Evaluation of the Cognitive Performance of Hypertensive Patients with Silent Cerebrovascular Lesions
Summary
Here, we present a protocol to assess whether various types of silent cerebrovascular lesions are differentially associated with deficits in certain cognitive domains in a cohort of 398 hypertensive elderly Chinese, using a combination of neuropsychological tests and multi-sequence 3T MRI scanning.
Abstract
Evidence accumulated from the last decade has proven that silent cerebrovascular lesions (SCLs) and their underlying pathogenic processes contribute to cognitive decline in the elderly. However, the distinct effects of each type of the lesions on cognitive performance remain unclear. Moreover, research data from Chinese elderly with SCLs is scarce. In this study, 398 otherwise healthy hypertensive elderly subjects (median age 72 years) were included and assessed. All participates were required to complete a battery of structured neuropsychological assessment, including forward and backward digit span tests, symbol digit modalities test, Stroop test, verbal fluency test and Montreal Cognitive Assessment. These tests were used to assess attention, executive function, information processing speed, language, memory and visuospatial function. A multi-sequence 3T MRI scanning was arranged within one month of the neuropsychological assessment to evaluate the burden of SCLs. SCLs were rated visually. Cerebral microbleeds (CMBs) and silent lacunes (SLs) were identified as strictly lobar CMBs and SLs or deep CMBs and SLs according to their locations, respectively. Similarly, white matter hyperintensities (WMHs) were separated into periventricular WMHs (PVHs) and deep WMHs (DWMHs). A series of linear regression models were used to assess the correlation between each type of SCLs and individual cognitive function domain. The results showed that CMBs tend to impair language-related cognition. Deep SLs affect executive function, but this association disappeared after controlling for other types of SCLs. PVHs, rather than DWMHs, are associated with cognitive decline, especially in executive function and processing speed. It is concluded that different aspects of SCLs have differential impact on cognitive performance in hypertensive elderly Chinese.
Introduction
Silent lacunes (SLs), cerebral microbleeds (CMBs) and white matter hyperintensities (WMHs) are referred to as silent cerebrovascular lesions (SCLs). Two types of WMHs are recognized: periventricular WMHs (PVHs) and deep WMHs (DWMHs). SCLs were once regarded as benign lesions without clinical significance. After decades of research, SCLs are now confirmed to be linked to varying functional impairment and cognitive deficits1,2. Nevertheless, consistent evidence is still limited in the spectrum and magnitude of cognitive effects of different types of SCLs. Moreover, the underlying mechanisms are elusive.
Most previous studies either recruited hospital patients with severe medical conditions3,4,5 or included participants with advanced cerebral small vessel diseases6,7. The heterogeneity of the participants among different studies has partly contributed to the inconsistent results. To exclude these confounding factors, we conducted the current one-centered study as an attempt to provide a clear picture through assessment of a relatively large, pure cohort recruited from a primary care setting. Furthermore, previous studies have predominantly focused on one or two types of SCLs and did not fully evaluate the independent associations between individual SCLs and specific cognitive functions. Therefore, we assessed various types of SCLs in the current study.
Neuropsychological tests are widely used to assess cognitive function of specific domains. They are useful in differentiation between normal aging and early cognitive impairment. Results of properly conducted neuropsychological assessment are sensitive in discerning behavioral and functional deficits. A battery of structured neuropsychological tests was chosen, including forward and backward digit span tests, symbol digit modalities test (SDMT), Stroop test, verbal fluency test and Montreal Cognitive Assessment (MoCA). Scores from these tests were grouped and combined to represent performance in different cognitive domains8,9. Such a method is widely used and is time efficient. A major drawback is that different neuropsychological tests may partly overlap in their tested domains. A more specific alternative is to use computer-based assessment with well-designed modules constructed using the E-Prime system, which is time-consuming and may not be suitable for screening purposes.
In conclusion, we aimed to assess the associations between the burden of different SCLs and impairment of various cognitive domains. Furthermore, vascular risk factors and other types of SCLs were controlled for to determine the distinct and independent profile of cognitive impairment of each type of SCLs.
Protocol
The study protocol was approved by the Institutional Review Board of the University of Hong Kong / Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB) for human research.
1. Participants
- Recruit otherwise healthy elderly Chinese subjects (from 65 to 99 years old, mean age 72) with a history of hypertension for at least 5 years.
- Exclude participants with any disease affecting the cognitive function and/or with any disability hindering the completion of the required assessment, including but not limited to stroke, dementia, encephalitis, depression, diabetes mellitus and coronary heart diseases.
- Inform the participant of the scope of the study before obtaining the written consent.
2. Neuropsychological assessment
- Arrange an interview for each participant to administer a battery of neuropsychological tests focusing on six cognitive domains (Table 1) and to collect the demographic and clinical data. Review the participant's medical records to ensure the reliability of relevant information.
- Forward/Backward digit span tests
- Prepare groups of random digit sequences of increasing length (Figure 1A). Start with a three-digit sequence. Read out the digit sequence aloud at a rate of one digit per second. Ask the participant to immediately recall the digit sequence verbally in the Forward digit span test10.
- Have the participant recall progressively longer digit sequences with one more digit each time the participant has successfully recalled the digit sequence without any error.
- Give a different digit sequence of the same length if the participant has failed in the first trial of a specific length. End the test if the participant has failed again. Discontinue the test also when the participant has failed up to three times in total.
- Record the longest length of the digit sequence the participant has successfully recalled without any error.
- Start with a three-digit sequence and ask the participant to recall the digit sequence in a reverse order in the Backward digit span test. Follow the steps of Forward digit span test otherwise.
- MoCA
- Administer MoCA using the validated version. Use the Cantonese version to measure global cognitive function in our protocol and to construct compound domain scores11,12.
- MoCA verbal learning task: Read five words from different categories (
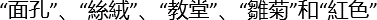 as Chinese characters for face, cloth, church, daisy and red color in our protocol, respectively) to the participant. Ask the participant to immediately recall the words. Repeat the reading and immediate recall a second time. Remind the participant about a delayed recall 5 minutes later. Assign one point to each correct word during the delayed recall.
as Chinese characters for face, cloth, church, daisy and red color in our protocol, respectively) to the participant. Ask the participant to immediately recall the words. Repeat the reading and immediate recall a second time. Remind the participant about a delayed recall 5 minutes later. Assign one point to each correct word during the delayed recall. - MoCA naming task: Show pictures of three animals (lion, rhinoceros and camel in our protocol) and ask the participant to tell their names. Assign one point to each correct name.
- MoCA repetition task: Read a simple sentence to the participant and ask the participant to immediately repeat it. Repeat the procedure with a more complex sentence. Assign one point to each correct repetition.
- MoCA drawing a cube task: Ask the participant to copy a cube printed on a sheet of paper in nearby blank space. Assign one point if the cube is copied correctly.
- MoCA drawing a clock task: Ask the participant to draw a clock face with time at 11:10. Assign one point each for accurate completion of the clock face, numbers and pointers, respectively.
- Stroop test
- Use the Chinese Translated Victoria Version of the Stroop test in our protocol13.
- Inform the participant to finish three sessions each with 24 stimuli printed in four different colors in 6 rows within a sheet of paper (Figure 1B). Start with dots (color naming subtask), next with four Chinese characters (of meaning not related to any color; neutral color subtask), and finally with four Chinese characters (of meaning related to a color but in another color different from their meaning, e.g.,
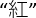 as a Chinese character for "red" printed in green; interference subtask). Remind the participant to name the color of the printed stimuli (i.e., green, blue, yellow or red) and disregard their meaning.
as a Chinese character for "red" printed in green; interference subtask). Remind the participant to name the color of the printed stimuli (i.e., green, blue, yellow or red) and disregard their meaning. - Allow the participant to use the first 4 stimuli in each session as a practice to ensure a full understanding of the rules. Point out any error during the practice stage and encourage the participant to correctly name the color.
- Remind and encourage the participant to complete the remaining 20 stimuli as quickly and accurately as possible. Record the time used by the participant to complete each session (excluding the practice stage).
- SDMT
- Pair 1 to 9 digits in the numeric order with nine unassociated symbols14.
- Print a list of the nine symbols in a random order without the corresponding digits (Figure 1C). Ask the participant to fill in the blank with the correctly paired digit below each symbol. Allow the participant to check back and forth the printed pairs for reference at any time of the test.
- Allow the participant to try filling the first 10 blanks as a practice to ensure a full understanding of the rules. Point out any error during the practice stage and encourage the participant to be correct.
- Remind and encourage the participant to fill in the blank as quickly and accurately as possible in the next 90 seconds. Record the number of correct responses in the written-SDMT.
- Continue the test but ask the participant to provide the correctly paired digit verbally. Record the number of correct responses in the oral-SDMT.
- Verbal fluency
- Ask the participant to provide a verbal list of names belonged to each of the three categories (i.e., animals, vegetables and fruits) separately in one minute for each category15.
- Record the total number of names for each category.
3. MRI acquisition and Visual rating of SCLs on MRI
- Perform a multi-sequence 3-Tesla MRI scanning for the participant using the parameters and including the sequences summarized in Table 2. Complete the MRI scanning within one month of the neuropsychological assessment.
- Identify and visually rate SCLs on MRI according to standard criteria by experienced raters in an anonymous manner. Ensure good intra- and inter-rater reliability.
- Use T1-weighted and fluid-attenuated inversion recovery (FLAIR) images to identify SLs (as hypointense foci of 2-15 mm diameter on both sequences, usually with a hyperintence rim on FLAIR images) and their locations (Figure 2A). Re-confirm the SLs on T2-weighted images (as hyperintense foci at the same locations).
- Search all brain regions in a pre-specified order from anterior to posterior and from one side to the other to avoid any omission (i.e., starting from frontal lobe, island lobe, basal ganglion, thalamus, temporal lobe, parietal lobe, occipital lobe, cerebellum and finally to brain stem, and starting from the left side and then to the right side).
- Use susceptibility-weighted imaging (SWI) to identify CMBs (as punctuate or small round/oval hypointense foci of 2-10 mm diameter) and their locations (Figure 2B). Divide the whole brain region into 7 anatomical locations (i.e., cortex and grey-white junction, subcortical white matter, basal ganglia grey matter, internal and external capsule, thalamus, brain stem and cerebellum) according to the Brain Observer MicroBleed Scale (BOMBS)16.
- Label SLs and CMBs as strictly lobar SLs and CMBs, respectively, when they are confined to the lobar white matter. Label them as deep SLs and CMBs, respectively, when deep or infratentorial lesions are observed with and without additional lobar lesions17,18.
- Use T2-weighted and FLAIR images to identify WMHs (bilateral, almost symmetrical hyperintense areas) (Figure 2C). Re-confirm WMHs on T1-weighted images (as isointense or hypointense areas at the same locations). Recognize PVHs and DWMHs separately. Use the Fazekas scale to rate the severity of WMHs19.
- Rate PVHs appearing as "caps" or pencil-thin lining, smooth "halo" and irregular signal extending into the deep white matter as grade 1, 2 and 3, respectively. Rate DWMHs appearing as punctate foci, small confluent areas and large confluent areas as grade 1, 2 and 3, respectively.
4. Statistical analysis
- Perform all analyses using the statistical package SPSS 22.0 for MacBook.
- Transform the participant's score for each test using z transformation:
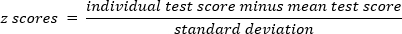
- Invert the Stroop test scores so that a higher score represents better performance.
- Calculate a compound score for each cognitive domain by averaging the mean z score of all component tests under the same domain8,9:
The compound score for executive function = (z score of backward digit span + z score of Stroop interference + z score of verbal fluency) / 3 - Use linear regression models to explore the association between each type of SCLs and cognitive function, adjusting for age, sex, and educational level. Perform further analyses after adjusting for vascular risk factors if significant associations are identified.
- Conduct additional analyses after further adjustment for the other types of SCLs in order to assess the independence of the association between the load of a specific type of SCLs and cognition.
Representative Results
The mean age of the 398 participants was 72.0 (from 65 to 99, SD = 5.1) years, and there were 213 men (53. 5%; Table 3). Table 4 summarizes the neuropsychological assessment results. Only 5 participants had all four types of SCLs. One or more types of SCLs were found in 169 (42.5%) participants, and 35 (8.8%) and 17 (4.3%) participants had 2 and 3 types of SCLs, respectively (Table 5).
The degree of PVHs and DWMHs were separately examined for their associations with performance in different cognitive domains. The data confirmed an independent association between the burden of PVHs and worse performance in executive function and information processing speed (Table 6). An increasing load of CMBs was associated with impaired language-related performance. Additional adjustment for vascular risk factors and other types of SCLs did not affect the independent impact of CMBs on language function (Table 6). Although there was a significant association between the presence of SLs and worse performance on executive function, this association was lost following additional correction for other types of SCLs (Table 6).
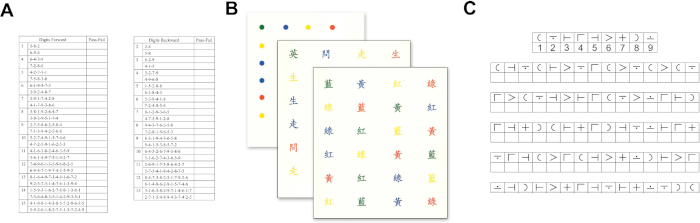
Figure 1: Test sheets for the neuropsychological assessment. (A) Forward digit span test. (B) Stroop test. (C) Symbol digit modalities test. Please click here to view a larger version of this figure.
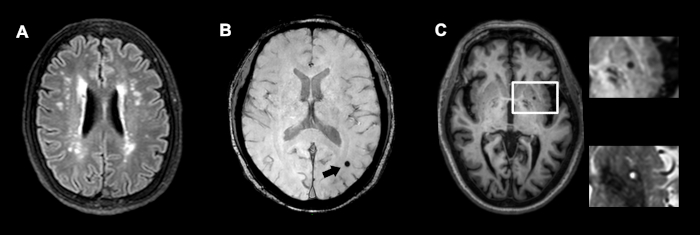
Figure 2: MRI Images of different kinds of silent cerebrovascular lesions. (A) Fazekas grade 2 PVHs and DWMHs on a FLAIR image. (B) A CMB on SWI. (C) A SL on T1-weighted image magnified on both T1-weighted and T2-weighted imaging. CMB, cerebral microbleed; DWMHs, deep white matter hyperintensities; PVHs, periventricular hyperintensities; SL, silent lacune. Please click here to view a larger version of this figure.
| Cognitive domains | Neuropsychological tests |
| Attention | forward digit span, backward digit span |
| Executive function | backward digit span, Stroop interference subtask, verbal fluency |
| Information processing speed | Stroop color naming subtask, Stroop neutral color subtask, symbol digit modalities oral test, symbol digit modalities written test |
| Language-related function | MoCA naming, MoCA repetition, verbal fluency |
| Memory | MoCA verbal learning test |
| Visuospatial function | MoCA drawing a clock, MoCA copying a cube |
Table 1: Neuropsychological tests of six different cognitive domains. MoCA, Montreal cognitive assessment. Original source: Reference20.
| MRI sequences | Repetition time | Echo time | Inversion time | Slices | Slice thickness | Acquisition matrix size |
| Axial three dimensional T1-weighted magnetization prepared rapid gradient echo | 7000 ms | 3.2 ms | / | 155 | 1 mm | 240 x 240 |
| Axial proton density/T2 turbo spin echo run twice | 5000 ms | 16/80 ms | / | 50 | 2.5 mm | 480 x 480 |
| Fluid attenuated inversion recovery sequence | 11000 ms | 120 ms | 2800 ms | 50 | 1 mm | 768 x 768 |
| Susceptibility weighted imaging | 27.9 ms | 23 ms | / | 135 | 2 mm | 704 x 704 |
Table 2: MRI sequences and main parameters.
| Demographic characteristics | Number of participants |
| Male (%) | 213 (53.5) |
| Mean age in years (SD) | 72.0 (5.1) |
| Mean SBP in mmHg (% on drugs) | |
| <120 | 21 (5.3) |
| 120-139 | 302 (75.8) |
| ≥140 | 75 (18.9) |
| Mean DBP in mmHg (% on drugs) | |
| <80 | 265 (66.6) |
| 80-89 | 114 (28.7) |
| ≥90 | 19 (4.7) |
| History of smoking status (%) | 84 (20.0) |
| History of heavy alcohol consuming (%) | 14 (3.5) |
| BMI distribution (%) | |
| <25 | 228 (57.3) |
| 25-29.9 | 146 (36.7) |
| ≥30 | 24 (6.0) |
| Median educational level in years (IQR) | 8 (6) |
Table 3: Demographic characteristics and vascular risk factors of 398 participants. BMI, body mass index; DBP, diastolic blood pressure; IQR, interquartile range; SBP, systolic blood pressure; SD, standard deviation. Original source: Reference20.
| Neuropsychological tests | Mean score | Standard deviation |
| Backward digit span | 4.6 | 1.6 |
| Forward digit span | 8 | 1.5 |
| MoCA copying a cube and drawing a clock | 3.4 | 0.9 |
| MoCA naming | 2.9 | 0.3 |
| MoCA repetition | 2.7 | 0.5 |
| MoCA verbal learning test | 12.5 | 2.4 |
| Stroop color naming in s | 18.7 | 5.9 |
| Stroop neutral color in s | 25.9 | 10.4 |
| Stroop interference in s | 43.1 | 17.5 |
| Symbol digit modalities oral test | 41.0 | 12.8 |
| Symbol digit modalities written test | 32.2 | 11.9 |
| Verbal fluency | 14.2 | 3.2 |
Table 4: Neuropsychological assessment results. MoCA, Montreal cognitive assessment. Original source: Reference20.
| Types of SCLs | n (%) |
| PVHs | |
| Fazekas grade 1 | 176 (44.2) |
| Fazekas grade 2 | 191 (48.0) |
| Fazekas grade 3 | 31 (7.8) |
| DWMHs | |
| Fazekas grade 1 | 326 (81.9) |
| Fazekas grade 2 | 56 (14.1) |
| Fazekas grade 3 | 16 (4.0) |
| CMBs | |
| Strictly lobar | 53 (13.3) |
| Deep | 17 (4.3) |
| Both | 15 (3.8) |
| SLs | |
| Strictly lobar | 65 (14.8) |
| Deep | 6 (1.50) |
| SCLs | |
| One type | 112 (28.1) |
| Two types | 35 (8.8) |
| Three types | 17 (4.3) |
| All four types | 5 (1.3) |
Table 5: Prevalence and distribution of different types of SCLs. CMBs, cerebral microbleeds; DWMHs, deep white matter hyperintensities; PVHs, periventricular hyperintensities; SCLs, silent cerebrovascular lesions; SLs, silent lacunes. Original source: Reference20.
| Executive function | Information processing speed | Language-related function | ||||||||||
| B | SE | β | p-value | B | SE | β | p-value | B | SE | β | p-value | |
| PVHs severity1 | -0.143 | 0.059 | -0.13 | 0.016* | -0.159 | 0.059 | -0.131 | 0.007* | -0.147 | 0.059 | -0.128 | 0.014* |
| Strictly lobar CMBs1 | NA | NA | -0.275 | 0.108 | 0.134 | 0.012* | ||||||
| Deep SLs1 | -0.235 | 0.012 | -0.121 | 0.021* | NA | NA | ||||||
| PVHs severity2 | -0.126 | 0.063 | -0.106 | 0.046* | -0.149 | 0.064 | -0.116 | 0.020* | -0.107 | 0.062 | -0.09 | 0.088 |
| Strictly lobar CMBs2 | NA | NA | -0.202 | 0.102 | -0.098 | 0.049* | ||||||
| Deep SLs2 | -0.197 | 0.106 | -0.098 | 0.064 | NA | NA | ||||||
Table 6: Association between severity of PVHs, presence of deep SLs or strictly lobar CMBs and the Z score of selected cognitive domains. B, unstandardized beta coefficient; β, standardized beta coefficient; CMBs, cerebral microbleeds; NA, not applicable; PVHs, periventricular hyperintensities; SCLs, silent cerebrovascular lesions; SLs, silent lacunes; SE, standard error. 1, single variable linear regression models controlled for age, gender, educational levels and vascular risk factors (body mass index, hyperlipidemia, impaired glucose tolerance, smoking, drinking, systolic blood pressure and diastolic blood pressure); 2, multiple variables linear regression models controlled for age, gender, educational levels and the other two types of SCLs. *, p < 0.05. Original source: Reference20.
Discussion
In the study, we have combined the results of a battery of neuropsychological assessment and findings of a multi-sequence MRI examination to evaluate the impact of different types of SCLs on various cognitive functions. The major types of SCLs were examined (i.e., CMBs, SLs and WMHs). As previous studies have revealed that SCLs in different locations may represent different pathology and lead to different consequences, we categorized CMBs and SLs into strictly lobar (i.e., lobar only without deep ones) and deep ones (with or without lobar ones), and separated WMHs into PVHs and DWMHs. A battery of structured neuropsychological tests was chosen to provide a comprehensive assessment of cognitive functions covering six domains (i.e., attention, executive function, information processing speed, language, memory and visuospatial function). Compound scores for each domain were constructed for statistical analyses.
PVHs adversely affect executive function and information processing speed. Strictly lobar CMBs are linked to impaired language dysfunction. SLs are associated with impaired executive function. We additionally controlled for the vascular risk factors and other types of SCLs to determine the independent effects of each type of SCLs on cognitive functions. All the above-mentioned associations are independent of the vascular risk factors except that the association between SLs and executive function has disappeared when controlled for PVHs; other associations are not affected by controlling for other types of SCLs. In conclusion, the protocol has successfully confirmed that the type of SCLs could differentially affect the cognitive performance in different domains. In other words, different types of SCLs are associated with distinct profiles of cognitive impairments. As previous studies have observed clinical differences between patients with hypertensive and non-hypertensive ischemic stroke21, the results of the present study are relevant to patients with hypertension.
Other limitations of the current research must be noted. First, the incidence and number of lesions in individual participants are relatively low despite choosing a cohort of hypertensive elderly who should have a higher incidence of SCLs than healthy non-hypertensive elderly. A possible explanation is the exclusion of the participant with significant diseases such as dementia and other overt cardiovascular diseases. Such exclusion criteria have omitted the participant at an advanced stage of SCLs and therefore could have underestimated the burden and impact of the SCLs. Another explanation is that the burden of SCLs may be lower in Asians than Caucasians. In any case, a lower burden of SCLs in the cohort has hindered the opportunity for further exploration of the impact of individual type of SCLs and their strategic locations. The chosen battery of neuropsychological assessment has led to another limitation. Some of these tests have inherent overlaps in their evaluated domains, while others have been used in different protocols to assess different domains. These could have contributed to the inconsistencies between the present and published results. We have adopted neuropsychological tests that were most frequently used in the literature for specific cognitive domains. Modules using computer-based tests or functional neuroimaging studies developed for different individual domains should be used in future studies. Focal cerebral atrophy is a potentially important type of SCLs relevant to both hypertension and cognitive functions22, warranting further studies.
It is crucial to ensure that the participant knows precisely what is required to do when a start signal is given during the neuropsychological assessment. A practice stage is generally available before the formal test, during which the participant's errors are pointed out for corrections. A unified standard should be adopted for different tests in all participants, and this was achieved by having the same person (M. ZHANG) administer all the neuropsychological tests. The standard assessment procedures were reviewed every three months to ensure uniformity.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by matching and donation funds (Cerebrovascular Research Fund, SHAC Matching Grant, UGC Matching Grant, and Dr. William Mong Research Fund in Neurology awarded to Professor R.T.F. Cheung).
References
- Jokinen, H., et al. Incident lacunes influence cognitive decline: the LADIS study. Neurology. 76 (22), 1872-1878 (2011).
- Lawrence, A. J., et al. Longitudinal decline in structural networks predicts dementia in cerebral small vessel disease. Neurology. 90 (21), e1898-e1910 (2018).
- Chen, Y. K., et al. Atrophy of the left dorsolateral prefrontal cortex is associated with poor performance in verbal fluency in elderly poststroke women. Neural Regeneration Research. 8 (4), 346-356 (2013).
- Dufouil, C., et al. Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke. 40 (6), 2219-2221 (2009).
- Mungas, D., et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 65 (4), 565-571 (2005).
- Benjamin, P., et al. Lacunar Infarcts, but Not Perivascular Spaces, Are Predictors of Cognitive Decline in Cerebral Small-Vessel Disease. Stroke. 49 (3), 586-593 (2018).
- Carey, C. L., et al. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke. 39 (2), 397-402 (2008).
- Zi, W., Duan, D., Zheng, J. Cognitive impairments associated with periventricular white matter hyperintensities are mediated by cortical atrophy. Acta Neurologica Scandinavica. 130 (3), 178-187 (2014).
- Vernooij, M. W., et al. White Matter microstructural integrity and cognitive function in a general elderly population. Archives of General Psychiatry. 66 (5), 545-553 (2009).
- Blackburn, H. L., Benton, A. L. Revised administration and scoring of the digit span test. Journal of Consulting and Clinical Psychology. 21 (2), 139-143 (1957).
- Hachinski, V., et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 37 (9), 2220-2241 (2006).
- Wong, A., et al. The Validity, Reliability and Utility of the Cantonese Montreal Cognitive Assessment (MoCA) in Chinese Patients with Confluent White Matter Lesions. Hong Kong Medical Journal. 14 (6), (2008).
- Lee, T. M., Chan, C. C. Stroop interference in Chinese and English. Journal of Clinical and Experimental Neuropsychology. 22 (4), 465-471 (2000).
- Gawryluk, J. R., Mazerolle, E. L., Beyea, S. D., D’Arcy, R. C. Functional MRI activation in white matter during the Symbol Digit Modalities Test. Frontiers in Human Neuroscience. 8, 589 (2014).
- Chiu, H. F., et al. The modified Fuld Verbal Fluency Test: a validation study in Hong Kong. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 52 (5), P247-P250 (1997).
- Cordonnier, C., et al. improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke. 40 (1), 94-99 (2009).
- Poels, M. M., et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 78 (5), 326-333 (2012).
- Yamashiro, K., et al. Cerebral microbleeds are associated with worse cognitive function in the nondemented elderly with small vessel disease. Cerebrovascular Diseases Extra. 4 (3), 212-220 (2014).
- Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., Zimmerman, R. A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. American Journal of Roentgenology. 149 (2), 351-356 (1987).
- Zhang, M., et al. Distinct profiles of cognitive impairment associated with different silent cerebrovascular lesions in hypertensive elderly Chinese. Journal of the Neurological Sciences. 403, 139-145 (2019).
- Arboix, A., Roig, H., Rossich, R., Martinez, E. M., Garcia-Eroles, L. Differences between hypertensive and non-hypertensive ischemic stroke. European Journal of Neurology. 11 (10), 687-692 (2004).
- Grau-Olivares, M., et al. Progressive gray matter atrophy in lacunar patients with vascular mild cognitive impairment. Search Results. 30 (2), 157-166 (2010).

