Performing Injection into Palpebral Portion of the Superior Lacrimal Gland: A Technique for Inducing Dry Eye Disease in Rabbit Model
Abstract
Source: Honkanen, R. A., et al. A Rabbit Model of Aqueous-Deficient Dry Eye Disease Induced by Concanavalin A Injection into the Lacrimal Glands: Application to Drug Efficacy Studies. J. Vis. Exp. (2020).
In this video, we show a procedure to induce aqueous-deficient dry eye disease in a rabbit model by injecting the palpebral portion of the superior lacrimal gland with Concanavalin A. This model is useful for pharmacokinetic and biodistribution assays of drugs for treating dry eye disease.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Animals and housing
- Acquire New Zealand White (NZW) rabbits weighing 2-3 kg.
- House the rabbits singly in cages with strict temperature (65 ± 5 °F) and humidity (45 ± 5%) control. Lighting should have a 12 h on/off cycle.
- Provide unlimited access to water and standard rabbit chow. Eliminate dietary enrichments as they may contain vitamin A that affects the eye.
- Acclimate the animals for at least 2 weeks prior to baseline measures or induction of dry eye.
2. Methods of anesthesia and euthanasia
NOTE: All procedures require mild sedation except for Con A injection that requires moderate sedation.
- For mild sedation, inject acepromazine (1 mg/kg) subcutaneously over the shoulders using a 26-gauge needle. Endpoint for mild sedation: animals maintain a relaxed head position with ear lobes no longer fully upright.
NOTE: If the appropriate endpoint is not reached, an additional injection of acepromazine may be given. Animals should always remain awake, responsive to touching of their whiskers, and never show slowed breathing. - For moderate sedation, first give the animals acepromazine as above. After the endpoint is reached (see the note above), give isoflurane using a gas mask with O2 flow set at 1 L/min and isoflurane delivery set to 5% (Figure 1).
- Administer isoflurane until the rabbit's body tone is completely relaxed and ears are completely floppy.
NOTE: No compensatory muscle movements should occur when the animal is turned on its side; breathing always remains spontaneous. - Spontaneous recovery occurs within 2-5 min: signs include spontaneous head movements and increased or normal muscular tone. After the experimental procedure is completed with moderate sedation, observe the rabbits for about 30 min or until their behavior returns to normal.
NOTE: Ophthalmic ointment is not required during either form of sedation. 1) In mild sedation, animals are still alert and maintain a blink reflex. In moderate sedation, the inhibition of the blink reflex is so short that the ocular surface is not at risk. 2) Placement of ophthalmic ointment on the ocular surface precludes visualization of structures assessed during testing. - Euthanasia: Use an overdose of intravenous pentobarbital (100 mg/kg).
3. Induction and treatment of dry eye
NOTE: Three portions of the orbital lacrimal gland system are injected.
- Sedate the rabbits with acepromazine 0.2 mg/kg subcutaneously.
- Shear off the fur in the periorbital and scalp area and completely remove any residual fur using Nair. Leave the skin entirely smooth for better visualization of the anatomical hallmarks and US-guided injection of concanavalin A (Figure 2).
- Induce moderate sedation as described above.
- Injection of the palpebral portion of the superior lacrimal gland (PSLG)
NOTE: Perform injection of PSLG first.- Apply to the appropriate eye 25 µL of preservative-free lidocaine 1% with a micropipette.
- Evert the upper eyelid and apply gentle medial pressure to the posterior orbital rim until the protuberance marking the palpebral portion of the gland is seen. The PSLG appears as a small bulbous elevation in the posterior (temporal) portion of upper lid.
NOTE: To view the gland tissue during the learning process, apply 5% fluorescein to the area (Figure 3A). Tears can be seen streaming from the bulbous PSLG. Application of fluorescein is not needed for the administration of Con A; it is done only for illustration purposes to show the gland tissue. - Using fine-toothed forceps and a 27-gauge needle on a tuberculin syringe, directly penetrate the gland using a transconjunctival approach. Advance the needle 2 mm into the tissue and inject 500 µg of Con A in a volume of 0.1 mL (Figure 3B).
NOTE: This injection can sometimes be painful. If necessary, keep the animals under isoflurane until this injection is completed.
Representative Results
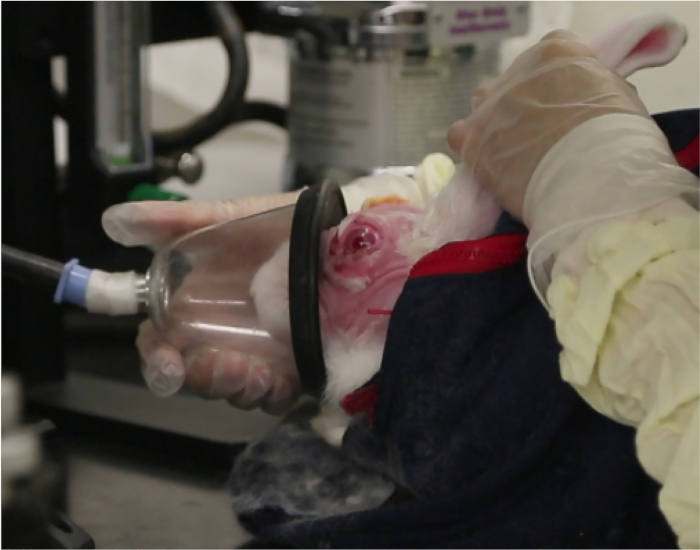
Figure 1: Gas mask sedation. This photograph shows the gas mask providing brief moderate sedation with isoflurane.
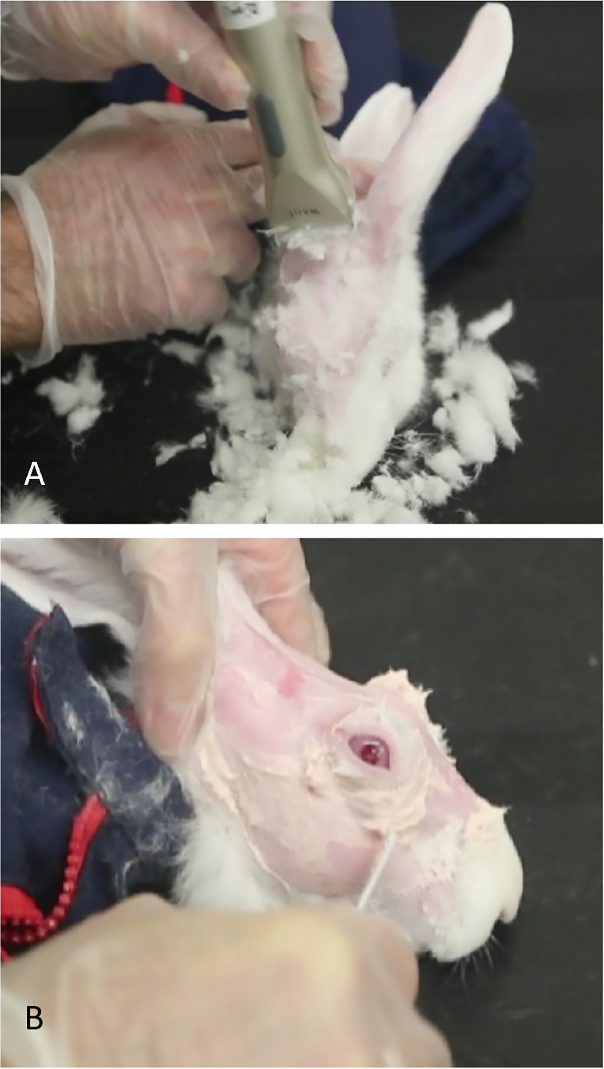
Figure 2: Preparation of rabbit for concanavalin A injections. (A) Small shears are used to remove fur, allowing easier visualization of landmarks to identify the orbital superior lacrimal gland. (B) Nair is used to remove hair that remains after shearing.
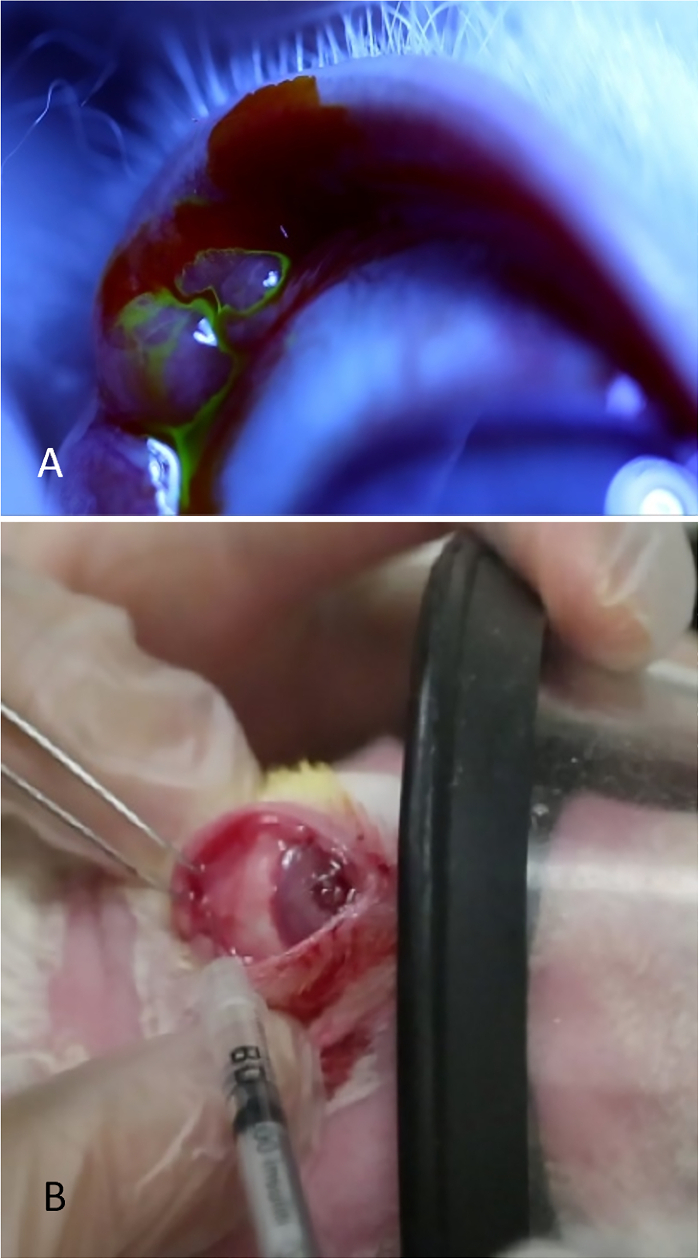
Figure 3: Injection of the palpebral lacrimal gland. (A) The palpebral lacrimal gland, appearing as a bulbous elevation in the posterior temporal portion of the upper lid. Tears are seen streaming from the surface of this gland after applying a drop of 2% fluorescein. (B) The palpebral lacrimal gland is being injected while the rabbit is receiving moderate sedation. One investigator retracts the eyelid, optimizes exposure of the gland, and secures the mask while the second investigator injects the gland.
Divulgazioni
The authors have nothing to disclose.
Materials
| 27 gauge needles (5/8) | Becton Dickinson and Company, Franklin Lakes, NJ | 305921 | Needles for injecting ConA into the lacrimal glands |
| Aceproinj (acepromazine) | Henry Schein Animal Health, Dublin, OH | NDC11695-0079-8 | 0.1ml/kg subcutaneously injection for rabbit sedation |
| Anesthesia vaporizer | VetEquip, Pleasanton, CA | Item #911103 | |
| Bishop Harmon Forceps | Bausch and Lomb (Storz), Bridgewater, NJ | E1500-C | Tissue forceps |
| Concanavalin A | Sigma, St. Louis, MO | C2010 | Make 5mg/ml in PBS for injection into rabbit lacrimal glands |
| fluorescein | AKRON, Lake Forest, IL | NDC17478-253 | Dilute to 0.2% with PBS to measure TBUT |
| Isoflurane | Henry Schein, Melville, NY | 29405 | |
| lidocaine | Sigma, St. Louis, MO | L5647 | 1% in PBS for anesthesia agent |
| PBS (phosphate buffered saline) | Mediatech, Inc. Manassas, VA | 21-031-CV | |
| Rabbit, New Zealand White or Dutch Belted (as described in text) | Charles River Labs, Waltham, MA | 2-3 kg | Research animals |
| Surgical Loupes +1.50 | Designs for Vision, Bohemia, NY | Specialty item | Provide magnificantion of ocular surface while observing tear break up and performing Concanavalin A injections. |

