the desired features with a chromium hard mask.
7. Processing of the other side of the wafer
- After rinsing the backside of the wafer with a copious amount of acetone and ethanol, blow-dry with an N2 gun, then repeat steps 4.1 and 4.2.
8. Manual back alignment
- Align the desired features on the backside with the front side of the wafer using the alignment marks in the design and the "Manual back alignment with crosshair" module in the contact aligner (EVG 6200).
NOTE: Manual back alignment is a crucial step in the microfabrication protocol. Thus, the designed alignment features on the photomask must be used effectively to avoid offset in pore alignment.
9. Lithography on the backside of the wafer
- For the backside of the wafer, repeat steps 4.3–4.7, section 5, and section 6 to generate the required design with chromium on both sides of the wafer. Note that the part of surface covered with chromium does not undergo etching; thus, spots in which chromium is absent on the wafer define the inlets and outlets of the pore.
10. Etching
- Undergo etching of the exposed SiO2 layer on both sides of the wafer by an inductively coupled plasma (ICP) reactive ion etcher (RIE) that employs fluorine (C4F8) and oxygen (O2) chemistries. The duration is 16 min (ICP-RIE parameters listed in Table 3) for each side.
- Process the wafer with five cycles of anisotropic etching using the Bosch process to create a notch in the silicon layer. This process is characterized by a flat sidewall profile using alternating depositions of C4F8 and sulfur hexafluoride (SF6) gases. By alternating anisotropic etching and polymer deposition, the silicon etches straight down (etching parameters listed in Table 3).
- Immerse the wafer in a bath of piranha solution (H2SO4:H2O2 = 3:1 by volume) maintained at a temperature of 388 K for 10 min. This removes the polymers deposited in the anisotropic step.
- To create the undercut, which yields the reentrant profile, undergo isotropic etch using an SF6-based recipe for a duration of 165 s (etching parameters listed in Table 3).
NOTE: This step is performed on each side of the wafer. - Anisotropic silicon etching
- Transfer the wafer to deep-ICP-RIE (Oxford instruments) to etch 150 µm of silicon using 200 cycles of deep etching using the Bosch process (etching parameters are listed in Table 3).
- Repeat step 10.4.1 with the backside of the wafer.
- Undergo piranha cleaning of the wafer in the wet bench for 10 min to remove polymeric contaminants deposited from the etching process, which ensures uniform etching rates.
- Repeat steps 10.4.1–10.4.3 to realize through pores (which can be visualized by naked eyes under a light source) in the wafer having reentrant inlets and outlets.
- Immerse the wafer in a 100 ml bath of chrome etchant for 60 s to remove chromium from both sides of the wafer.
| Parameter | Silica Etching | Anisotropic silicon etching /cycle | Isotropic silicon etching | |
| Deposition | Etching | |||
| RF power (W) | 100 | 5 | 30 | 20 |
| ICP power (W) | 1500 | 1300 | 1300 | 1800 |
| Etching pressure (mTorr) | 10 | 30 | 30 | 35 |
| Temperature (°C) | 10 | 15 | 15 | 15 |
| C4F8 flow (sccm) | 40 | 100 | 5 | – |
| O2 flow (sccm) | 5 | – | – | – |
| SF6 flow (sccm) | – | 5 | 100 | 110 |
| Etching time (s) | 960 | 5 | 7 | 165 |
Table 3: Parameters for SiO2/Si dry etching.
11. Final cleaning
- After the microfabrication process, clean the wafer with 100 mL of freshly prepared piranha solution (H2SO4:H2O2 = 3:1 by volume; T = 388 K) in a glass container for 10 min, then further blow-dry with a 99% pure N2 pressure gun.
- Place the samples in a glass Petri dish inside a clean vacuum oven at T = 323 K until the intrinsic contact angle of water on smooth SiO2 is stabilized at θo ≈ 40° (after 48 h).
- Store the obtained dry samples (silica GEMs) in a N2 cabinet.
NOTE: The entire fabrication workflow is depicted in Figure 4.
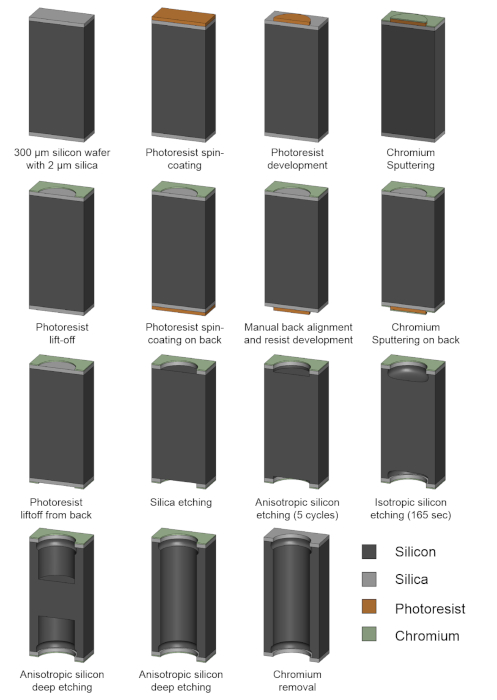
Figure 4: Schematic illustration of the GEM microfabrication process. Please click here to view a larger version of this figure.
Representative Results
This section presents the underwater performance of silica-GEMs microfabricated using the abovementioned protocol. The pores of these GEMs were vertically aligned, the inlet/outlet diameters were D = 100 μm, the center-to-center distance between the pores (pitch) was L = 400 μm, the separation between the reentrant edges and the wall was w = 18 µm, and length of the pores was h = 300 μm (Figure 5). Due to the inhomogeneities incurred during etching steps and minor misalignment during microfabrication, the middle portion of the pores was a bit narrower compared to the portion below the inlets and outlets of the pores, however, it did not affect the mass flux significantly.
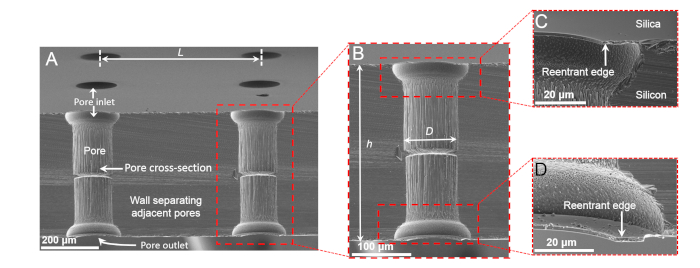
Figure 5: Scanning electron micrographs of silica-GEMs. Shown are (A) a tilted cross-sectional view of silica-GEMs, (B) a magnified cross-sectional view of a single pore, and (C,D) magnified views of reentrant edges at the inlets and outlets of a pore. Panels (C) and (D) are reprinted from Das et al.37. Please click here to view a larger version of this figure.
Immersing silica-GEMs in water
Silica (SiO2) is hydrophilic, as characterized by the intrinsic contact angle of water drops on it under saturated water vapor, θo ≈ 40°. Thus, if cylindrical pores are created on a silica sheet, water would imbibe them, reaching the fully-filled, or the Wenzel state32. To test this, a custom-built module was employed that can secure a test-membrane between a reservoir of dyed salty water (~0.6 M NaCl with food coloring) and deionized water (T = 293 K and p = 1 atm). It also logs the electrical conductivity of the deionized water reservoir into a computer to monitor pore filling in situ (Figure 6A). Here, silica membranes with simple cylindrical holes were not able to prevent the mixing of the two reservoirs, since water infiltrated instantaneously as reflected by the release of the dye (Supplemental Movie). In sharp contrast, when silica-GEMs were tested under the same conditions, they robustly entrapped air and held it intact for over 6 weeks, confirmed by electrical conductivity measurements (detection limit = ± 0.01 μS/cm), after which the experiment was discontinued (Figure 6B). These findings establish that the GEMs architecture can enable hydrophilic materials to robustly entrap air upon immersion in water. Also, a pore level scenario was presented in which the low solubility of the entrapped air in water and the curvature of the air-water interface prevented the liquid meniscus from intruding further into the pore (Figure 6C).
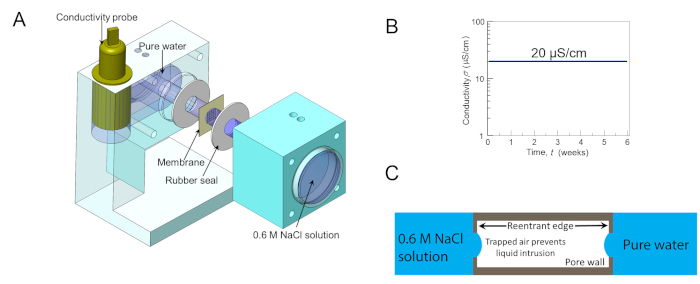
Figure 6: Membrane robustness testing. (A) Schematic of the 3D-printed customized cell for testing the robustness of membranes at separating dyed salty water (~0.6 M NaCl with food coloring) from pure deionized water (T = 293 K, p = 1 atm), while simultaneously logging the electrical conductivity of DI water reservoir into a computer. (B) A semi-logarithmic plot of the electrical conductivity of the DI water reservoir overtime when silica-GEMs were used to separate the two reservoirs. Remarkably, silica-GEMs robustly entrapped air in every pore, such that water could not penetrate even a single pore for over 6 weeks, evidenced by the electrical conductivity data. (C) Pore level schematic, showing the air-water interface at either end. Panels (A) and (B) are reprinted from Das et al.37. Please click here to view a larger version of this figure.
To gain deeper insight into the pinning and displacements of the air-water interface at the inlets and outlets of silica-GEMs underwater (~5 mm column), confocal microscopy was utilized. It is well-known that the laser used for illumination in confocal microscopy also heats up the system39, which can accelerate wetting transitions. Nevertheless, the high spatial resolution can yield useful insight. For comparison, the behavior of silica surfaces with reentrant cavities was also investigated29,40. In both scenarios, the additional heat supplied to the water reservoir above enhances the capillary condensation of water vapor inside the microtexture. In the case of reentrant cavities, the condensation of water vapor displaced the entrapped air, which caused bulging of the air-water interface upwards and destabilized the system (Figure 7A,C). Under those experimental conditions, water intruded into all the cavities in less than 2 h. In contrast, silica-GEMs remained free from bulging for a much longer period, even though the rate of heating was similar. These results were rationalized on the basis of preferential condensation of water vapor from the laser-heated reservoir onto the cooler air-water interface at the opposite side of the pore (Figure 7B,D). However, it was not possible to measure the rate of mass transfer in this experimental configuration.
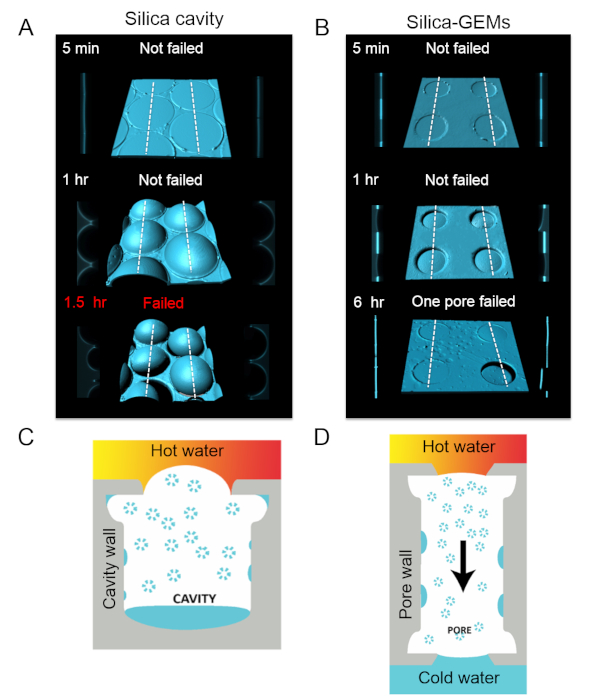
Figure 7: Air-water interfaces. (A) Computer-enhanced 3D reconstructions of the air-water interface at inlets of silica-GEMs underwater (column height, z ≈ 5 mm; laser power = 0.6 mW) along with cross-sectional views along the white dotted lines (on left and right sides of the central image). Due to heating from the laser on the top side, water vapor condensed inside the cavities, displacing the entrapped air. This caused the air-water meniscus to bulge upward and become unstable. After 1.5 h, most of the cavities were intruded by water. (B) Confocal micrographs of silica-GEMs under similar conditions as in (A). (C) Schematic of the bulging of the air-water meniscus in the case of reentrant cavities underwater. (D) Schematic for a pore in silica-GEMs under similar conditions. Hot water vapor condenses everywhere, most notably on the cooler air-water interface on the side further from the laser. As a result of this mass transfer, there is a minimal pressure build-up in the pore. Please click here to view a larger version of this figure.
Direct contact membrane distillation with GEMs
Having established that silica-GEMs can robustly separate two water reservoirs on either side, a static DCMD configuration was tested, in which the salty feed side (0.6 M NaCl at T = 333 K) and deionized permeate side (T = 288 K) were static reservoirs. Even though silica-GEMs prevented water intrusion, measurable fluxes were not observed. This was due to the fact that the thermal conductivity of silicon (k = 149 W-m-1 K-1)41 is orders of magnitude higher than that of typical DCMD membranes (i.e., k < 1 W-m-1-K-1)2. Thus, the experimental set-up with silica-GEMs suffered from what is known as temperature polarization, wherein the hot side loses heat to the cold side, lowering the flux31.
It may be possible to reduce the thermal conductivity of silicon through nanostructuring42 (for instance, to enhance its thermoelectric properties43), but these avenues were not explored. Instead, the design principles from silica-GEMs were translated to polymethylmethacrylate (PMMA) sheets (θo ≈ 70° for water, k = 0.19 W-m-1-K-1)40 to create PMMA-GEMs37. Indeed, the first (proof-of-concept) batch of PMMA-GEMs with a low porosity (of 0.08) exhibited robust separation of feed side and permeate and yielded a flux of 1 L-m2-h-1 over 90 h. Thus, it is possible to translate these silica-GEMs based studies to using more common materials for generation of greener, lower cost membranes for desalination.
Supplemental Movie. Please click here to view this file (Right click to download).
Discussion
This work presents the design and fabrication of silica-GEMs, the first-ever DCMD membranes derived from hydrophilic materials. Microfabrication with the SiO2/Si system provides immense flexibility to create microtextures to test creative ideas. Of course, the scope of this work is limited to the proof-of-concept for GEMs, because SiO2/Si/SiO2 wafers and cleanroom microfabrication protocols are impractical for desalination membranes.
It should be noted that, even though GEMs architecture can prevent the intrusion of water upon immersion when the intrinsic contact angle is θo ≥ 40°, this strategy fails if the surface is made superhydrophilic. For example, after exposure to oxygen plasma, silica surfaces exhibit θo ≈ 5°, and these silica-GEMs lose air that is entrapped inside the pores spontaneously as bubbles, because the liquid meniscus is no longer pinned at the reentrant edges. However, common plastics, such as polyvinyl alcohol (θo ≈ 51°) and poly(ethylene terephthalate) (θo ≈ 72°), should be amenable to this approach. Thus, design principles learned from silica-GEMs can be scaled up using 3-D printing44, additive manufacturing45, laser micromachining46, and CNC milling37, etc.
Next, some crucial aspects of the microfabrication of silica-GEMs are discussed, which require special attention. The manual back alignment (section 8) of the features should be performed with as much care as possible to achieve vertically aligned pores. Offsets may result in pore-throats, and in the worst case, the misalignment may lead to only cavities on either side (no pores). Thus, it is suggested to use multi-scale alignment marks, with the smallest alignment mark being at least four times smaller than the pore diameter.
During the etching of the silica layer with C4F8 and O2 (step 10.1), prior usage (i.e., cleanliness) of the reaction chamber can influence etching rates. This is because of the presence of contaminants in the reaction chamber, a common occurrence in shared user facilities such as universities. Thus, it is recommended that this step is performed first on a dummy wafer to ensure that the system is clean and stable. Also, it is advised to use short periods for etching (e.g., no more than 5 min while monitoring the thickness of the silica layer using reflectometry). For example, if it takes 16 min to completely remove a 2 µm SiO2 layer from a SiO2/Si/SiO2 wafer, then the etching process should be divided into four steps comprising three 5 min cycles followed by reflectometry, and one 1 min (optional) etching step, based on the reflectometry results.
To preserve the silica reentrant features during the Bosch process that is used to etch the silicon layer (step 10.4), it is crucial that a chromium hard mask is used. The Bosch process entails the deposition of C4F8 to ensure the anisotropic profile. However, over long etching cycles, this layer can become very thick and difficult to remove. Thus, it is recommended that the Bosch process should not be run for more than ~200 cycles, and it should be followed by piranha cleaning. It has also been observed that long cycles of deep etching also reduce the thickness of the silica layer, despite the presence of a chromium hard mask.
Most dry etching tools fail to achieve spatial uniformity in terms of etching rates. Thus, the features obtained in the center of a SiO2/Si/SiO2 wafer may not be the same as those at the boundary of the wafer. Here, high quality features were realized in the center of 4" wafers, and samples were periodically observed under a microscope. In the case that some regions are etched more than others, the wafer should be broken into pieces that should be etched separately.
This fabrication protocol can be applied to SiO2/Si/SiO2 wafers of any thickness; however, a thicker layer means that a higher number of etching cycles is needed. It is suggested to use silicon wafers of <300 µm thickness, as long as this does not compromise the mechanical integrity of the wafer during handling and characterization.
Divulgations
The authors have nothing to disclose.
Acknowledgements
H.M. acknowledges funding from King Abdullah University of Science and Technology under BAS/1/1070-01-01 and KAUST access to nanofabrication core lab facilities.
Materials
| 3D Printer | BCN3D | 020.180510.3103 | BCN3D Sigma 3D printer for printing test module with PLA (polylactic acid) filament. |
| Acetone | BASF | ||
| AZ-5214 E photoresist | Merck | ||
| AZ-726 MIF developer | Merck | ||
| Chrome Etchant | MicroChemicals | TechniEtch Cr01 | To remove chromium from silicon wafer and mask |
| Conductivity Meter | Hanna | HI98192 | To measure conductivity of pure water during leak testing. |
| Confocal microscope | Zeiss | ZEISS LSM 710 | For fluorescence imaging of water. |
| Contact Aligner | EVG | EVG6200 | Mask aligner |
| Deep ICP-RIE | Oxford Instruments | Plasmalab system100 | |
| DI water | |||
| Direct writer | Heidelberg Instruments | µPG501 direct-writing system | UV exposure |
| Food Dye | Kroger | Green food dye to label salty water. | |
| Glass Petri dish | VWR | ||
| HMDS vapor prime | Yield Engineering systems | ||
| Hot plate | Cost effective equipments | Model 1300 | |
| Hydrogen peroxide 30% | VWR chemicals | To prepare piranha solution. | |
| Imaris software | Bitplane | Version 8 | Postprocess confocal microscopy images |
| Nitrogen gas | |||
| Optical surface profiler | Zygo | Zygo newview 7300 | |
| Photomask | Nanofilm | 5-inch soda lime glass mask | |
| Profilometer | Veeco | Detak 8 | Stylus profilometer |
| Reactive Sputter | Equipment Support Company Ltd | Chromium sputtering | |
| Reactive-Ion Etching (RIE) | Oxford Instruments | Plasmalab system100 | |
| Reflectometer | Nanometrics | Nanospec 6100 | To check remaining oxide layer thickness. |
| Rhodamine B | Merck | 81-88-9 | Dye for imaging water meniscus under confocal microscope. |
| SEM stub | Electron Microscopy Sciences | ||
| SEM-Quanta 3D | FEI | Quanta 3D FEG Dual Beam (SEM/FIB) | |
| Silicon wafer | Silicon Valley Microelectronics | Double side polished, 4" diamater, 300 µm thickness, 2 µm thick oxide layer, p-doped, <100> orientation. | |
| Sodium Chloride | Merck | 7647-14-5 | For preparing NaCl solution |
| Sonicator | Branson | 1510 | |
| Spin coater | Headway Research,Inc. | ||
| Spin dryer | MicroProcess | Avenger Ultra Pure 6 | Spin drying in Nitrogen environment. |
| Sputter | Quorum Technologies | Q150T S | Iridium sputter for SEM. |
| Sulfuric acid 96% | Technic | 764-93-9 | To prepare piranha solution. |
| Tanner EDA L-Edit software | Tanner EDA, Inc. | For designing photomask | |
| Tweezers | Excelta | ||
| UV Cure | Tamarack Scientific Co. Inc. | PRX-2000-20 | For flood exposure of wafer and photomask |
| Vaccum oven | Thermo Scientific | 13-258-13 | Lindberg/Blue M |
| Wet bench | JST Manufacturing Inc. | 17391-015-00 | Wet bench used for piranha cleaning |
References
- Fisher, J. B., et al. The future of evapotranspiration: Global requirements for ecosystem functioning, carbon and climate feedbacks, agricultural management, and water resources. Water Resources Research. 53 (4), 2618-2626 (2017).
- Deshmukh, A., et al. Membrane distillation at the water-energy nexus: limits, opportunities, and challenges. Energy & Environmental Science. 11 (5), 1177-1196 (2018).
- Ali, A., Tufa, R. A., Macedonio, F., Curcio, E., Drioli, E. Membrane technology in renewable-energy-driven desalination. Renewable and Sustainable Energy Reviews. 81, 1-21 (2018).
- Ghaffour, N., et al. Renewable energy-driven innovative energy-efficient desalination technologies. Applied Energy. 136, 1155-1165 (2014).
- Chen, J., et al. Recovery of dilute aqueous butanol by membrane vapor extraction with dodecane or mesitylene. Journal of Membrane Science. 528, 103-111 (2017).
- Wang, P., Chung, T. -. S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. Journal of Membrane Science. 474, 39-56 (2015).
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Advances in Colloid and Interface Science. 164 (1-2), 56-88 (2011).
- Drioli, E., Ali, A., Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination. 356, 56-84 (2015).
- Souhaimi, M. K., Matsuura, T. . Membrane Distillation. 1st edn. , (2011).
- Janssen, D., De Palma, R., Verlaak, S., Heremans, P., Dehaen, W. Static solvent contact angle measurements, surface free energy and wettability determination of various self-assembled monolayers on silicon dioxide. Thin Solid Films. 515 (4), 1433-1438 (2006).
- Hendren, Z. D., Brant, J., Wiesner, M. R. Surface modification of nanostructured ceramic membranes for direct contact membrane distillation. Journal of Membrane Science. 331 (1-2), 1-10 (2009).
- Verho, T., et al. Mechanically Durable Superhydrophobic Surfaces. Advanced Materials. 23, 673-678 (2011).
- Boinovich, L., Emelyanenko, A. M., Pashinin, A. S. Analysis of Long-Term Durability of Superhydrophobic Properties under Continuous Contact with Water. ACS Applied Materials & Interfaces. 2 (6), 1754-1758 (2010).
- Lindstrom, A. B., Strynar, M. J., Libelo, E. L. Polyfluorinated compounds: past, present, and future. Environmental Science & Technology. 45 (19), 7954-7961 (2011).
- Gryta, M. Influence of polypropylene membrane surface porosity on the performance of membrane distillation process. Journal of Membrane Science. 287 (1), 67-78 (2007).
- An, A. K., et al. Enhanced vapor transport in membrane distillation via functionalized carbon nanotubes anchored into electrospun nanofibres. Scientific Reports. 7, (2017).
- Hammami, M. A., et al. Engineering Hydrophobic Organosilica Nanoparticle-Doped Nanofibers for Enhanced and Fouling Resistant Membrane Distillation. ACS Applied Materials & Interfaces. 9 (2), 1737-1745 (2017).
- Shi, Y., et al. A 3D Photothermal Structure toward Improved Energy Efficiency in Solar Steam Generation. Joule. 2 (6), 1171-1186 (2018).
- Wang, W., et al. Simultaneous production of fresh water and electricity via multistage solar photovoltaic membrane distillation. Nature Communications. 10 (1), 3012 (2019).
- Hensel, R., Neinhuis, C., Werner, C. The springtail cuticle as a blueprint for omniphobic surfaces. Chemical Society Reviews. 45 (2), 323-341 (2016).
- Helbig, R., Nickerl, J., Neinhuis, C., Werner, C. Smart Skin Patterns Protect Springtails. PLoS ONE. 6 (9), 25105 (2011).
- Andersen, N. M., Cheng, L. The marine insect Halobates (Heteroptera: Gerridae): biology, adaptations, distribution, and phylogeny. Oceanography and Marine biology: an Annual Review. 42, 119-180 (2004).
- Cheng, L. Marine and Freshwater Skaters: Differences in Surface Fine Structures. Nature. 242, 132 (1973).
- Gao, X., Jiang, L. Water-repellent legs of water striders. Nature. 432 (7013), 36 (2004).
- Golovin, K. B., Gose, J., Perlin, M., Ceccio, S. L., Tuteja, A. Bioinspired surfaces for turbulent drag reduction. Philosophical Transactions of the Royal Society A – Mathematical Physical and Engineering Sciences. 374 (2073), (2016).
- Liu, T. Y., Kim, C. J. Turning a surface superrepellent even to completely wetting liquids. Science. 346 (6213), 1096-1100 (2014).
- Domingues, E. M., Arunachalam, S., Mishra, H. Doubly Reentrant Cavities Prevent Catastrophic Wetting Transitions on Intrinsically Wetting Surfaces. ACS Applied Materials & Interfaces. 9 (25), 21532-21538 (2017).
- Arunachalam, S., Das, R., Nauruzbayeva, J., Domingues, E. M., Mishra, H. Assessing omniphobicity by immersion. Journal of Colloid and Interface Science. 534, 156-162 (2019).
- Domingues, E. M., Arunachalam, S., Nauruzbayeva, J., Mishra, H. Biomimetic coating-free surfaces for long-term entrapment of air under wetting liquids. Nature Communications. 9 (1), 3606 (2018).
- Gonzalez-Avila, S. R., Nguyen, D. M., Arunachalam, S., Domingues, E. M., Mishra, H., Ohl, C. D. Mitigating cavitation erosion using biomimetic gas-entrapping microtextured surfaces. Science Advances. , (2020).
- Subramanian, N., et al. Evaluating the potential of superhydrophobic nanoporous alumina membranes for direct contact membrane distillation. Journal of Colloid and Interface Science. 533, 723-732 (2019).
- Wenzel, R. N. Resistance of solid surface to wetting by water. Industrial and Engineering Chemistry. 28 (8), 7 (1936).
- Cassie, A. B. D., Baxter, S. Wettability of porous surfaces. Transactions of the Faraday Society. 40, 0546-0550 (1944).
- Kaufman, Y., et al. Simple-to-Apply Wetting Model to Predict Thermodynamically Stable and Metastable Contact Angles on Textured/Rough/Patterned Surfaces. The Journal of Physical Chemistry C. 121 (10), 5642-5656 (2017).
- Mishra, H., et al. Time-Dependent Wetting Behavior of PDMS Surfaces with Bioinspired, Hierarchical Structures. ACS Applied Materials & Interfaces. 8 (12), 8168-8174 (2016).
- Seo, D., et al. Rates of cavity filling by liquids. Proceedings of the National Academy of Sciences. , (2018).
- Das, R., Arunachalam, S., Ahmad, Z., Manalastas, E., Mishra, H. Bio-inspired gas-entrapping membranes (GEMs) derived from common water-wet materials for green desalination. Journal of Membrane Science. 588, 117185 (2019).
- Tanner Research. . Manual, L.-E.U. , (1996).
- Xu, M. C., Sun, G. U., Kim, C. J. Infinite Lifetime of Underwater Superhydrophobic States. Physical Review Letters. 113 (13), (2014).
- Arunachalam, S., Domingues, E. M., Das, R., Nauruzbayeva, J., Buttner, U., Syed, A., Mishra, H. Rendering SiO2/Si Surfaces Omniphobic by Carving Gas-Entrapping Microtextures Comprising Reentrant and Doubly Reentrant Cavities or Pillars. Journal of Visualized Experiments. , e60403 (2020).
- Dean, J. A. . Lange’s Handbook of Chemistry. , (1999).
- Yu, J. K., Mitrovic, S., Tham, D., Varghese, J., Heath, J. R. Reduction of thermal conductivity in phononic nanomesh structures. Nature Nanotechnology. 5 (10), 718-721 (2010).
- Mishra, H., et al. Thermomechanical and Thermal Contact Characteristics of Bismuth Telluride Films Electrodeposited on Carbon Nanotube Arrays. Advanced Materials. 21 (42), 4280 (2009).
- Liu, X., et al. 3D Printing of Bioinspired Liquid Superrepellent Structures. Advanced Materials. , 1800103 (2018).
- Jafari, R., Cloutier, C., Allahdini, A., Momen, G. Recent progress and challenges with 3D printing of patterned hydrophobic and superhydrophobic surfaces. The International Journal of Advanced Manufacturing Technology. , 1-14 (2019).
- Vorobyev, A., Guo, C. Multifunctional surfaces produced by femtosecond laser pulses. Journal of Applied Physics. 117 (3), 033103 (2015).

