Measuring Calpain Activity in Fixed and Living Cells by Flow Cytometry
Summary
This article will detail the protocol for measuring calpain activity in fixed and living cells using flow cytometry.
Abstract
Protocol
Prepare Reagents
Note: All PBS contains Ca2+ and Mg2+
- Detection Reagent
Add 20μM 7-amino-4-chloromethyl coumarin, t-BOC-Leucine-methionine amide (BOC-LM-CMAC) to room temperature PBS. Each cell suspension will require 2mL detection reagent. - 1% Paraformaldehyde (Fixed Cells)
Dilute 4% paraformaldehyde stock solution in room temperature PBS. Aliquot 1mL 1% paraformaldehyde to each FACS tube. Three FACS tubes are required for each experimental condition (ex. 32Dkit, 32Dkit + PD150606, 32Dkit + PD98059 need 9 FACS tubes) - PD150606 (calpain inhibitor)
Reconstitute PD150606 in DMSO to a final concentration of 100mM. Protect this reagent and all cells treated with it from light for the duration of this experiment. - PD98059 (MEK1 inhibitor)
Reconstitute PD98059 in DMSO to a final concentration of 50mM.
Prepare Cells
- Grow 32Dkit cells at 5 x 105 to 1 x 106 cells per mL in Opti-MEM media supplemented with 5% fetal bovine serum, WEHI-3 supernatant as a source of IL-3 (or any other source for IL-3 such as recombinant protein), 0.1% 2-mercaptoethanol and antibiotics.
- Collect 12 x 106 32Dkit cells into three 15mL Falcon tubes (4 x 106 cells per tube).
- Pellet the cells at 1000 RPM for 5 minutes at 15°C.
- Aspirate the supernatant. Resuspend the cells in 2mL room temperature PBS.
- Treat one cell suspension with 50μM PD150606. Ensure the sample is protected from light by covering the Falcon tube with aluminum foil. Vortex briefly then incubate for twenty to thirty minutes at room temperature in the dark.
- Treat the second cell suspension with 20μM PD98059. Plate the cells in a 35mm tissue culture dish and incubate for 2 hours at 37°C.
Calpain Assay in Fixed Cells
- While the samples are incubating with PD150606, begin the calpain assay on the untreated samples. Begin by adding 2mL detection reagent to each cell suspension. Immediately add 1mL of the cell suspension/detection reagent mixture to the previously prepared FACS tubes with 1% paraformaldehyde (0 minutes time point).
- Incubate cell suspensions with detection reagent for 5 minutes at room temperature. Then add 1mL of the cell suspension to a FACS tube with 1mL 1% paraformaldehyde.
- Continue incubating the cell suspensions with detection mixture for an additional 5 minutes at room temperature. Then add 1mL of the cell suspension to a FACS tube with 1mL 1% paraformaldehyde.
- Repeat calpain assay on 32Dkit cells that have been treated with inhibitors.
- Fix cells overnight in the dark at 4°C.
- The next day pellet the cells at 1800 RPM for 5 minutes at 15°C.
- Aspirate the supernatant. Resuspend the cells in 750uL PBS. Place cells on ice and keep in the dark.
Acquire Data for Fixed Cells
- Analyze the cells using an LSR II (BD Bioscience). Filters are set for the Lightwave Xcyte (UV) laser, excitation line 355nm and laser power 25mW, emission of 405 and 450 nm for substrate and product respectively. Band pass filters for substrate and product are 405/20 and 450/50. Long pass filters for substrate and product are 405 and 450. The PMT voltage was set on 350-375 for substrate and 325-350 for the product.
- Set-up a BD FACSDiva experiment with the following parameters: forward scatter; side scatter; Indo-1 Blue (log); Indo-1 Violet (log). On the worksheet set up the following graphs: forward scatter vs. side scatter dot plot with a gate on live cells (Figure 1a), Indo-1 Blue histogram, Indo-1 Violet histogram, Indo-1 Blue vs. Indo-1 Violet dot plot.
- Calibrate the LSR II with AlignFlow and AlignFlow plus fluorescent beads (Invitrogen). The PMT voltage should be adjusted to place the peak of the bead fluorescence histogram in the same channel prior to every experiment.
- Begin acquiring data using the 32Dkit cells at the 0 minute time point. Adjust parameter voltages as necessary. Acquire and record 10,000 events per sample.
- Repeat acquisition for each sample, rinsing the machine with FACS buffer between each tube.
- Export the data. Ensure LSR II is cleaned according to institute guidelines.
Calpain Assay in Live Cells
- Prepare the cell suspensions as above, with no PD150606. Bring the samples to the LSR II and set up filters and parameters as above. Set up the worksheet as above. Include a large dot plot showing Indo-1 Blue vs. time. Set the program to acquire the maximum number of events (no stop gate).
- Add 50μM PD150606 to one cell suspension of 32Dkit cells. Keep the sample in the dark and incubate at room temperature for 20-30 minutes.
- Begin acquiring data on the 32Dkit cell suspension with no PD150606. Use a low flow rate of 200-300 events per second. After 30 seconds to 1 minute remove the tube from the machine. Add 20μM BOC-LM-CMAC to the cells. Vortex briefly then continue acquiring the data.
- Acquire data for 10-20 minutes or until the calpain activity plateaus.
- Repeat steps 3 and 4 with 32Dkit cells treated with PD150606.
- Export the data. Ensure LSR II is cleaned according to institute guidelines.
Calpain Assay in Complex (Mixed) Cell Populations
*This experiment will identify 32Dkit cells in a cell suspension harvested from a mouse spleen.
- Harvest the spleen from each mouse. Lyse the red blood cell with NH4Cl for 10 minutes at room temperature.
- Prepare single cell suspensions in 2mL PBS.
- Divide each cell suspension in two. Treat 1 cell suspension with 50μM PD150606 for 20-30 minutes at room temperature in the dark.
- Proceed as above for the Calpain Assay in Fixed Cells (Steps 1-6)
- Aspirate the supernatant. Wash the cells in 500μL PBS. Aspirate the supernatant.
- Resuspend the cells in 200μL staining buffer with 2% BSA. Incubate for 15 minutes at room temperature.
- Prepare the primary antibody (FITC anti-mouse CD117). The antibody should be used at a 1:200 dilution in staining buffer. Ensure the antibody is kept in the dark.
- Add 200μL primary antibody to the cells. Incubate for 30 minutes at room temperature in the dark.
- Add 400μL PBS to increase the volume for flow cytometry. Place the cells on ice and keep in the dark.
- Proceed as described in Acquire Data for Fixed Cells. Add FITC to the parameters and include a histogram for FITC on the worksheet.
Analysis and Representative Results
- Analyze data using FlowJo (Treestar). Gate on live cells based on forward and side scatter profile (Figure 1a). Determine the mean fluorescence for Indo-Blue from the viable cell gate (Figure 1b).
- a) For the Mixed Cell Population
- use FITC fluorescence on histograms and dot plots to identify the 32Dkit population
- Determine the mean fluorescence for Indo-Blue of the 32Dkit population
- Use Microsoft Excel to graph the mean fluorescence (Figure 2a). Calculate the slope of the line between 5 and 10 minutes. Graph the slope as a bar graph to determine calpain activity in the cells (Figure 2b).
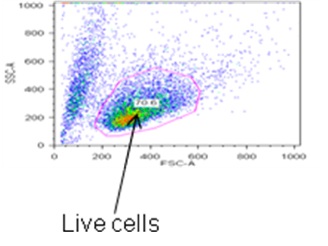
Figure 1a. Determining live cells based on forward and side scatter profile.
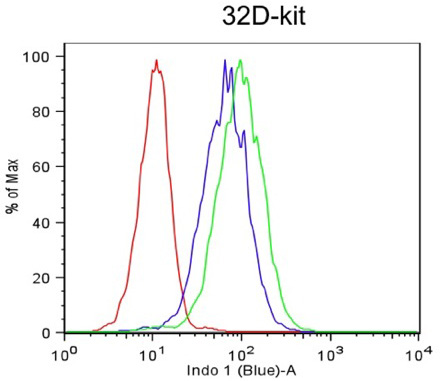
Figure 1b. Mean fluorescence change over 10 minute time course.
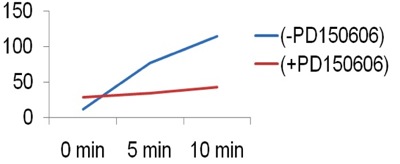
Figure 2a. Representative graph of mean fluorescence.
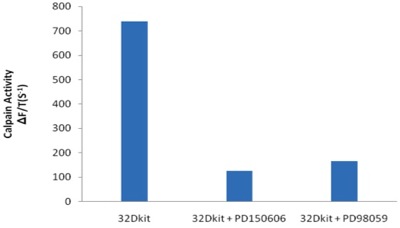
Figure 2b. Calpain activity in 32Dkit cells with inhibitors.
Discussion
The calpain activity in fixed and living 32Dkit cells has been determined in this video protocol. Treatment of the cell line with the calpain inhibitor PD150606 resulted in complete inhibition of calpain activity in the cells. In addition, treatment with the MEK1 inhibitor PD98059 resulted in complete inhibition of calpain activity in the cells. ERK is a major activator of calpain-2 3. These results suggest that calpain-2 activity is predominant in the 32Dkit cells. Current work in our laboratory is investigating the consequences of increased calpain activity and the contributions of calpain-1 and calpain-2 in leukemia.
This protocol details the first flow cytometric based assay to measure calpain activity levels in fixed and living cells and can be used to understand the mechanism of calpain regulation and its role in pathological processes using intact cells.
This protocol can be modified to examine the effects of inhibitors or gene manipulations on calpain activity. In addition, this assay can be utilized to measure calpain activity in a complex mixture of cells such as cord blood or to identify and measure calpain activity in cell subsets isolated from the spleen.
This assay does not distinguish between activity generated by different isoforms of calpain. Genetic or pharmacological reagents that knock down or inhibit specific calpains can be used to address this issue.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Supported by grants from the Canadian Institutes of Health Research, The Leukemia and Lymphoma Society of Canada and the Lymphoma Foundation of Canada.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| PBS BOC-LM-CMAC 4% paraformaldehyde |
Invitrogen | Dissolve 4g paraformaldehyde in 100mL PBS. In fume hood, heat sample with stirring until clear. Let cool then store at 4°C |
||
| PD150606 | Invitrogen | Use freshly reconstituted PD150606 for maximal calpain inhibition. | ||
| PD98059 | Cell Signaling Technology | |||
| AlignFlow, AlignFlow plus fluorescent beads FACS tubes |
Invitrogen | |||
| LSR II | BD Bioscience | Supernatant of WEHI-3 cells is used as a source of IL-3 in these experiments. Each collection of WEHI-3 supernatant is titrated to determine the optimal concentration used for 32Dkit cell growth. |
Riferimenti
- Goll, D. E., Thompson, V. F., Li, H., Wei, W., Cong, J. . The Calpain System. Physiol. Rev. 83, 731-801 (2003).
- Niapour, M., Berger, S. A. Flow Cytometric Measurement of Calpain Activity in Living Cells. Cytometry A. 71A, 475-485 (2007).
- Leloup, L., Daury, L., Mazéres, G., Cottin, P. Involvement of the ERK/MAP kinase signalling pathway in milli-calpain activation and myogenic cell migration. The International Journal of Biochemistry and Cell Biology. 39, 1177-1189 (2007).

