Ensayo sobre la muerte celular: Ensayo de liberación de cromo de la capacidad citotóxica
English
Diviser
Vue d'ensemble
Fuente: Frances V. Sjaastad1,2, Whitney Swanson2,3y Thomas S. Griffith1,2,3,4
1 Programa de Posgrado en Microbiología, Inmunología y Biología del Cáncer, Universidad de Minnesota, Minneapolis, MN 55455
2 Centro de Inmunología, Universidad de Minnesota, Minneapolis, MN 55455
3 Departamento de Urología, Universidad de Minnesota, Minneapolis, MN 55455
4 Centro De Cáncer Masónico, Universidad de Minnesota, Minneapolis, MN 55455
Una de las principales funciones de las células del sistema inmunitario es eliminar las células diana que han sido infectadas con virus o han sufrido transformación en una célula tumoral. Los ensayos in vitro para medir la capacidad citotóxica de las células inmunitarias han sido un elemento básico en los laboratorios durante muchos años. Estos ensayos se han utilizado para determinar la capacidad de las células T, células NK, o cualquier otra célula inmune para matar las células diana de una manera específica de antígeno o -no específico. Los ligandos de la muerte (por ejemplo, Fas ligando o TRAIL), las citoquinas (por ejemplo, IFNg o TNF) o gránulos citotóxicos (es decir, perforin/granzyme B) expresados por las células efectoras son algunas formas en que se puede inducir la muerte celular diana. Con la explosión en la investigación de inmunoterapia tumoral en los últimos años, hay un creciente interés en encontrar agentes para aumentar la actividad citotóxica de las células inmunitarias para mejorar los resultados del paciente. Por el contrario, algunas enfermedades se caracterizan por la actividad excesivamente exuberante de la actividad citotóxica de las células inmunitarias, lo que resulta en esfuerzos para identificar agentes para templar estas respuestas. Por lo tanto, tener un ensayo en el que el usuario puede integrar fácilmente cualquier número de diferentes células efectoras, células diana y / o modificadores de respuesta en el diseño experimental puede servir como un medio valioso de evaluar rápidamente la capacidad citotóxica de las células efectoras y / o la capacidad de respuesta de la célula objetivo.
Estos ensayos in vitro implican la mezcla de diferentes poblaciones celulares, así como el uso de un número relativamente bajo de células efectoras y dianas. Por lo tanto, una necesidad del ensayo es etiquetar las celdas de destino de una manera que pueda ser fácilmente detectada y cuantificada, permitiendo al usuario determinar el ‘porcentaje específico de lisis’ mediado por las células del efector. La radiactividad – especialmente, cromo 51(51Cr) en forma de Na251CrO4– es una manera económica de etiquetar rápida e inespecíficamente las proteínas celulares dentro de las células diana (1). El etiquetado corto y los tiempos totales de ensayo reducen el potencial de cambios significativos en el número y/o fenotipo de las células diana, lo que podría influir en el resultado del ensayo. Tras la pérdida de la integridad de la membrana de las células diana como resultado de la actividad citotóxica de las células efectoras, las 51proteínas celulares etiquetadas con Cr dentro de las células diana se liberan en el sobrenadante del cultivo, Cuantificación. Al igual que con cualquier ensayo que examine la función de las células inmunitarias in vitro,hay una serie de consideraciones importantes a considerar la mejora del rendimiento del experimento. Una de las características más críticas es utilizar células efectora saludable (para la máxima actividad citotóxica) y células diana (para máxima capacidad de respuesta y mínima muerte espontánea / liberación de51Cr). Se requiere contacto de células objetivo y efector (lo que lleva al uso común de placas de 96 pocillos de fondo redondo para fomentar el contacto con células celulares) (2). Por último, el análisis de datos depende de la inclusión de poblaciones de células objetivo de control positivo y negativo.
El siguiente protocolo describirá los pasos para realizar un ensayo de liberación estándar de 51Cr para medir la capacidad citotóxica de una población de células efectoras, aunque recientemente se ha desarrollado una versión no radioactiva con Europium. 51 Cr es un emisor de radiación de gran alcance. En consecuencia, el uso de este ensayo requiere un entrenamiento adecuado de seguridad de la radiación, un espacio de laboratorio dedicado, un contador gamma y la eliminación de muestras radiactivas.
La secuencia general de eventos en este ensayo es: 1) preparar 51objetivos con etiqueta Cr; 2) preparar las células efectoray y añadir a la placa mientras las células objetivo están etiquetando; 3) añadir objetivos etiquetados a la placa; 4) placa de incubación; 5) cosechar supernatantes; y 6) analizar los datos después de ejecutar muestras en el mostrador. Las muestras se preparan comúnmente en triplicado, y luego se promedian para tener en cuenta cualquier diferencia sutil de pipeteo.
El EPP adecuado es importante para este ensayo. Específicamente, el usuario debe usar una capa de laboratorio y guantes. Es posible que se requieran gafas de seguridad en función del laboratorio o la institución. Debe haber un amplio blindaje de plomo para el almacenamiento seguro y el uso de la 51Cr durante todos los pasos. Por último, debe haber espacio de laboratorio dedicado y equipo reservado para el uso de 51Cr, incluyendo toda la señalización adecuada para indicar dónde se guardan muestras con 51Cr y un contador Geiger equipado con sonda gamma para inspeccionar el espacio para Contaminación.
En este ejercicio de laboratorio, determinaremos la capacidad de las células mononucleares de sangre periférica humana (PPBCM), (CpG estimulado frente a no estimulado) para matar las células del melanoma, utilizando la línea celular de melanoma humano WM793 como modelo y el ensayo de liberación de cromo.
Procédure
Résultats
In this example, effector cells stimulated with CpG (Figure 1, black circles) killed the target cells more effectively, as the ratio of effector cells to target cells increased. This increase was not observed in the unstimulated PBMCs (white circles), indicating that CpG stimulation is necessary for the observed increase in target cell lysis.
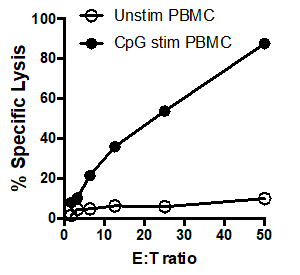
Figure 1: 51Cr assay scatter plot: Tumoricidal activity by human PBMCs, unstimulated (white circles) and after stimulation with CpG (black circles), tested at different effector: target cell ratios (E: T) ratios (ranging from. 50:1 to 1.5:1).
Applications and Summary
The assay described here has considerable flexibility, as a variety of effector and target cells can be used depending on the question being asked. For example, effector cell specificity can be determined by using different target cells or the mechanism of effector cell killing can be determined by using cells deficient in specific proteins or using protein specific inhibitors. A major problem with the 51Cr release assay is the potential for a high spontaneous release rates by the target cells. When cultured alone (without effector cells), the spontaneous release of 51Cr by the target cells should ideally be no more than 30% of the total ("maximal") release by the target cells immediately lysis. Higher spontaneous release rates may be due to using unhealthy target cells, either due to poor health (e.g., extended culture of a cell line) or an overly long labelling period.
References
- Brunner, K. T., Mauel, J., Cerottini, J. C. and Chapuis. B. Quantitative assay of the lytic action of immune lymphoid cells on 51Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology, 14 (2):181-196, (1968).
- Kemp, T. J., B. D. Elzey, and T. S. Griffith. Plasmacytoid dendritic cell-derived IFN-alpha induces TNF-related apoptosis-inducing ligand/Apo-2L-mediated antitumor activity by human monocytes following CpG oligodeoxynucleotide stimulation. The Journal of Immunology, 171 (1): 212-218, (2003).
Transcription
In this video you will observe how to perform the chromium release assay and determine the cytotoxic potential of the effector cells.
Immune cells are responsible for identifying and removing potentially harmful cells, like cancer or virus-infected cells, from the body, which is an integral part of the immune response. Several immune cells, like T-cells and NK cells, possess a property known as cytotoxic potential, which is the ability to identify target cells and secrete proteins that induce protein degradation, lysis, and death of those target cells. Quantifying cytotoxic potential is critical for measuring immune cell activation and potency, and the chromium release assay is commonly used for this purpose.
This method enables users to compare cytoxicity induced by specific types of immune cells under different conditions, which is valuable for studying cancer immunotherapy and immunity related diseases. To begin, the target cells, like cancer cells, are incubated with a radioactive isotope, chromium 51, which is taken up by the cells. Next, these radio labeled cells are co-cultured with the isolated immune cells of interest, also called the effector cells, in a round bottom, 96- well plate to facilitate interaction between the two cell types.
The overall setup of the assay involves incubating a specific number of target cells with different concentrations of the immune cells, along with appropriate controls. The co-culture allows the effector cells to induce apoptosis and lysis in the target cells, resulting in the release of the intracellular chromium 51 into the supernatant. Then, at a preoptimized time point, the supernatant containing the released chromium is harvested from all the wells. The chromium 51, being radioactive, spontaneously undergoes radioactive decay to emit gamma radiation. The gamma radiation levels in the supernatants from all the wells in the assay plate represent a quantifiable output of the lysis of the target cells. This is measured using a gamma counter, which is then used to determine the cytotoxic potential of the immune cells.
To begin, the target cells, human melanoma cell line WM793 in this example, are prepared into a single cell suspension. To do this, first remove the media from the tissue culture flask and wash the cells with five milliliters of 1X PBS. Decant the PBS and then add one milliliter of trypsin to the plate for approximately two minutes. Gently tap the flask to loosen the cells from the flask surface and then add five milliliters of RPMI media to the flask. Pipette the media up and down to collect the cells and add this suspension to a 15 milliliter conical tube.
Place the tube in the centrifuge for five minutes at 1200 RPM. Next, remove the media from the tube making sure not to disrupt the cell pellet. Gently flick the bottom of the tube to disrupt the cell pellet and add 10 milliliters of media to the tube. Then, gently pipette the media up and down to bring the cells into suspension. Next, determine the cell concentration using a hemocytometer and transfer two milliliters of the original cell suspension to a new 15 milliliter conical tube. Place the tube into a centrifuge and pellet the cells at 12 hundred RPM for five minutes. After centrifugation, pour the excess media out of the tube into a waste container. Briefly vortex the tube to resuspend the cell pellet in the small volume of medium left behind.
Next, prepare to use Chromium 51 by moving to a lab space dedicated for this particular radioactivity. There should be ample lead shielding for safe storage and use of the Chromium 51 during all steps, as well as proper signage to indicate where samples with Chromium 51 are being kept. A Geiger counter equipped with a pancake probe is also necessary to serve in the space for possible contamination.
Once set up for the proper use of radioactivity, add 100 microcuries of Chromium 51 directly to the target cell suspension. Then, add a small piece of radioactive tape to the tube to indicate that the sample and tube are now radioactive. Place the tube in a 37 degree celsius incubator with a lead shield and incubate for an hour, flicking the tube every 15 to 20 minutes.
While the target cells are labeling, prepare a single cell suspension of effector cells. In this example, human peripheral blood mono nuclear cells, or PDMCs, were isolated from whole blood by standard density gradient centrifugation to a concentration of 5 times 10 to the 6th. Transfer this effector cell suspension into a disposable reagent reservoir and then add 200 microliters of this suspension into each well of row B in a 96-well round-bottom plate. Next, add 100 microliters of RPMI to each well in row C through G of the plate.
Now, begin performing serial dilutions of the PBMCs to have a range of effector cell numbers by first removing 100 microliters of the cells in the wells in row B and adding this to row C. Then, further dilute the effector cells by transferring 100 microliters of cells from row C to row D. Continue the serial dilution. Once row G is reached, move 100 microliters from the wells to leave a final volume of 100 microliters in each well in that row. Next, add 100 microliters of tissue culture medium to the wells in row A to serve as a control for the spontaneous release of Chromium 51 from the target cells, as no effector cells should be added to this row. Then, place a plate into a 37 degree celsius incubator until the target cells are ready to be added.
After the incubation period, remove the target cells from the incubator and wash with 5 milliliters of FBS to remove any excess Chromium 51. Then, place the tube in a designated centrifuge and spin at 1200 rpm for 5 minutes. Remove the radioactive FBS wash into an appropriate waste container and repeat the wash step by resuspending the pellet in a fresh 5 milliliters of FBS. Place the tube in a designated centrifuge and spin the cells again at 1200 rpm for 5 minutes. Remove the second wash and check the pellet for incorporated radioactivity using a Geiger counter. Finally, Resuspend the pellet in 10 milliliters of complete medium and pour the Chromium 51 labeled, target cell suspension into a disposable reagent reservoir. Then, add 100 microliters of these labeled target cells to every well of the 96-well effector cell plate. Next, add 100 microliters of 1% NP-40 in water to the wells in row H to lyse all the target cells this each row. These wells will be used as a control to determine the total counts per minute, or cpm.
Now that the plate is prepared, secure the lid by adding a small piece of tape to the each side of the plate and place a piece of radioactive tape on the lid to indicate it contains chromium 51. Then, place the plate in a centrifuge marked to handle radioactive samples. If only one experimental plate is being used, add a balance plate to the centrifuge. Set the centrifuge to 1200 rpm, and bring the plate up to speed. Once at the speed, stop the machine. Remove the plate from the centrifuge. Then, place the plate in a 37 degree celsius incubator with a small piece of lead shielding over the plate for additional safety. Incubate for 16 hours to allow the target cells to lyse.
At the end of the incubation period, carefully remove the tape around the edge of the plate, and remove the lid. Next, place the harvesting frame on the plate making sure to confirm the small filter discs are in place for each of the cotton plugs. Now, slowly and gently press the cotton plugs into the wells. After approximately ten seconds, release the pressure on the cotton plugs, and then transfer the cotton plugs to tube strips. Place each of these tubes into a secondary FACS tube. Finally, load the FACS tubes onto a gamma counter and run the samples to quantitate the amount of chromium 51 released in each condition. Carefully record the order in which the tubes were loaded into the counter.
Here, unstimulated PBMCs were added to the first 3 lanes and CPG stimulated PMBCs were added to lanes 4 through 6. In this example, the counts per minute were entered into the cells of a spreadsheet in the same manner as the samples were laid out in the original plate and the averages of the triplicates were calculated. For example, for the first condition, cells A1, A2, and A3 were averaged in cell I3. Once the averages are determined, the percent of specific lysis for each condition can be calculated using this formula. For example, to calculate the percent specific lysis for the unstimulated cells that had a ratio of 50 to 1 effector cells to target cells the spontaneous CPM, which in this example, is 1164.67, was subtracted from the experimental CPM, 1129. 67. This number can then be divided by the difference between the maximum CPM and the spontaneous CPM, and then multiplied by 100 to give the percent specific lysis. This is then calculated for each condition. These data can then be graphed to show comparison of the E to T ratio with the percent specific lysis for both the unstimulated PBMCs, and the CPG stimulated PBMCs. In this example, effector cells stimulated with CPG more effectively killed target cells as the ratio of effector cells to target cells increased. This increase was not observed in the unstimulated PBMCs, indicating that CPG stimulation is necessary for the observed increase in target cell lysis.
