A Package of Established Analytical Tools to Investigate the Solid-State Alteration of Lipid-Based Excipients
Summary
This publication shows the application of x-ray diffraction and differential scanning calorimetry as gold standards for investigating the solid-state of lipid-based excipients (LBEs). Understanding the solid-state alteration in LBEs and its effect on the performance of pharmaceutical products thereof is the key factor for manufacturing robust lipid-based dosage forms.
Abstract
Lipid-based excipients (LBEs) are low-toxic, biocompatible, and natural-based, and their application supports the sustainability of pharmaceutical manufacturing. However, the major challenge is their unstable solid-state, affecting the stability of the pharmaceutical product. Critical physical properties of lipids for their processing-such as melt temperature and viscosity, rheology, etc.-are related to their molecular structure and their crystallinity. Additives, as well as thermal and mechanical stress involved in the manufacturing process, affect the solid-state of lipids and thus the performance of pharmaceutical products thereof. Therefore, understanding the alteration in the solid-state is crucial. In this work, the combination of powder x-ray diffraction and differential scanning calorimetry (DSC) is introduced as the gold standard for the characterization of lipids’ solid state. X-ray diffraction is the most efficient method to screen polymorphism and crystal growth. The polymorphic arrangement and the lamella length are characterized in the wide- and small-angle regions of x-ray diffraction, respectively. The small-angle x-ray scattering (SAXS) region can be further used to investigate crystal growth. Phase transition and separation can be indicated. DSC is used to screen the thermal behavior of lipids, estimate the miscibility of additives and/or active pharmaceutical ingredients (API) in the lipid matrix, and provide phase diagrams. Four case studies are presented in which LBEs are either used as a coating material or as an encapsulation matrix to provide lipid-coated multiparticulate systems and lipid nanosuspensions, respectively. The lipid solid-state and its potential alteration during storage are investigated and correlated to the alteration in the API release. Qualitative microscopical methods such as polarized light microscopy and scanning electron microscopy are complementary tools to investigate micro-level crystallization. Further analytical methods should be added based on the selected manufacturing process. The structure-function-processability relationship should be understood carefully to design robust and stable lipid-based pharmaceutical products.
Introduction
Lipids are a class of materials that contain long-chain aliphatic hydrocarbons and their derivatives. They cover a broad range of chemical structures, including fatty acids, acylglycerols, sterols and sterol esters, waxes, phospholipids, and sphingolipids1. The use of lipids as pharmaceutical excipients started in 1960 for embedding drugs in a wax matrix to provide sustained release formulations2. Since then, lipid-based excipients (LBEs) have gained extensive attention for diverse applications, such as modified drug release, taste-masking, drug encapsulation, and enhanced drug bioavailability. LBEs can be applied in a broad range of pharmaceutical dosage forms via versatile manufacturing processes, namely, hot-melt coating, spray-drying, solid lipid extrusion, 3D printing, tableting, and high-pressure homogenization, among others. Dosage forms such as tablets, orally disintegrating films, multiparticulate systems, nano and microparticles, pellets, and 3D-printed forms are the result2,3,4.
LBEs possess the "General Recognized as Safe" status, low toxicity, good biocompatibility, and improved patient tolerance. Their natural origin and broad availability allow them to empower green and sustainable pharmaceutical manufacturing. Nevertheless, the use of LBEs has been associated with unstable dosage forms. Alterations in the properties of lipid-based products after storage have been widely reported. The solid-state of LBEs and the existence of lipid polymorphism are considered the main reasons for the instability of lipid-based dosage forms5,6,7,8.
The mechanical and physical properties of lipids are closely related to their crystallization properties and the structure of their crystal network, which shows distinct hierarchies of structural organization. When lipids are used in the manufacturing of pharmaceutical products, the crystal structure is affected by the process parameters applied, such as temperature, organic solvents, shear, and mechanical forces, which in turn affects the performance of the pharmaceutical product5,7,9,10,11,12. To understand this structure-function relationship, it is important to know the fundaments of lipid crystallization and crystal structure and analytical methods to screen them.
At the molecular level, the smallest unit of a lipid crystal is termed a "unit cell." A regular three-dimensional repetition of unit cells builds the crystal lattices, with stronger molecular interactions alongside their lateral directions than the longitudinal ones, explaining the layered construction of lipid crystals. The repeated cross-sectional packing of hydrocarbon chains is known as sub-cell1,12,13 (Figure 1). Lamellae are the lateral packing of lipid molecules. In the crystal package, the interfaces between different lamellae are made of methyl end groups, whereas the polar glycerol groups are placed at the interior parts of the lamella14. To differentiate each fatty-acid chain in the lamella, the term leaflet is employed, which represents a sublayer composed of single fatty-acid chains. Acylglycerols can be arranged in double (2L) or triple (3L) leaflet chain lengths14. The surface energy of the lamellae drives them to epitaxially stack to each other, to provide nano-crystallites. Different processing factors such as cooling temperature and rate affect the number of stacked lamellae and thereby, the crystallite thickness (~10-100 nm). Aggregation of crystallites leads to the formation of spherulites in micro-scale, and the aggregation of spherulites provides the crystal network of LBEs with defined macroscopic behavior13.
Solid-state transitions start at the molecular level. The geometrical transition from one sub-cell to another is called polymorphism. Three major polymorphs of α-, β'-, and β-form are usually found in acylglycerols, ordered according to increased stability. Tilting of the lamella with respect to end-groups occurs during polymorphic transitions1,13. Storage and melt-mediated polymorphic transitions are experienced by LBEs. Storage transitions occur when the metastable form is stored below its melting temperature, whereas melt-mediated transitions happen as the temperature rises above the melting point of a metastable form provoking melting and successive crystallization of the more stable form.
Furthermore, phase separation and crystal growth can also occur. Phase separation is driven by initial multiphasic crystallization and growth of one phase or more. Particle-particle interactions, including sintering, molecular interactions, microstructural features, and foreign components, can also trigger crystal growth1,5.
Monitoring the solid-state transitions of LBEs and their impact on the performance of dosage forms is of significant importance. Among others, differential scanning calorimetry (DSC) and x-ray diffraction, specifically simultaneous small and wide-angle X-ray scattering (SWAXS), are two gold standards for assessing lipid solid-state.
DSC is commonly used to measure the enthalpy changes of the material of interest associated with the heat flow as a function of time and temperature. The method is widely used for the screening of thermal behavior of lipids, such as possible pathways of melting and crystallization, corresponding temperature and enthalpy of different polymorphic forms, as well as minor and main fractions of lipid compositions. These data can be used to depict the heterogeneity, multiple phases, and lipid polymorphism5,7,13.
X-ray diffraction techniques are the most powerful methods for structure determination in the solid state. Possessing ordered nanostructure with repeated lamellae, the reflection of x-ray beam from lipid crystals can be investigated using Bragg's law:
d = λ/2sinθ (Equation 1)
where λ is the x-ray wavelength of 1.542 Å, θ is the diffraction angle of the scattered beam, and d is the interplanar spacing of repeated layers, defined as lamella length in lipids. The small-angle region of the x-ray can be perfectly used to detect the long-spacing pattern and calculate the lamella length (d). The larger the repeated distance d, the smaller the scattering angle (1-15°, small-angle region) since d is inversely proportional to sin θ. The sub-cell arrangement of lipids can be characterized as the short-spacing pattern in the wide-angle region of the x-ray diffraction. Both the long- and short-spacing patterns of lipids (lamella length and sub-cell arrangement) can be used to indicate the monotropic polymorphic transformation. For example, the α-form (hexagonal) can be altered to β (triclinic) due to a change in the angle of tilt of the chains, with alterations in the lamella length (long-spacing pattern, in the small-angle region, 1-15°) and in the cross-sectional packing mode (short-spacing pattern, in the wide-angle region, 16-25°) (Figure 2).
The information obtained from the SAXS region can further be used to investigate the crystal growth by measuring its thickness (D) via the Scherrer equation15:
D = Kλ/FWHMcosθ (Equation 2)
Where, FWHM is the width in radians of the diffraction maximum measured at a half-way height between the background and the peak, generally known as full-width at half-maximum (FWHM); θ is the diffraction angle; λ is the X-ray wavelength (1.542 Å) and K (Scherrer constant) is a dimensionless number that provides information about the shape of the crystal (in case of the absence of detailed shape information K = 0.9 is a good approximation). Please note that the Scherrer equation can be used to estimate mean crystal sizes of up to about 100 nm since the peak broadening is inversely proportional to the crystallite size. Therefore, its application is useful for determining the thickness of nanoplatelets and, indirectly, the number of aggregated lamellae. Examples of using this well-known approach for screening the crystal properties of lipids in the pharmaceutical formulation development and the corresponding instability in product performance can be found in5,12,16,17,18.
Monitoring the solid-state of LBEs within each developmental stage through well-established analytical techniques provide an effective strategy for designing high-performance manufacturing processes and stable lipid-based pharmaceutical products.
This publication presents the critical application of a comprehensive solid-state analysis of LBEs for monitoring the changes in solid-state and its correlation to the alteration in the release profile of active pharmaceutical ingredient (API) from the pharmaceutical dosage form. Multiparticulate systems based on lipid-coated API crystals via hot-melt coating, and nano-lipid suspensions produced via high-pressure homogenization are taken as case studies. The focus of this publication is the application of powder x-ray diffraction and DSC as analytical tools. The first two examples show the effect of polymorphic transformation and crystal growth, respectively, on the alteration in API release from coated samples. The last example reveals the correlation between the stable solid-state of lipid and the stable performance of the pharmaceutical product in lipid-coated multiparticulate systems and in nano-lipid suspensions.
Protocol
1. Differential scanning calorimetry (DSC)
- Instrument preparation
- Use a differential scanning calorimeter equipped with an intracooler, an autosampler, and the software for instrument control and data analysis.
- Switch on the nitrogen gas supply and set the pressure between 0.2–0.5 bar and power up the DSC instrument and the automatic sample changer.
- Open the software and activate the standby mode by clicking on the Yes button. Allow equilibration of the device for at least one hour
- Purge the furnace with nitrogen, click on the New Method icon and go to Method Definition. Activate the Temperature Modulation option in the overview window. Go to header tab and select the method by clicking on “sample”.
- Go to the tab Temperature Program, select Purge 2 MFC and on Protective MFC, both at 50 mL/min.
- Insert the following measurement method: Standby at 20 °C, heating cycle at 5 K/min from 20 °C to above the melting temperature of lipid, isothermal holding at this temperature for 5 min, cooling cycle to 0 °C at 5 K/min to -20 °C, final emergency reset temperature at a temperature 10 degrees °C above the highest temperature of the program, and final standby temperature at 20 °C.
- Go to the calibration tab and select the appropriate temperature and sensitivity file. Save the method
- Sample preparation and measurement
- Weigh 3–4 mg of each sample into aluminum crucibles. Record the exact weight loaded into each crucible and seal the aluminum crucible with a pierced lid.
- Place the crucibles in the autosampler tray and activate the autosampler mode in the software and load related method for each sample.
- Fill out the sample position, sample name, and weight of each sample, and the position of reference crucible in the Sample Tray View window and start the measurements.
- Data analysis
- Open the raw data using the software for data analysis and plot the temperature versus heat flow, by clicking on the button “X-time / X-temperature”
- On the popped out window, click on “Hide isothermal segments”. On the left side of the screen, select only the curves that are to be analyzed (e.g. unclick the “Additional” data).
- Check the thermal behavior of lipids as endothermic and exothermic events of absorbed or released energy in the form of heat, respectively, as a function of temperature.
- Click on the curve, followed by Evaluation and Area, to calculate the enthalpy of fusion as the area under the curve of endotherms.
- Select the integration boundaries by moving the vertical lines around 2 to 3 degrees Celsius before and after the onset and endpoint of the peak.
- Select a linear baseline for the peak integration. The area between the curve and the baseline is proportional to the change in enthalpy. Click on Apply to finish the calculation. Similarly, calculate the enthalpy of crystallization as the area under the curve of exotherms
- Determine the onset of melting temperature (To) by clicking on the curve to be analyzed and then on Evaluation and Onset.
- Select the quantification boundaries by moving the vertical lines to the most straight section of the curve. This is usually around 5-10°C before and after the peak. Then, determine the melting temperature by clicking on the curve to be analyzed, followed by Evaluation and Peak. The obtained value is the peak maximum.
2. Small- and wide-angle x-ray scattering (SWAXS)
- Instrument preparation
- Use an x-ray scattering system, composing a point-focusing camera fixed to a sealed-tube x-ray generator and equipped with a control unit and related software.
- Use cooper (λ = 1.54 Å) at 50 kV and 1 mA as an x-ray source and two linearly positioned sensitive detectors to cover both small- and wide-angle x-ray scattering regions.
- Ensure safety requirements to protect from x-ray exposure.
- Switch on the cooling water system on the control unit, the vacuum pump, the gas valves, and the power and safety control system.
- Switch on the voltage control and purging valves for the detectors at a gas flow between 10–20 mL/min.
- Switch on the x-ray tube and the standby option and wait approximately 10 min. Switch off the standby mode and power up the x-ray tube to full power (>50kV) and wait at least 30 minutes.
- Start the control software and click on RESET TPF. Choose Tugsten filter and set position. Go to Position to fix the position of the Tungsten filter
- Sample preparation and measurement
- Make sure that the samples are available as fine powder. If necessary, grind the samples gently at low temperatures to provide a fine powder.
- Fill the samples into special glass capillaries with an external diameter of approximately 2 mm, avoiding any air entrapment in the capillaries. Seal the glass capillary with wax and place it carefully into the capillary holder.
- Switch on the motor for sample rotation and close the vacuum valve until the pressure is beneath 5 mbar.
- In the software, fix the measurements setting by selecting a position resolution of 1024. Fix the exposure time to 1200 s.
- Setup the energy limits by clicking on the tap Tools, then click on energy and resolution, and click on restart. Setup the energy limits to a suitable range between 400-900.
- Open the safety shutter and start the measurements. Ensure that the measurement window shows a maximum of 80 counts per second. If this is not given, adapt the filter position.
- Data analysis
- Export the data as p00 files. The data consists of the intensity of transmission and absorption versus the channel number and the diffraction angle.
- Transfer the data for evaluation to a statistical software and correct the data by normalizing the intensities using the scattering mass measured with the Tungsten filter.
- Create a plot of normalized intensity versus two times the diffraction angle [(2Θ) 2xtheta].
- Use the function of “screen reader” to find diffraction peaks in SAXS and WAXS regions.
- Apply Bragg's equation to compute the diffraction peaks with maximum intensity into short and long d-spacing for WAXS and SAXS regions, respectively.
- Calculate the ratios of the peak position of the SAXS region to find out the crystal symmetry of the lipids (e.g., lamellar, hexagonal, cubic).
- Use the main diffraction peak of the SAXS region to quantify the crystallite thickness (D). Fit the peak into a Gaussian function via classical least squares and obtain the FWHM by clicking on Analysis, Peaks and baselines, Peak analyzer, Open dialog.
- On the popped out window, select the option “Fit Peaks Pro”. Select a constant baseline with y = 0, and select the main diffraction peak of the SAXS region and click on “Fit control” to select the peak fit parameters.
- Choose the GaussAmp function. Set the parameters y_0, xc_1 and A_1 as fixed and obtain the FWHM from the fit. Use the Scherrer equation to compute the crystallite thickness.
3. Dissolution tests
- API release from coated multiparticulate systems
- Use USP apparatus 2 (paddle) for dissolution studies.
- Fill the dissolution test vessels with phosphate buffer pH 6.8, and heat to 37 °C.
- Weigh triplicates of samples of coated particles equivalent to a single dose of API, and place the samples into dissolution test vessels.
- Start the paddle at a speed of 100 rpm.
- Set the auto-sampler to take samples of 1 mL at the following sampling points: 30 min, 60 min, 90 min, 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 18 h, and 24 h.
- Analyze the samples via a suitable HPLC method5,7,17.
- Analyze the data by plotting the cumulative API release versus time.
- Carry out the experiments for stored samples under long-term (25 °C, 60% relative humidity) and accelerated (40 °C and 70% relative humidity).
- API release from solid lipid-nanoparticles (SLN)
- Prepare simulated lung fluid (SLF) by mixing 0.02% (w/w) of dipalmitoylphosphatidylcholine in Dulbecco's phosphate buffer saline (D-PBS), with the following composition: KCl (2.683 mM), KH2PO4 (1.47 mM), NaCl (136.893 mM), Na2HPO4·2H2O (8.058 mM), CaCl2·2H2O (0.884 mM), and MgCl2·6H2O (0.492 mM). Pre-heat it at 37 °C.
- Use dialysis cassettes with a cellulose membrane bag of a controlled cut-off of 7,000 Da in triplicate for each sample.
- Assign one dialysis cassette for each sampling time: 0.5 h, 1.5 h, 3 h, 5 h, 7 h, and 24 h. Hydrate the dialysis cassettes for 2 min by immersing them in SLF. Then, carefully dry their surface with soft paper towels.
- Inject 3 mL of the sample (lipid-nanosuspension), equivalent to 600 µg of dexamethasone, into each cassette.
- Immerse each cassette in 200 mL of SLF at 37 °C (sink conditions) and agitate the system at 125 rpm.
- Take 200 mg samples from inside the cassette using a syringe at each determined sampling time.
- Determine the API content using the developed HPLC-MS method18.
- Calculate the API released from SLN by mass balance, according to18, briefly as the difference between the total amount of API in SLN and the remaining amount of API after sampling.
- Repeat the process for stored samples.
Representative Results
Correlation between polymorphic transition of lipid and API release in lipid-coated API crystals:
API crystals coated with glycerol monostearate are measured via DSC and x-ray directly after coating and after 3 months of storage under accelerated conditions (40 °C, 75% relative humidity)7. Glyceryl monostearate is a multiphasic system containing 40%-55% monoglycerides, 30%-45% diglycerides, and 5%-15% glycerides, mainly tristearin19. The polymorphic forms of sub-α, α, β-prime, and β are reported for monostearin20. Tristearin and 1, 2-distearin show α, β-prime, and β polymorphic forms14.
The DSC data of T0 samples and samples stored under accelerated conditions are shown in Figure 3A. The heating cycle on the T0 sample showed a wide endotherm event up to 10 °C, which can be correlated to the reversible sub-α / α transition described for 1-monostearin and 1-monopalmitin21. Two endothermic events at To = 54.0 °C and 46.7 are correlated to the β-form and a coexisting phase of lower melting point. A coexisting phase can be seen in the x-ray data as the short d-spacing of 4.16 Å corresponding to the polymorphic α-form, organized in a lamellar phase of 57.3 Å and corresponding to different components of the mixture. The lamellar arrangement of lipid coating of T0 sample is given due to the available harmonic peak at 18.7 Å corresponding to the third order reflection in SAXS diffractogram7 (Figure 3B).
DSC data of samples after 3 months of storage under accelerated conditions show an endotherm at To = 55.7 °C, with two overlapped events at Tm = 60.2 °C for the remaining α-form and at Tm = 63.8 °C as the main event for the melting of β-form. The polymorphic transition is confirmed with the x-ray data by detecting short d-spacings of 4.66 Å, 4.58 Å, 4.37 Å, 3.92 Å, and 3.83 Å, typical for the β-form, combined with the reduction in the lamella thickness from 57.3 Å to 50.4 Å, due to the molecular tilting7.
Comparing the release profile of API from coating at T0 and after 3 months of storage under accelerated conditions (Figure 3C) show significant alteration in the release profiles, which can be explained by the evident polymorphic transformation of α-form to β-form with a denser sub-cell arrangement, resulting in a water repellent surface7,21.
Correlation between crystal growth of lipid coating, potential phase separation, and release alteration in lipid-coated API crystals: API crystals are coated with a mixture of tripalmitin and polysorbate 65 in 90:10 %w/w ratio. Tripalmitin is a triglyceride with a purity of 99%5. Triglycerides commonly show the α, β-prime, and β polymorphic forms, ordered by the increased density of crystal pack and increased stability.
Polysorbate 65 is an emulsifier with a hydrophilic-lipophilic balance (HLB) value of 10.5 and a melting temperature of 32 °C. Triglycerides are commonly crystallized in their α-polymorph from the melt. Certain additives induce the transformation of α to β of TAGs, among them polysorbate 65. Moreover, polysorbate 65 acts as an impurity in the system, causing heterogeneous crystallization of tripalmitin at lower driving forces and triggering crystal growth.
The DSC and x-ray data of T0 samples and samples stored under accelerated conditions are shown in Figure 4A,B. The heating cycle of DSC measurements on the T0 sample shows a sharp endothermic event with a peak at 64.8 °C, corresponding to the polymophic β-form of tripalmitin5. This is also detectable in the WAXS region, showing the short-spacing at 4.6 Å, characteristic of the sub-cell of the β-form (Figure 4A,B). The data show, clearly, the induced polymorphic β-form of tripalmitin in the presence of polysorbate 65 at T0 samples and, of course, in stored samples. The corresponding lamellar thickness is calculated using Bragg's equation as d = 2π/q001 = 42 Å5.
The crystal thickness (D) of T0 samples and stored samples can be measured using the Scherrer equation described above. The calculations show a crystal thickness of 24 nm in T0 samples and an increased thickness of 37 nm in stored samples, corresponding to 5.7 and 8.8 lamellae, respectively.
Comparing the release profile of API from coating at T0 and after 3 months of storage under accelerated conditions again shows significant alteration in the release profiles after storage (Figure 4C).
Due to the fact that the mixture of tripalmitin and polysorbate 65 is a two-phasic system, the crystallite growth of tripalmitin is triggered by the existence of polysorbate phase, specially under the accelerated condition (40 °C, 75% relative humidity) where polysorbate 65 is in its liquid molten form. The phase transition and the growth of polysorbate phase under accelerated condition are most probably due to motion of liquid material due to the capillarity and gravity forces5,22. The consequence is the alteration in the API release from coating5.
Correlation between stable solid-state of lipids and stable performance of lipid-based pharmaceutical products: Two different lipid-based pharmaceutical products are assessed: (a) a solid dosage form composed of API crystals coated with lipid-based excipients17 and (b) a liquid dosage form composed of suspended solid-lipid nanoparticles loaded with an API18. The LBEs employed for both products are polyglycerol esters of fatty acids (PGFAs), a group of lipid molecules consisting of oligomeric hydroxyethers of glycerol fully or partially esterified with fatty acids. PGFAs are characterized by monophasic crystallization into α-form, absence of polymorphic transitions, and overall stability of their molecular, nano, and microstructure23.
In the first product, API crystals were coated with PG3-C16/C18p, a PGFA composed of 3 glycerol units partially esterified with palmitic and stearic acid. The DSC and x-ray data of T0 and 3-month stored samples under accelerated conditions are shown in Figure 5. DSC analysis (Figure 5A) shows a single melting peak in the first heating cycle corresponding to the existence of only one polymorphic form of PG3-C16/C18p with To = 54.2 °C. The cooling cycle reveals the monophasic crystallization of the lipid through the existence of one single peak with Tc = 45.4°C. Stored samples reveal unchanged thermograms too, which depict no polymorphism and no phase separation. The stable solid-state of PG3-C16/C18p is confirmed by the SWAXS patterns (Figure 5B). WAXS region shows a peak corresponding to a short-spacing of d = 4.15 Å in T0 and stored samples17. Such short d-spacing is associated with the α-form of TAGs1,13. The unaltered WAXS signal after storage confirms the absence of polymorphism of PG3-C16/C18p. SAXS region reveals a main peak at a long d-spacing of d = 63.7 Å, corresponding to a lamellar structure with 2L-configuration. The crystallite size (D) of T0 samples obtained via Scherrer analysis depicts 23 nm, corresponding to four stacked lamellae. No alterations of lamella thickness (63.5 Å) or crystal growth (four lamellae) are shown after storage. A comparison of the release profile of T0-samples and after storage (Figure 5C) shows the developed product's outstanding stability. The stable solid-state of the lipid matrix provided by PG3-C16/C18p results in the stable performance of the release profile of the product17.
For the second product, API-loaded solid lipid-nanoparticles (SLN) in the form of aqueous nanosuspension were prepared using PG2-C18f as lipid matrix and Poloxamer 188 as emulsifier18. PG2-C18f is a PGFA molecule composed of 2 glycerol units fully esterified with stearic acid. Poloxamer 188 is a non-ionic block polymer with a high HLB of 29. The chemical structure is composed of polyoxypropylene and polyoxyethylene parts. The API is encapsulated into the lipid matrix. Within this product, the solid-state of the lipid can be impacted not only by the processing conditions but also by water-nanoparticle interactions and emulsifier-lipid interactions. The DSC and X-ray data of nanosuspensions at T0 and after 3 months of storage under accelerated conditions are shown in Figure 6. DSC analysis shows an endothermic event at To = 55.3 °C followed by a wide endotherm extended up to 100 °C. The first event attributed to the melting of SLN of PG2-C18f and the wide endotherm is due to water evaporation. Since Poloxamer 188 is dissolved in the water phase, no endotherm is depicted in the first cycle. Stable thermal behavior is depicted in the DSC analysis of stored samples, which show no alterations. Although lipid polymorphism is usually accelerated in nanosized systems, SWAXS analysis confirms the stable behavior of the lipid matrix. The measured short d-spacing of 4.15 Å for PG2-C18f after its crystallization in the freshly manufactured SLN and after 6 months storage of samples under accelerated condition (6m/AC) indicates the presence of the stableα-form. The lamella thickness of PG2-C18f within SLN at T0 (56.5 Å) and after storage (56.3 Å) shows no alterations. The lamellar structure of the lipid is evidenced by a harmonic signal at 18.7 Å. The crystallite size (D) of PG2-C18f by Scherrer analysis is found to be 10.8 nm (two lamellae), showing no crystal growth after storage of the nanosuspensions (11.7 nm, two lamellae)18. The use of SLN in the pharmaceutical industry has been hindered due to well-reported stability issues after storage, such as particle agglomeration and gelation, loss of encapsulation (API expulsion), and unstable release profile. Instead, the application of a stable lipid matrix, PG2-C18f, as herein shown, results in the product performance presented in Figure 7. No particle agglomeration, stable release profile, and stable encapsulation efficiency are depicted. The general instability of SLN has been attributed to lipid polymorphism and other solid-state transitions24. Polymorphic lipids suffer transitions during storage from less dense crystal forms (α-form) to more dense (β-prime and β). This polymorphic transition can affect the surface area of manufactured nanoparticles, especially, if the surface area is not sufficiently stabilized by emulsifier. The consequent can be instabilites such as agglomeration or gelation. Also, the alteration of crystal density from α to β causes the loss of sufficient space for the API within the lipid matrix, which leads to API expulsion, alterations in the encapsulation efficiency, and the release profile. Considering the small size of SLN (in this study x50 = 234.3 nm), the effects of crystal growth on product performance become critical too. The use of a lipid matrix with a stable solid-state resulted in stable product performance18.
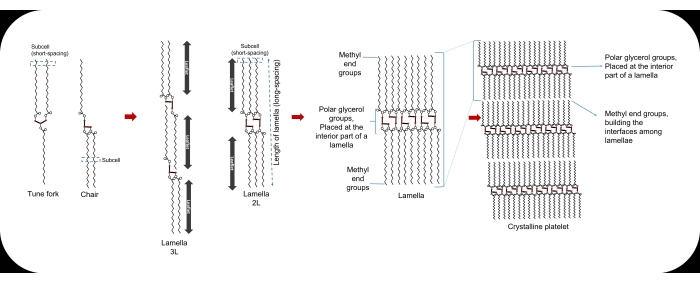
Figure 1: The tune fork and chair configurations of a TAG molecule, the sub-cell, the lamella, and the crystalline platelet. Please click here to view a larger version of this figure.
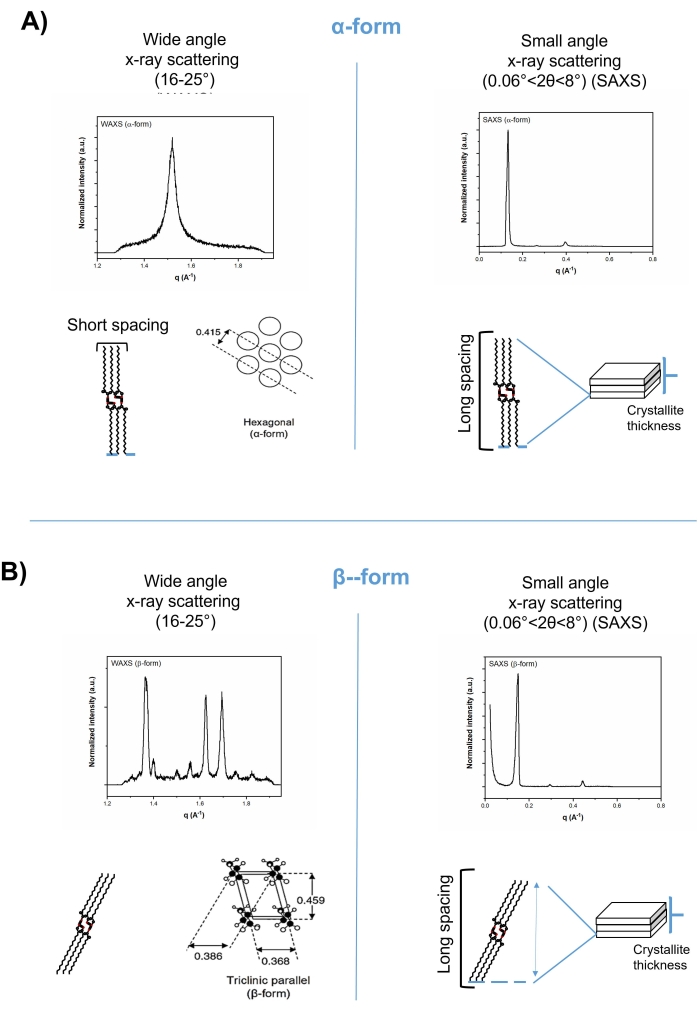
Figure 2: Short spacing (left-hand) and long spacing (right-hand) patterns of tripalmitin in wide-angle and small-angle regions of x-ray diffractograms, respectively. (A) The alpha-form, and (B) the beta-form. Please click here to view a larger version of this figure.
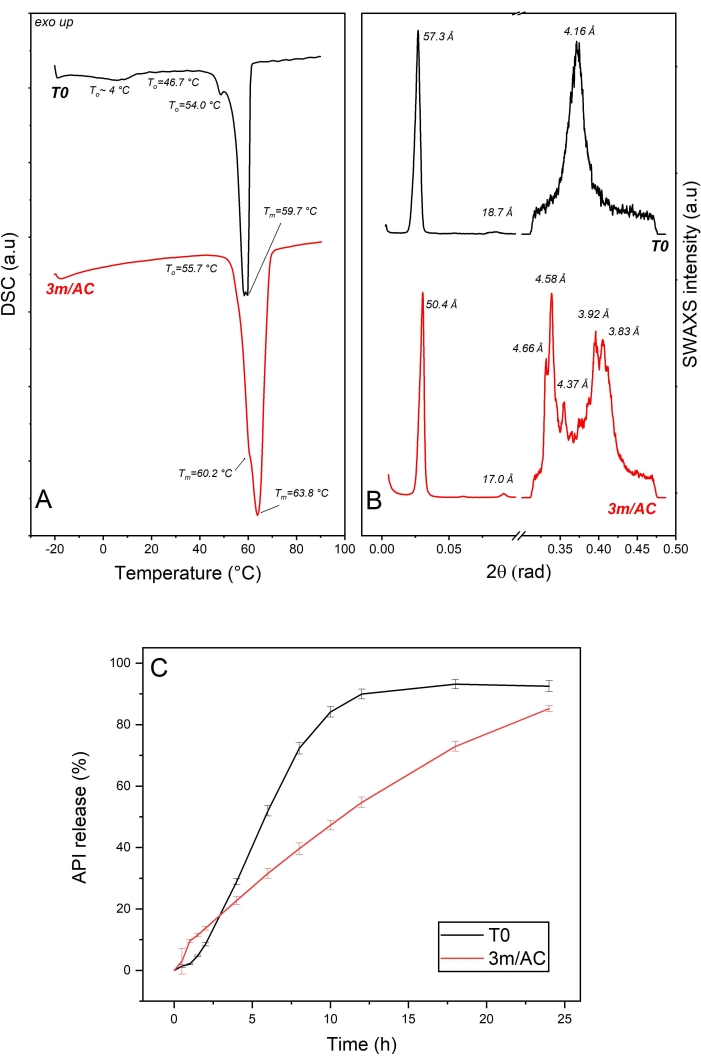
Figure 3: API crystals coated with glycerol monostearate: solid state analysis of lipid as coating material and API release profile of freshly prepared samples (T0) and after 3-month storage under accelerated conditions (AC). (A) DSC, (B) SWAXS, and (C) release profile. This figure has been modified from7. Please click here to view a larger version of this figure.
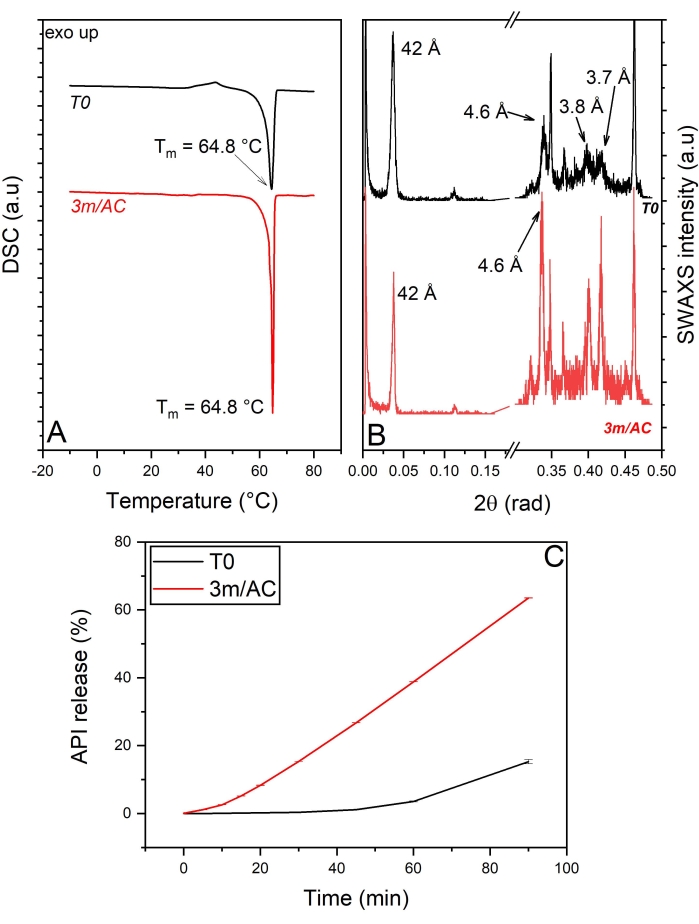
Figure 4: API crystals coated with tripalmitin and polysorbate 65 (90:10 %w/w): Solid state analysis of coating material and API release of freshly prepared samples (T0) and after 3-month storage under accelerated conditions (AC). (A) DSC, (B) SWAXS, (C) API release profile. This figure has been modified from5. Please click here to view a larger version of this figure.
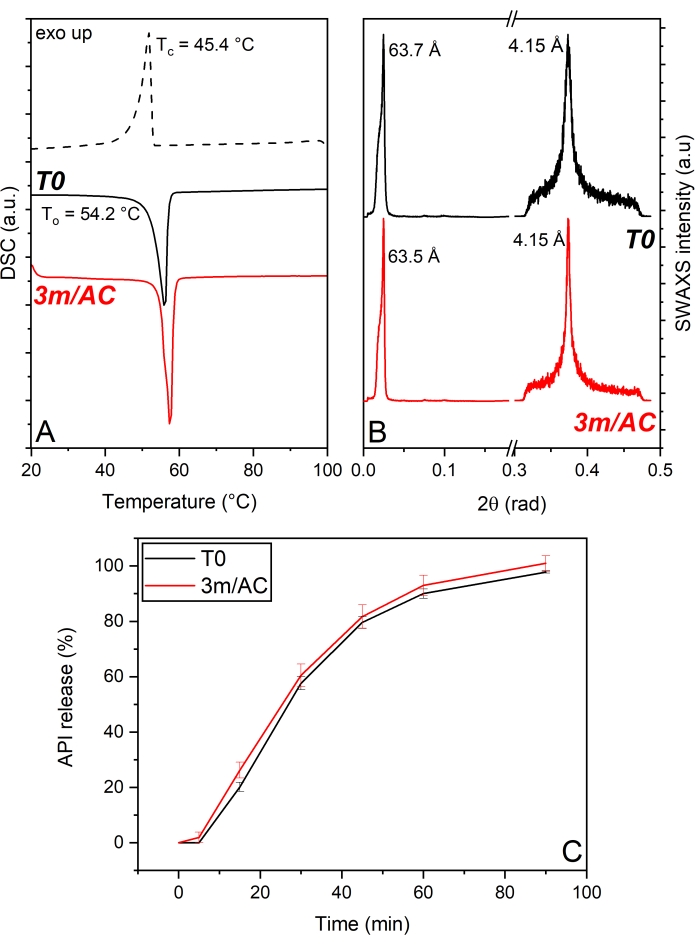
Figure 5: API crystals coated with PG3-C16/C18p: Solid state analysis of PG3-C16/C18p as coating material and API release profile of freshly prepared samples (T0) and after 3-month storage under accelerated conditions (AC). (A) DSC, (B) SWAXS, (C) API release profile. This figure has been modified from17. Please click here to view a larger version of this figure.
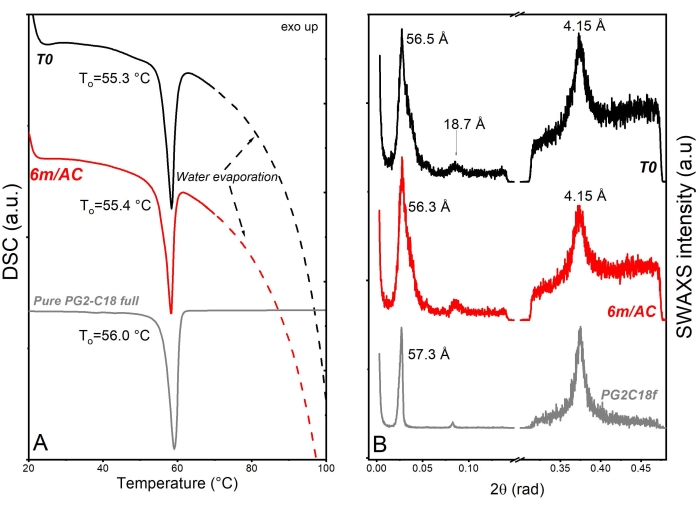
Figure 6: Solid state analysis of freshly prepared SLN samples (T0), after 3-month storage under accelerated conditions (AC), and raw lipid excipient. (A) DSC and (B) SWAXS. This figure has been modified from18. Please click here to view a larger version of this figure.
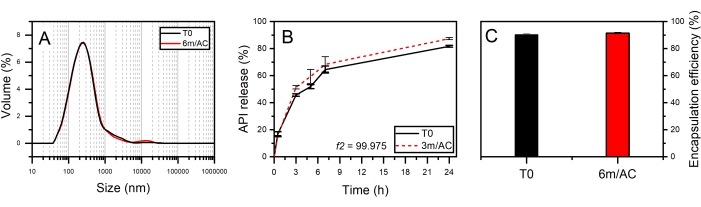
Figure 7: Product performance of freshly prepared SLNs (T0) and after 3 and 6-month storage under accelerated conditions (3m/AC, 6m/AC). (A) Particle size distribution, (B) release profile, (C) encapsulation efficiency. This figure has been modified from18. Please click here to view a larger version of this figure.
Discussion
Powder x-ray diffraction and DSC were described in this manuscript as gold standards for the solid-state analysis of LBEs. Powder x-ray diffraction has the outstanding advantage of processing the measurements in situ, with minimum solid-state manipulation of samples during the measurements. Moreover, the same-filled capillaries can be stored under different conditions after initial measurements to investigate the solid-state alteration during storage. In this work, we focused on the wide- and small-angle regions of x-ray, enabling us to deliver structural data of dimensions up to approximately 100 nm.
Ultra SAXS (USAXS) can be used for tracking the crystallite nanoplatelets' (CNP) aggregation and crystalline growth in larger dimensions. The method has been successfully applied in particular systems to analyze CNP sizes in the region of approximately 100 to 1,000 nm15,26,27. Characterization of lipid crystals in liquid systems requires higher resolution. Synchrotron radiation and providing x-ray flux with higher intensity is normally used for such characterization28. Synchrotron x-ray diffraction and small angle neutron scattering (SANS) are powerful tools for the characterization of liquid crystals and multilayer self-emulsifying systems such as liposomes, which are not the scope of this article25,28,29. The liquid systems can also be characterized using the x- ray set-up described in the protocol by adjusting the radiation time for a longer period.
The following points are to be noted for implementing the x-ray for screening the solid-state of lipids and their compositions: (i) Generally, the radiation time should be selected individually, based on the nature of samples and equipment settings. (ii) The intensity of signals is directly proportional to the material concentration in a blend. Therefore, it is important to screen, at first, the physical mixture of a multiphasic composition. This will avoid the wrong interpretation of data in amorphous solid dispersions (ASDs), if the recrystallization of amorphous API in its processed composition with a LBE is investigated. To detect small fractions of crystals in such compositions, it is important to zoom in on regions the signals are expected in. (iii) Grinding the samples to provide fine powder for filling in capillaries should be performed at a low temperature to avoid external heat and stress. This may cause alteration in the solid-state of the lipid in the sample. The dense filling of capillaries is important to avoid air entrapment between particles and to ensure the immaculate scattering of x-ray by particles.
DSC is a powerful tool for screening the thermal behavior of lipids, estimating the miscibility of additives and/or API in the lipid matrix, and providing phase diagrams. The thermodynamic event, including the melting and crystallization onsets and peaks, as well as the enthalpy of each event, provides useful information on the available polymorphic forms, possible polymorphic transitions, and different phase transitions. However, contrary to x-ray diffraction, the applied heat in the DSC measurements can manipulate the solid-state behavior of lipids and cause polymorphic and phase transition during the measurements. Therefore, it is strongly recommended to avoid the sole usage of this technique for lipid solid-state analysis. This method should be used as a complementary technique to x-ray diffraction. Coupled DSC and x-ray diffraction has been broadly used in the food industry for lipid solid-state analysis30,31,32,33,34. Its application in the pharmaceutical industry is rather limited to the detection of polymorphic changes in APIs35,36,37. The other disadvantage of the sole use of DSC is the characterization of multiphasic lipid systems, because the intensity of thermal events is concentration-dependent. Moreover, overlapping thermal events can occur. Temperature-modulated-DSC can be used for the characterization of multiphasic systems, which enables the separation of kinetic events and overlapping transitions38,39.
The following points are to be noted for implementing the DSC trials described in the protocol: (i) Based on the experiments, a second heating cycle can be applied if necessary. (ii) Due to the constant behavior of the specific heat capacity (Cp) of lipids during analysis, the selection of a linear baseline is appropriated. (iii) To obtain the melting onset (To), the limits of calculation should be defined. The minimum and maximum limits should include the extreme point of the derivative curve and the most linear range of the baseline. The intersection point between the inflectional tangent and the baseline is determined as To.
In the case of thermograms with well-separated peaks, it is recommended to consider the enthalpy of each event by calculating the area under the curve. The data are useful to explain the degree of polymorphic or phase transition in the system, in combination with the x-ray diffraction data.
This manuscript deals with the gold standards for analysis of LBEs and their pharmaceutical products. Other analytical methods can be used as complementary methods. Examples are qualitative microscopical methods such as polarized light microscopy and scanning electron microscopy, to investigate the effect of process stress on the crystallization rate and, consequently the shape and morphology of crystals. The approach of lipid-based pharmaceutical product development should be based on the characterization of physicochemical LBEs, to define their critical attributes relevant for selected manufacturing processes, and to predict their processability. The excipient-API interactions should also be screened carefully for each individual API40. Further analytical methods should be added based on the selected manufacturing process. The structure-function-processability relationship should be understood carefully to design robust and stable lipid-based pharmaceutical products.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The Research Center Pharmaceutical Engineering (RCPE) is funded within the framework of COMET – Competence Centers for Excellent Technologies by BMK, BMDW, Land Steiermark and SFG. The COMET program is managed by the FFG.
Materials
| CaCl2·2H2O | Sigma-Aldrich | 223506 | |
| Cassettes with a cellulose membrane bag with a cut-off of 7000 Da, Thermo Scientific Slide-A-Lyzer 7K | Fisher Scientific Inco, USA | ||
| Control software of x-ray system | HECUS dedicated house equipment | ||
| Control unit of x-ray system | HECUS dedicated house equipment | ||
| Differential scanning calorimeter (DSC) aluminum crucibles and lids | Netzsch, Germany | ||
| Differential scanning calorimeter DSC 204 F1 Phoenix (NETZSCH, Germany). | Netzsch, Germany | ||
| Dipalmitoylphosphatidylcholine (DPPC) | Sigma-Aldrich | 850355P | |
| Dissolution paddle apparatus II, Erweka DT 828 LH | Erweka GmbH, Langen, Germany | ||
| Dynasan 116 | IOI OLEO | Tripalmitin | |
| Geleol | Gattefosse | Glyceryl monosterarate | |
| KCl | Sigma-Aldrich | 529552 | |
| KH2PO4 | Sigma-Aldrich | P0662 | |
| Kolliphor P 188 | BASF Chem Trade | Poloxamer 188 | |
| MgCl2·6H2O | Sigma-Aldrich | M2670 | |
| Na2HPO4·2H2O | Sigma-Aldrich | S9763 | |
| NaCl | Sigma-Aldrich | S9888 | |
| Netzsch DSC 204F1 Software Version 8.0.1 | Netzsch, Germany | 6.239.2-64.51.00 | |
| Origin Pro (OriginLab, Northampton, MA) (statistical software | OriginLab, Northampton, MA | ||
| Proteous Analysis Software | Netzsch, Germany | ||
| Tween 65 | Polysorbate 65 | ||
| Witepsol PMF 1683 | IOI OLEO | Triglycerol ester of stearatic/palmitic acid (partially esterified) | |
| Witepsol PMF 282 | IOI OLEO | Diglycerol ester of stearic acid | |
| X-ray HECUS system composed of a point-focusing camera and two linearly positioned sensitive detectors | HECUS dedicated house equipment |
References
- Sato, K. Crystallization behaviour of fats and lipids a review. Chemical Engineering Science. 56 (7), 2255-2265 (2001).
- Becker, K., Salar-Behzadi, S., Zimmer, A. Solvent-free melting techniques for the preparation of lipid-based solid oral formulations. Pharmaceutical Research. 32 (5), 1519-1545 (2015).
- Rosiaux, Y., Jannin, V., Hughes, S., Marchaud, D. Solid lipid excipients – Matrix agents for sustained drug delivery. Journal of Controlled Release. 188, 18-30 (2014).
- Siepmann, J., et al. Lipids and polymers in pharmaceutical technology: lifelong companions. International Journal of Pharmaceutics. 558, 128-142 (2019).
- Lopes, D., et al. Microphase separation in solid lipid dosage forms as the cause of drug release instability. International Journal of Pharmaceutics. 517 (1-2), 403-412 (2017).
- Reitz, C., Kleinebudde, P. Solid lipid extrusion of sustained release dosage forms. European Journal of Pharmaceutics and Biopharmaceutics. 67 (2), 440-448 (2007).
- Salar-Behzadi, S., Corzo, C., Schaden, L., Laggner, P., Zimmer, A. Correlation between the solid state of lipid coating and release profile of API from hot melt coated microcapsules. International Journal of Pharmaceutics. 565, 569-578 (2019).
- Windbergs, M., Gueres, S., Strachan, C. J., Kleinebudde, P. Two-step solid lipid extrusion as a process to modify dissolution behavior. AAPS PharmSciTech. 11 (1), 2-8 (2010).
- Schertel, S., Salar-Behzadi, S., Zimmer, A. Impact of surface properties of core material on the stability of hot melt-coated multiparticulate systems. Pharmaceutics. 13 (3), 366 (2021).
- Tang, D., Marangoni, A. G. Microstructure and fractal analysis of fat crystal networks. Journal of the American Oil Chemists’ Society. 83, 377-388 (2006).
- Corzo, C., et al. Lipid-microparticles for pulmonary delivery of active pharmaceutical ingredients: Impact of lipid crystallization on spray-drying processability. International Journal of Pharmaceutics. 610, 121259 (2021).
- Acevedo, N. C., Marangoni, A. G. Characterization of the nanostructure of triacylglycerol crystal networks. Structure-Function Analysis of Edible Fats. , (2012).
- Marangoni, A. G. Structure-function analysis of edible fats. Structure-Function Analysis of Edible Fats. , (2018).
- Sato, K., Sato, K. Crystallization of lipids. Fundamentals and Applications in Food, Cosmetics, and Pharmaceuticals. , (2018).
- Acevedo, N. C., Marangoni, A. G. Toward nanoscale engineering of triacylglycerol crystal networks. Crystal Growth and Design. 10 (8), 3334-3339 (2010).
- Lopes, D. G., et al. Role of lipid blooming and crystallite size in the performance of highly soluble drug-loaded microcapsules. Journal of Pharmaceutical Sciences. 104 (12), 4257-4265 (2015).
- Salar-Behzadi, S., et al. Novel approach for overcoming the stability challenges of lipid-based excipients. Part 2: Application of polyglycerol esters of fatty acids as hot melt coating excipients. European Journal of Pharmaceutics and Biopharmaceutics. 148, 107-117 (2020).
- Corzo, C., Meindl, C., Lochmann, D., Reyer, S., Salar-Behzadi, S. Novel approach for overcoming the stability challenges of lipid-based excipients. Part 3: Application of polyglycerol esters of fatty acids for the next generation of solid lipid nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics. 152, 44-55 (2020).
- Tylor, A. K., Rowe, R. C., Sheskey, P. J., Quinn, M. E. Glyceryl monostearate. Handbook of Pharmaceutical Excipients. , 290-293 (2009).
- Lutton, R. S., Jackson, F. L. The polymorphism of 1- monostearin and 1-monopalmitin. Journal of the American Chemical Society. 70 (7), 2445-2449 (1948).
- Fang, W., Mayama, H., Tsujii, K. Spontaneous formation of fractal structures on triglyceride surfaces with reference to their super water-repellent properties. The Journal of Physical Chemistry. B. 111 (3), 564-571 (2007).
- Maleky, F., Marangoni, A. Nanoscale effects on oil migration through triacylglycerol polycrystalline colloidal networks. Soft Matter. 7, 6012-6024 (2011).
- Corzo, C., et al. Novel approach for overcoming the stability challenges of lipid-based excipients. Part 1: Screening of solid-state and physical properties of polyglycerol esters of fatty acids as advanced pharmaceutical excipients. European Journal of Pharmaceutics and Biopharmaceutics. 148, 134-147 (2020).
- Gordillo-Galeano, A., Mora-Huertas, C. E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. European Journal of Pharmaceutics and Biopharmaceutics. 133, 285-308 (2018).
- Fan, Y., Marioli, M., Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. Journal of Pharmaceutical and Biomedical Analysis. 192, 113642 (2021).
- Peyronel, F., Pink, D. A., Marangoni, A. G. Triglyceride nanocrystal aggregation into polycrystalline colloidal networks: Ultra-small angle X-ray scattering, models and computer simulation. Current Opinion in Colloid & Interface Science. 19 (5), 459-470 (2014).
- Acevedo, N. C., Marangoni, A. G. Functionalization of non-interesterified mixtures of fully hydrogenated fats using shear processing. Food and Bioprocess Technology. 7 (2), 575-587 (2014).
- Dong, Y. D., Boyd, B. J. Applications of X-ray scattering in pharmaceutical science. International Journal of Pharmaceutics. 417 (1-2), 101-111 (2011).
- Di Cola, E., Grillo, I., Ristori, S. Small angle X-ray and neutron scattering: Powerful tools for studying the structure of drug-loaded liposomes. Pharmaceutics. 8 (2), 10 (2016).
- Lopez, C., Lesieur, P., Bourgaux, C., Ollivin, M. Thermal and structural behavior of anhydrous milk fat. 3. Influence of cooling rate. Journal of Dairy Science. 88 (2), 511-526 (2005).
- Kalnin, D., Garnaud, G., Amenitsch, H. Ollivon. Monitoring fat crystallization in aerated food emulsions by combined DSC and time-resolved synchrotron X-ray diffraction. Food Research International. 35 (10), 927-934 (2002).
- Bugeat, S., et al. Unsaturated fatty acid enriched vs. control milk triacylglycerols: Solid and liquid TAG phases examined by Synchrotron radian X-ray diffraction coupled with DSC. Food Research International. 67, 91-101 (2015).
- Brubach, J. B., et al. Structural and thermal characterization of glyceryl behenate by X-ray diffraction coupled to differential calorimetry and infrared spectroscopy. International Journal of Pharmaceutics. 336 (2), 248-256 (2007).
- Chong, C. L., et al. Thermal and structural behaviour of crude palm oil: Crystallisation at very low cooling rate. European Journal of Lipid Science and Technology. 109 (4), 410-421 (2007).
- Askin, S., et al. A simultaneous differential scanning calorimetry-X-ray diffraction study of olanzapine crystallization from amorphous solid dispersions. Molecular Pharmaceutics. 17 (11), 4364-4374 (2020).
- Clout, A., et al. Simultaneous differential scanning calorimetry – synchrotron X-ray powder diffraction: A powerful technique for physical form characterization in pharmaceutical materials. Analytical Chemistry. 88 (20), 10111-10117 (2016).
- Jendrzejewska, I., Goryczka, T., Pietrasik, E., Klimontko, J., Jampilek, J. X-ray and thermal analysis of selected drugs containing acetaminophen. Molecules. 25 (24), 5909 (2020).
- Righetti, M. C. Crystallization of Polymers Investigated by Temperature-Modulated DSC. Materials. 10 (4), 442 (2017).
- Sauer, B. B., Kampert, W. G., Neal Blanchard, E., Threefoot, S. A., Hsiao, B. S. Temperature modulated DSC studies of melting and crystallization in polymers exhibiting multiple endotherms. Polymer. 41 (3), 1099-1108 (2000).
- Ali, F., Kumar, R., Lal Sahu, P., Singh, G. N. Physicochemical characterization and compatibility study of roflumilast with various pharmaceutical excipients. Journal of Thermal Analysis and Calorimetry. 130, 1627-1641 (2017).

