The Fabrication and Operation of a Continuous Flow, Micro-Electroporation System with Permeabilization Detection
Summary
This protocol describes the microfabrication techniques required to build a lab-on-a-chip, microfluidic electroporation device. The experimental setup performs controlled, single-cell-level transfections in a continuous flow and can be extended to higher throughputs with population-based control. An analysis is provided showcasing the ability to electrically monitor the degree of cell membrane permeabilization in real-time.
Abstract
Current therapeutic innovations, such as CAR-T cell therapy, are heavily reliant on viral-mediated gene delivery. Although efficient, this technique is accompanied by high manufacturing costs, which has brought about an interest in using alternative methods for gene delivery. Electroporation is an electro-physical, non-viral approach for the intracellular delivery of genes and other exogenous materials. Upon the application of an electric field, the cell membrane temporarily allows molecular delivery into the cell. Typically, electroporation is performed on the macroscale to process large numbers of cells. However, this approach requires extensive empirical protocol development, which is costly when working with primary and difficult-to-transfect cell types. Lengthy protocol development, coupled with the requirement of large voltages to achieve sufficient electric-field strengths to permeabilize the cells, has led to the development of micro-scale electroporation devices. These micro-electroporation devices are manufactured using common microfabrication techniques and allow for greater experimental control with the potential to maintain high throughput capabilities. This work builds off a microfluidic-electroporation technology capable of detecting the level of cell membrane permeabilization at a single-cell level under continuous flow. However, this technology was limited to 4 cells processed per second, and thus a new approach for increasing the system throughput is proposed and presented here. This new technique, denoted as cell-population-based feedback control, considers the cell permeabilization response to a variety of electroporation pulsing conditions and determines the best-suited electroporation pulse conditions for the cell type under test. A higher-throughput mode is then used, where this ‘optimal’ pulse is applied to the cell suspension in transit. The steps for fabricating the device, setting up and running the microfluidic experiments, and analyzing the results are presented in detail. Finally, this micro-electroporation technology is demonstrated by delivering a DNA plasmid encoding for green fluorescent protein (GFP) into HEK293 cells.
Introduction
Current therapeutic innovations in biomedical research, such as CAR-T (Chimeric Antigen Receptor Engineered T cell) cell therapy and genetic editing using CRISPR (clustered regularly interspaced short palindromic repeat DNA sequences)/Cas9, heavily rely on the ability to deliver exogenous material both successfully and efficiently into the intracellular space1. In CAR-T therapy, the gold standard to perform the gene delivery step in cell therapy manufacturing is using viral vectors2. Though viral-mediated gene delivery is an efficient delivery modality, it also has several drawbacks. These include manufacturing costs, cytotoxicity, immunogenicity, mutagenesis/tumorigenesis potential, and size limitations on the gene(s) to be delivered3. These limitations have led to the research and development of alternative, non-viral delivery technologies.
Electroporation, an alternative to viral-mediated gene delivery, relies on the application of an optimal electrical pulse waveform to perform DNA, RNA, and protein transfections of cells. Following the application of an external electric field, the cell membrane is briefly compromised, making the cell susceptible to the intracellular delivery of otherwise impermeable exogenous materials4. Compared to viral-mediated delivery, electroporation is advantageous as it is generally safe, easy to operate, and has low operating costs. Electroporation can deliver both small and large molecular cargo and can be efficient in transfecting cells regardless of lineage5. To achieve desirable outcomes following electroporation, i.e., good viability and good electro-transfection efficiency, a variety of experimental parameters need to be co-optimized. These include cell type6, cell density, molecule concentration7, electroporation buffer properties (e.g., molecular composition, conductivity, and osmolarity)8, electrode size/geometry9, and electrical pulse waveform (shape, polarity, number of pulses)10 (refer to Figure 1 for an illustration). Although each of these parameters can have a significant effect on the outcomes of electroporation experiments, pulse waveform has been especially studied in great detail, as the electrical energy of the applied pulse(s) is the root of the intrinsic trade-off between the resulting cell viability and electro-transfection efficiency8.
Typically, electroporation experiments are performed on the macro-scale, where cells are suspended in 100s of microliters of buffer between a set of large, parallel-plate electrodes within an electroporation cuvette. The electrodes are commonly manufactured out of aluminum with an electrode distance of 1-4 mm. Once the cells are manually loaded via pipette, the cuvette is electrically connected to a bulky, electrical pulse generator where the user can set and apply the pulse waveform parameters to electroporate the cell suspension. Although macro-scale or bulk electroporation can process cell densities >106 cells/mL, this feature can be wasteful when optimizing the electrical pulse waveform settings. This is particularly of concern when electroporating primary cell types where the cell population numbers can be limited. Additionally, due to the large distance between the electrodes, the pulse generator must be able to supply large voltages to achieve electric field strengths >1kV/cm11. These high voltages cause resistive power dissipation through the electrolyte buffer resulting in Joule heating, which can be detrimental to the resulting cell viability12. Lastly, performing electroporation on a dense suspension of cells will consistently be burdened with an innate variability in the resulting electro-transfection efficiency and cell viability. Each cell in suspension could experience a different electric field strength due to the surrounding cells. Depending on whether the experienced electric field strength is either increased or decreased, the resulting cell viability or electro-transfection efficiency may each be negatively impacted11. These downsides to macro-scale electroporation have led to the pursuit and development of alternative technologies that operate on the micro-scale and allow for better control at the single-cell level.
The field of BioMEMS, or biomedical micro-electro-mechanical systems, stems from the technological advancements made in the microelectronics industry. Specifically, utilizing microfabrication processes to develop micro-devices for the advancement of biomedical research. These advancements include the development of micro-electrode arrays for in vivo electrical monitoring13, capacitive micro-electrodes for in situ electroporation14, miniaturized organ-on-a-chip devices15, microfluidic point-of-care diagnostics16, biosensors17, and drug delivery systems18, including nano- and micro-electroporation devices19,20,21. Due to the ability to design and manufacture devices at the same size scale as biological cells, nano- and micro-electroporation technologies are advantageous when compared to their macro-scale counterpart22,23. These electroporation devices eliminate the requirement of high voltage pulse applications, as electrode sets with spacings of 10s to 100s of micrometers are typically integrated. This feature drastically reduces the current through the electrolyte, which in turn reduces the accumulation of toxic electrolysis products and the effects of Joule heating in these systems. The micro-scale channels also ensure that a much more uniform electric field is reliably applied to the cells during pulse application, resulting in more consistent outcomes24. In addition, it is also commonplace for micro-electroporation devices to be integrated into a microfluidic platform which lends itself for future integration into a fully automated technology, a highly desirable capability in cell therapy manufacturing25. Lastly, micro-scale electroporation allows for the electrical interrogation of electroporation events. For example, the degree of cell membrane permeabilization can be monitored in real-time at a single cell level26,27. The purpose of this method is to describe the microfabrication, system operation, and analysis of a microfluidic, single-cell micro-electroporation device capable of measuring the degree of cell membrane permeabilization for optimizing electroporation protocols, yet increasing throughput over the previous state-of-the-art.
Performing single-cell level electroporation is no longer a novel technique, as it was first demonstrated by Rubinsky et al. in 2001 with the development of a static cell electroporation technology28. Their micro-device was innovative as they were the first to demonstrate the ability to electrically monitor the event of electroporation. This has further led to the development of static, single-cell electroporation technologies capable of electrically detecting the degree of cell membrane permeabilization in a parallelized manner to increase the throughputs of the devices. However, even with parallelization and batch processing, these devices severely lack the total number of cells they can process per unit time29,30. This limitation has led to the development of flow-through devices capable of performing single-cell level micro-electroporation at much greater throughputs31. This device transition, from static to flow-through environment, limits the capability of electrically monitoring the degree of cell membrane permeabilization following the application of the electroporation pulse. The method described in this work bridges the gap between these two technologies, a micro-electroporation technology capable of electrically detecting, pulsing, and monitoring the degree of cell membrane permeabilization of individual cells, in a continuous-flow, serial fashion.
This technology was recently described in Zheng et al. In that work, the capabilities of this technology were introduced with the completion of a parametric study, where both the amplitude and duration of the electroporation pulse were varied, and the ensuing electrical signal, indicative of cell membrane permeabilization, was explored32. The results showed that an increase in the intensity of the electroporation pulse (i.e., increase in applied electric field or increase in pulse duration) caused an increase in the measured cell membrane permeabilization. To further validate the system, a common fluorescent indicator of successful electroporation, propidium iodide33, was added to the cell suspension, and a fluorescence image was captured immediately following the application of the electrical pulse. The optical signal, i.e., the fluorescence intensity of propidium iodide inside the cell, was strongly correlated with the electrical measurement of the degree of cell membrane permeabilization, verifying the reliability of this electrical measurement. However, this work only considered the delivery of the small molecule propidium iodide, which has little to no translatable significance.
In this work, a new application of this technology is introduced to improve upon the throughput of the system while delivering a biologically active plasmid DNA (pDNA) vector and assessing the electro-transfection efficiency of cells replated and cultured following electroporation. Though the previous work outperforms existing micro-electroporation technologies that are capable of electrically measuring the event of electroporation, the current state of the device still requires long cell transit times between the electrode set (~250 ms) to perform the cell detection, pulse application, and the cell membrane permeabilization measurement. With a single channel, this limits the throughput to 4 cells/s. To combat this limitation, a new concept of cell-population-based feedback-controlled electroporation is introduced to perform pDNA electro-transfection. By using a hypo-physiologic conductivity electroporation buffer, this system allows for the electrical interrogation of single cells across a multitude of electroporation pulse applications. Based on the electrical response, an 'optimal' electroporation pulse is then determined. A 'high-throughput' mode is then implemented where the cell membrane permeabilization determination is nullified, the flow rate is increased, and the electroporation pulse duty cycle is matched to the cell transit time to ensure one pulse per cell in transit between the electrodes. This work will provide extensive details into the microfabrication steps for the manufacturing of the micro-device, the material/equipment and their setup required to perform the experimentation, and the operation/analysis of the device and its electro-transfection efficiency (eTE).
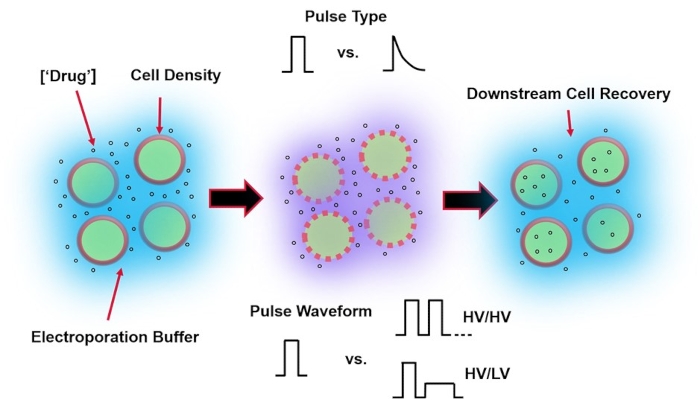
Figure 1: Experimental factors affecting electroporation outcomes. (Left) Cell Suspension-Important factors to consider prior to the onset of electroporation include: Payload (in this case, pDNA), concentration, cell density, and electroporation buffer properties. Electroporation buffer properties to consider are conductivity, osmolarity, and the exact molecular composition contributing to these values. (Middle) Pulse Application-The exact pulse-type (square wave vs. exponential decay) and pulse waveform (single pulse vs. pulse train) must be optimized to maximize both the resulting cell viability and electro-transfection efficiency. Common pulse trains implemented in electroporation processes are typically composed of a series of High Voltage (HV) pulses or series of pulses rotating between HV and Low Voltage (LV) pulse magnitudes. (Right) Cell Recovery-Down-stream processing steps, in particular, the recovery cell culture media that cells are transferred to, should be optimized. Not featured (Far Left), additional upstream cell processing steps can be implemented for overall electroporation process optimization. Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
The methodology presented within this protocol primarily focuses on the microfabrication of a microfluidic device that is then integrated into a specialized electroporation experimental setup. The term 'recipe', which is often used when describing the specifics of the microfabrication process, hints at the importance of following/optimizing each step to successfully fabricate a functioning device. However, certain critical steps within the process, when not optimized, such as UV exposure time/energy, PVD sputteri…
Divulgations
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge financial support by the National Science Foundation (NSF CBET 0967598, DBI IDBR 1353918) and the U.S. Department of Education's Graduate Training in Emerging Areas of Precision and Personalized Medicine (P200A150131) for funding graduate student J.J.S. on fellowship.
Materials
| 150-mm diameter petri dishes | VWR | 25384-326 | step 6.1.1 to secure wafer |
| 24-well tissue culture plates | VWR | 10062-896 | step 10.3.6 to plate electroporated cells |
| 33220A Waveform/Function generator | Agilent | step 9.2.3 electroporation pulse generator | |
| 4'' Si-wafers | University Wafer | subsection 2.1 for microfluidic channel fabrication | |
| 6-well tissue culture plates | VWR | 10062-892 | step 8.1.8 to plate cells |
| Acetone | Fisher Scientific | A18-4 | step 2.1.2 for cleaning and step 5.1 photoresist lift-off |
| Allegra X-22R Centrifuge | Beckman Coulter | steps 8.1.4 , 8.3.2. and 8.3.3. to spin down cells | |
| AutoCAD 2018 | Autodesk | subsection 1.1. to design transparency masks | |
| Buffered oxide etchant 10:1 | VWR | 901621-1L | subsection 3.1 for HF etching |
| CCD Monochrome microscope camera | Hamamatsu | Orca 285 C4742-96-12G04 | step 11.2.3. for imaging |
| CMOS camera- Sensicam QE 1.4MP | PCO | subsection 9.3 part of the experimental setup | |
| Conductive Epoxy | CircuitWorks | CW2400 | subsection 7.6. for wire attachement |
| Conical Centrifuge Tubes, 15 mL | Fisher Scientific | 14-959-70C | step 8.1.4. for cell centrifuging |
| Dektak 3ST Surface Profilometer | Veeco (Sloan/Dektak) | step 2.1.15 and 5.4 for surface profilometry | |
| Disposable biopsy punch, 0.75 mm | Robbins Instruments | RBP075 | step 6.2.3 for inlet access |
| Disposable biopsy punch, 3 mm | Robbins Instruments | RBP30P | step 6.2.3 for outlet access |
| DRAQ5 | abcam | ab108410 | step 11.2.2. for live cell staining |
| Dulbecco’s Modified Eagle’s Medium | ThermoFisher Scientific | 11885084 | step 8.1.2. part of media composition |
| E3631A Bipolar Triple DC power supply | Agilent | step 9.2.1.-9.2.2.part of the experimental setup | |
| Eclipse TE2000-U Inverted Microscope | Nikon | subsection 9.3. part of the experimental setup | |
| EVG620 UV Lithography System | EVG | step 2.1.9. and 2.2.7. for UV Exposure | |
| Fetal Bovine Serum | Neuromics | FBS001 | step 8.1.2. part of media composition |
| FS20 Ultrasonic Cleaner | Fisher Scientific | subsection 5.1. for photoresist lift-off | |
| Glass Media Bottle with Cap, 100mL | Fisher Scientific | FB800100 | step 8.2.1. for buffer storage |
| Glass Media Bottle with Cap, 500mL | Fisher Scientific | FB800500 | step 8.1.2.for media storage |
| HEK-293 cell line | ATCC | CRL-1573 | subsection 8.1 for cell culturing |
| HEPES buffer solution | Sigma Aldrich | 83264-100ML-F | step 8.2.1 part of electroporation buffer composition |
| Hexamethyldisilazane | Sigma Aldrich | 379212-25ML | step 2.2.3 adhesion promoter |
| HF2LI Lock-in Amplifier | Zurich Instruments | subsection 9.2 part of the experimental setup | |
| HF2TA Current amplifier | Zurich Instruments | subsection 9.2 part of the experimental setup | |
| Isopropyl Alcohol | Fisher Scientific | A459-1 | step 2.1.2 for cleaning, step 2.1.14 for rinsing wafer following SU-8 development, and step 6.3.1 for cleaning PDMS |
| IX81 fluorescence microscope | Olympus | step 11.2.3 for imaging | |
| L-Glutamine Solution | Sigma Aldrich | G7513-20ML | step 8.1.2. part of media composition |
| M16878/1BFA 22 gauge wire | AWC | B22-1 | subsection 7.5 for device fabrication |
| Magnesium chloride | Sigma Aldrich | 208337-100G | step 8.1.2 part of electroporation buffer composition |
| MF 319 Developer | Kayaku Advanced Materials | 10018042 | step 2.2.9. photoresist developer |
| Microposit S1818 photoresist | Kayaku Advanced Materials | 1136925 | step 2.2.4 positive photoresist for electrode patterning |
| Microscope slides, 75 x 25 mm | VWR | 16004-422 | step 2.2.1 electrode soda lime glass substrate |
| Model 2350 High voltage amplifier | TEGAM | 2350 | step 9.2.5. part of the experimental setup |
| National Instruments LabVIEW | National Instruments | data acquisition | |
| Needle, 30G x 1 in | BD Scientific | 305128 | step 10.1.1. part of the system priming |
| PA90 IC OPAMP Power circuit | Digi-key | 598-1330-ND | Part of the custom circuit |
| Penicillin-Streptomycin | Sigma Aldrich | P4458-20ML | step 8.1.2. part of media composition |
| Plasmid pMAX-GFP | Lonza | VCA-1003 | step 8.3.4. for intracellular delivery |
| Plastic tubing, 0.010'' x 0.030" | VWR | 89404-300 | step 10.1.2. for system priming |
| Platinum targets | Kurt J. Lesker | subsection 4.2. for physical vapor deposition | |
| Potassium chloride | Sigma Aldrich | P9333-500G | step 8.2.1. part of electroporation buffer composition |
| Pump 11 PicoPlus microfluidic syringe pump | Harvard Apparatus | MA1 70-2213 | step 10.1.4. for system priming |
| PVD75 Physical vapor deposition system | Kurt J. Lesker | subsection 4.1. for physical vapor deposition | |
| PWM32 Spinner System | Headway Research | steps 2.1.6 and 2.2.2. for substrate coating with photoresist | |
| PX-250 Plasma treatment system | March Instruments | subsection 7.2 for PDMS and glass substrate bonding | |
| SDG1025 Function/Waveform generator | Siglent | step 9.2.2. part of the experimental setup | |
| Sodium hydroxide | Sigma Aldrich | S8045-500G | step 8.2.1. part of electroporation buffer composition |
| SU-8 2010 negative photoresist | Kayaku Advanced Materials | Y111053 | step 2.1.7. for microfluidic channel patterning |
| SU-8 developer | Microchem | Y010200 | step 2.1.12. for photoresist developing |
| Sucrose | Sigma Aldrich | S7903-1KG | step 8.2.1. part of electroporation buffer composition |
| Sylgard 184 elastomer kit | Dow Corning | 3097358-1004 | step 6.2.1 10 : 1 mixture of PDMS polymer and hardening agent |
| Syringe, 1 ml | BD Scientific | 309628 | step 8.3.4. part of system priming |
| SZ61 Stereomicroscope System | Olympus | subsection 7.3. for channel and electrode alignment | |
| Tissue Culture Treated T25 Flasks | Falcon | 353108 | step 8.1.2 for cell culturing |
| Titanium targets | Kurt J. Lesker | subsection 4.2. for physical vapor deposition | |
| Transparency masks | CAD/ART Services | steps 2.1.9. and 2.2.7. for photolithography | |
| Trichloro(1H,1H,2H,2H-perfluorooctyl)silane | Sigma Aldrich | 448931-10G | step 6.1.2. for wafer silanization |
| Trypsin-EDTA solution | Sigma Aldrich | T4049-100ML | steps 8.1.3. and 8.3.1. for cell harvesting |
References
- Gao, Q. Q., et al. Therapeutic potential of CRISPR/Cas9 gene editing in engineered T-cell therapy. Cancer Medicine. 8 (9), 4254-4264 (2019).
- Aijaz, A., et al. Biomanufacturing for clinically advanced cell therapies. Nature Biomedical Engineering. 2 (6), 362-376 (2018).
- Milone, M. C., O’Doherty, U. Clinical use of lentiviral vectors. Leukemia. 32 (7), 1529-1541 (2018).
- Weaver, J. C., Chizmadzhev, Y. A. Theory of electroporation: A review. Bioelectrochemistry and Bioenergetics. 41 (2), 135-160 (1996).
- Kotnik, T., Rems, L., Tarek, M., Miklavcic, D. Membrane electroporation and electropermeabilization: mechanisms and models. Annual Review of Biophysics. 48, 63-91 (2019).
- Rosazza, C., Meglic, S. H., Zumbusch, A., Rols, M. P., Miklavcic, D. Gene electrotransfer: A mechanistic perspective. Current Gene Therapy. 16 (2), 98-129 (2016).
- Clauss, J., et al. Efficient non-viral T-cell engineering by sleeping beauty minicircles diminishing DNA toxicity and miRNAs silencing the endogenous T-cell receptors. Human Gene Therapy. 29 (5), 569-584 (2018).
- Sherba, J. J., et al. The effects of electroporation buffer composition on cell viability and electro-transfection efficiency. Scientific Reports. 10 (1), 3053 (2020).
- Lu, H., Schmidt, M. A., Jensen, K. F. A microfluidic electroporation device for cell lysis. Lab on a Chip. 5 (1), 23-29 (2005).
- Kar, S., et al. Single-cell electroporation: current trends, applications and future prospects. Journal of Micromechanics and Microengineering. 28 (12), (2018).
- Shi, J. F., et al. A review on electroporation-based intracellular delivery. Molecules. 23 (11), (2018).
- Wang, S. N., Zhang, X. L., Wang, W. X., Lee, L. J. Semicontinuous flow electroporation chip for high-throughput transfection on mammalian cells. Analytical Chemistry. 81 (11), 4414-4421 (2009).
- Wei, W. J., et al. An implantable microelectrode array for simultaneous L-glutamate and electrophysiological recordings in vivo. Microsystems & Nanoengineering. 1, (2015).
- Maschietto, M., Dal Maschio, M., Girardi, S., Vassanelli, S. In situ electroporation of mammalian cells through SiO2 thin film capacitive microelectrodes. Scientific Reports. 11 (1), (2021).
- Wu, Q. R., et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomedical Engineering Online. 19 (1), (2020).
- Pandey, C. M., et al. Microfluidics Based Point-of-Care Diagnostics. Biotechnology Journal. 13 (1), (2018).
- Vigneshvar, S., Sudhakumari, C. C., Senthilkumaran, B., Prakash, H. Recent advances in biosensor technology for potential applications – An overview. Frontiers in Bioengineering and Biotechnology. 4, (2016).
- Nuxoll, E. BioMEMS in drug delivery. Advanced Drug Delivery Reviews. 65 (11-12), 1611-1625 (2013).
- Kang, S., Kim, K. H., Kim, Y. C. A novel electroporation system for efficient molecular delivery into Chlamydomonas reinhardtii with a 3-dimensional microelectrode. Scientific Reports. 5, (2015).
- Zheng, M. D., Shan, J. W., Lin, H., Shreiber, D. I., Zahn, J. D. Hydrodynamically controlled cell rotation in an electroporation microchip to circumferentially deliver molecules into single cells. Microfluidics and Nanofluidics. 20 (1), (2016).
- Santra, T. S., Kar, S., Chang, H. Y., Tseng, F. G. Nano-localized single-cell nano-electroporation. Lab on a Chip. 20 (22), 4194-4204 (2020).
- Lee, W. G., Demirci, U., Khademhosseini, A. Microscale electroporation: challenges and perspectives for clinical applications. Integrative Biology. 1 (3), 242-251 (2009).
- Santra, T. S., Chang, H. Y., Wang, P. C., Tseng, F. G. Impact of pulse duration on localized single-cell nano-electroporation. Analyst. 139 (23), 6249-6258 (2014).
- Geng, T., Lu, C. Microfluidic electroporation for cellular analysis and delivery. Lab on a Chip. 13 (19), 3803-3821 (2013).
- Hsi, P., et al. Acoustophoretic rapid media exchange and continuous-flow electrotransfection of primary human T cells for applications in automated cellular therapy manufacturing. Lab on a Chip. 19 (18), 2978-2992 (2019).
- Khine, M., Ionescu-Zanetti, C., Blatz, A., Wang, L. P., Lee, L. P. Single-cell electroporation arrays with real-time monitoring and feedback control. Lab on a Chip. 7 (4), 457-462 (2007).
- Ye, Y. F., et al. Single-cell electroporation and real-time electrical monitoring on a microfluidic chip. 2020 33rd Ieee International Conference on Micro Electro Mechanical Systems (Mems 2020). , 1040-1043 (2020).
- Huang, Y., Rubinsky, B. Microfabricated electroporation chip for single cell membrane permeabilization. Sensors and Actuators a-Physical. 89 (3), 242-249 (2001).
- Guo, X. L., Zhu, R. Controllable in-situ cell electroporation with cell positioning and impedance monitoring using micro electrode array. Scientific Reports. 6, (2016).
- Punjiya, M., Nejad, H. R., Mathews, J., Levin, M., Sonkusale, S. A flow through device for simultaneous dielectrophoretic cell trapping and AC electroporation. Scientific Reports. 9, (2019).
- Wang, H. Y., Lu, C. Microfluidic electroporation for delivery of small molecules and genes into cells using a common DC power supply. Biotechnology and Bioengineering. 100 (3), 579-586 (2008).
- Zheng, M. D., et al. Continuous-flow, electrically-triggered, single cell-level electroporation. Technology. 5 (1), 31-41 (2017).
- Batista Napotnik, T., Miklavcic, D. In vitro electroporation detection methods – An overview. Bioelectrochemistry. 120, 166-182 (2018).
- MICROPOSIT™ S1800® G2 Series Photoresists. KAYAKU Available from: https://kayakuam.com/wp-content/uploads/2019/09/S1800-G2.pdf (2021)
- SU-8 2000 Permanent Negative Epoxy Photoresist. KAYAKU Available from: https://kayakuam.com/wp-content/uploads/2020/08/KAM-SU-8-2000-2000.5-2015-Datasheet-8.13.20-final.pdf (2001)
- Substrate Preparation. MicroChemicals Available from: https://www.microchemicals.com/technical_information/subtrate_cleaning_adhesion_photoresist.pdf (2021)
- Lisinenkova, M., Hahn, L., Schulz, J. . 4M 2006 – Second International Conference on Multi-Material Micro Manufacture. , 91-94 (2006).
- Beh, C. W., Zhou, W. Z., Wang, T. H. PDMS-glass bonding using grafted polymeric adhesive – alternative process flow for compatibility with patterned biological molecules. Lab on a Chip. 12 (20), 4120-4127 (2012).
- PA90 High Voltage Power Operational Amplifiers. APEX Available from: https://www.apexanalog.com/resources/products/pa90u.pdf (2021)
- Lissandrello, C. A., et al. High-throughput continuous-flow microfluidic electroporation of mRNA into primary human T cells for applications in cellular therapy manufacturing. Scientific Reports. 10 (1), 18045 (2020).

