Solid-phase Submonomer Synthesis of Peptoid Polymers and their Self-Assembly into Highly-Ordered Nanosheets
Summary
A simple and general manual peptoid synthesis method involving basic equipment and commercially available reagents is outlined, enabling peptoids to be easily synthesized in most laboratories. The synthesis, purification and characterization of an amphiphilic peptoid 36mer is described, as well as its self-assembly into highly-ordered nanosheets.
Abstract
Peptoids are a novel class of biomimetic, non-natural, sequence-specific heteropolymers that resist proteolysis, exhibit potent biological activity, and fold into higher order nanostructures. Structurally similar to peptides, peptoids are poly N-substituted glycines, where the side chains are attached to the nitrogen rather than the alpha-carbon. Their ease of synthesis and structural diversity allows testing of basic design principles to drive de novo design and engineering of new biologically-active and nanostructured materials.
Here, a simple manual peptoid synthesis protocol is presented that allows the synthesis of long chain polypeptoids ( up to 50mers) in excellent yields. Only basic equipment, simple techniques (e.g. liquid transfer, filtration), and commercially available reagents are required, making peptoids an accessible addition to many researchers’ toolkits. The peptoid backbone is grown one monomer at a time via the submonomer method which consists of a two-step monomer addition cycle: acylation and displacement. First, bromoacetic acid activated in situ with N,N’-diisopropylcarbodiimide acylates a resin-bound secondary amine. Second, nucleophilic displacement of the bromide by a primary amine follows to introduce the side chain. The two-step cycle is iterated until the desired chain length is reached. The coupling efficiency of this two-step cycle routinely exceeds 98% and enables the synthesis of peptoids as long as 50 residues. Highly tunable, precise and chemically diverse sequences are achievable with the submonomer method as hundreds of readily available primary amines can be directly incorporated.
Peptoids are emerging as a versatile biomimetic material for nanobioscience research because of their synthetic flexibility, robustness, and ordering at the atomic level. The folding of a single-chain, amphiphilic, information-rich polypeptoid into a highly-ordered nanosheet was recently demonstrated. This peptoid is a 36-mer that consists of only three different commercially available monomers: hydrophobic, cationic and anionic. The hydrophobic phenylethyl side chains are buried in the nanosheet core whereas the ionic amine and carboxyl side chains align on the hydrophilic faces. The peptoid nanosheets serve as a potential platform for membrane mimetics, protein mimetics, device fabrication, and sensors. Methods for peptoid synthesis, sheet formation, and microscopy imaging are described and provide a simple method to enable future peptoid nanosheet designs.
Protocol
1. Solid-Phase Submonomer Synthesis of Polypeptoids
Solid-phase synthesis (SPS) is a common technique used to synthesize sequence-specific biopolymers step-wise, directly on an inert solid-support such as a polymeric resin bead. High coupling yields and ease of excess reactant removal are major advantages of SPS. After a coupling reaction to the resin, excess reagents are simply drained and the beads are washed to be ready for the next reaction step. After the final synthesis reaction, the full-length oligomers are cleaved from the resin and the solution-phase material can be further studied. Here, we adapt the SPS procedure to generate sequence-specific peptoid polymers.
- Setup: All steps of the manual peptoid synthesis can be carried out in a disposable, polypropylene (PP) fritted cartridge or a fritted glass reaction vessel equipped with a 3-way stopcock. Perform all operations in a fume hood. For incubations in the glass vessel or plastic cartridge, connect one arm to a nitrogen supply to gently bubble the solution for proper mixing. Alternatively, for reaction incubations in the disposable cartridge, seal both ends of the cartridge with caps and place on a rotary shaker. To drain reaction mixtures or washes, connect to house vacuum via a waste trap. The vessel should be fritted with a coarse frit. Siliconize the glass reaction vessel to avoid beads from sticking to the walls of the glass vessel. Prepare a solution of 5% dichlorodimethylsilane in dichloroethane (v/v). Fill a clean and dry reaction vessel to the top with siliconizing solution, let sit for 30 minutes, then drain. Wash the vessel once with DCE and then once with methanol. The siliconizing solution can be reused, so it should be saved. Either air dry or shake off any excess solution and bake the glassware until dry after removing the stopcock. Cool reaction vessel before adding resin.
- Add 100 mg (0.06 mmol) of Rink amide resin to a fritted reaction vessel. Swell the resin by adding 2 mL of dimethylformamide (DMF). Agitate by shaking or bubbling for 10 minutes. Drain the solution by vacuum to isolate the swelled resin.
- Add 1 mL of 20% 4-methylpiperidine in DMF (v/v) to deprotect the Fmoc group. Agitate for 2 minutes and drain. Repeat with a 12 minute incubation.
- Rinse the resin by adding 2 mL of DMF, agitating for 15 seconds, and draining. Repeat 3x.
- Bromoacetylation: Add 1 mL of 0.6 M bromoacetic acid (0.6 mmol) in DMF and 86 μL of N,N’-diisopropylcarbodiimide (0.93 equivalent, 0.56 mmol). Incubate with gentle bubbling for 30 minutes, then drain and rinse with 2 mL of DMF (repeat 4x).
- Displacement: Add 1 mL of 1-2 M amine in N-methylpyrrolidinone. Incubate with bubbling for 30-120 minutes, then drain and rinse with DMF (4x 2 mL).
- Continue to grow the peptoid chain by repeating the submonomer cycle, steps 1.5 (bromoacetylation) and 1.6 (displacement).
- After the final displacement is done, rinse with 2 mL of DMF (repeat 4x), then 2 mL of dichloromethane (repeat 3x). Cap and store the reaction vessel until cleavage.
- Pause in synthesis (optional): To pause during a peptoid synthesis, finish the displacement reaction and continue to step 1.8. To continue growing the peptoid chain, restart the synthesis by re-swelling the dried resin (step 1.2) and repeating the submonomer cycle (step 1.5 and 1.6). The resin can be dried and stored after any displacement except the 2nd displacement because the resin-peptoid conjugate may form a cyclic diketopiperazine side product.
- For multiple simultaneous syntheses, a solid-phase extraction vacuum manifold is recommended to maximize efficiency. Peptoid synthesis can also be automated by properly programming methods in commercially available peptide synthesizers, such as the Aapptec Apex 396, CEM Liberty microwave synthesizer and Protein Technologies Inc. Prelude synthesizer.
2. Cleavage and Side-Chain Deprotection
- Transfer all of dried resin to a 20 mL scintillation glass vial.
- Working inside a hood and using proper personal protective equipment, add 4 mL of trifluoroacetic acid (TFA) cleavage cocktail1 (e.g. 95% aq. TFA, see discussion) to the scintillation glass vial and cap tightly. Shake for 10 minutes to 2 hours at room temperature (see discussion).
- Collect the TFA cleavage solution by filtering the resin through a disposable, PP fritted cartridge into a new, pre-weighed 20 mL scintillation glass vial. A disposable, PP pipette is convenient to transfer the cleavage cocktail solutions.
- Add 1 mL of fresh cleavage cocktail to rinse the resin and collect any residual peptoid. Repeat 2x.
- Evaporate TFA by blowing a gentle stream of nitrogen or by using a Biotage V10 evaporator.
- Redissolve the crude oil in 6 mL of acetonitrile/water 1:1 (v/v) for HPLC. Freeze and lyophilize. Repeat.
- Record the weight of the crude product. Store as a dry powder at -20 °C
- Test cleavage (optional): A test cleavage on 0.5% of the resin can be performed to quickly determine the purity and mass of the synthesized peptoid and whether correct cleavage conditions were chosen. Test cleavages are especially useful to monitor the progress of the synthesis.
3. Characterization and Purification of the Polypeptoid
- Through a combination of analytical HPLC, electrospray LC-MS, and/or MALDI-TOF, determine the purity of the crude product and whether the desired molecular weight is present.
- Prepare a ˜5-10 mg/mL solution of the dry peptoid powder in water with minimal acetonitrile as needed for solubility. Filter clear solution of crude peptoid product with 0.45 μm syringe filter to remove dust and particles.
- Analytical HPLC and electrospray LC-MS: Prepare a ˜ 20 μg/mL crude peptoid solution. Filter 200 μL with a 0.45 μm filter and inject 20 μL.
- MALDI: Mix 1 μL of ˜20 mg/mL peptoid with 1 μL matrix. Spot 1 μL on MALDI plate and allow to air dry. Matrix and acquisition mode is dependent on sample (Fig. 5).
- Purify the crude peptoid mixture with reverse-phase prep HPLC. Choose the gradient and column (C4 or C18) based on the hydrophobicity of polypeptoid. Combine purified fractions, freeze, and lyophilize, resulting in a fluffy white powder. Record the weight of the final product.
- Formation of HCl salt (optional): Redissolve the lyophilized powder in 100 mM HCl (aq.) with minimal acetonitrile. Transfer to pre-weighed glass vial. Freeze and re-lyophilize. Repeat 2x. Reweigh to determine mass of peptoid powder.
4. Peptoid Nanosheet Formation
This section describes the protocol to form sheets from a single-chain, sequence specific, amphiphilic 36-mer peptoid (Fig. 1). After the peptoid strand is synthesized, purified, and lyophilized as described above, the resulting white powder is dissolved in DMSO to make a 2 mM stock solution.
- Prepare 500 μL of 20 μM peptoid solution in sheet formation buffer (10 mM Tris-HCl, 100 mM NaCl, pH 8.0 in water) in a 1 dram glass vial. First, add 445 μL of Milli-Q water, 50 μL of 10x sheet formation buffer, and vortex to mix. Then, add 5 μL of 2 mM peptoid stock solution and gently swirl solution. Cap the glass vial.
- Sheets are formed by the gentle agitation of the dilute aqueous peptoid solution. Slowly tilting the glass vial from the horizontal position to the upright position results in sheets. Gentle shaking also yields sheets; however, the sheets tend to be smaller and with fewer straight edges. A more thorough analysis of the sheet formation mechanism is reported separately.2
- For many high-quality sheets, rotate the glass vials about the horizontal axis slowly (<1 RPM) for one to three days. An Appropriate Technical Resources RKVSD Rotamix tube rotator or a customized rocker can perform this continuously.
- Dialysis of Nanosheets (optional): In certain applications, it may be necessary to remove any free peptoid chains or buffers/salts. Soak a Float-a-Lyzer 100 kD membrane in the desired buffer for 15 minutes. Load 500 μL peptoid sheet solution into the sample chamber. Soak in 500 mL of desired buffer, stirring with a magnetic stir bar at 60 rpm. Allow dialysis of sheets to proceed for 4 hours. Every hour, exchange with a fresh stock of buffer solution.
5. Fluorescence Microscopy of Nanosheets
- Fluorescence images of the nanosheets were imaged with Nile Red, an environmentally sensitive dye whose fluorescence intensity increases when it is localized in hydrophobic environments (Fig. 2).
- Add 1 μL of 100 μM Nile Red to 100 μL of the nanosheet solution to obtain a final concentration of 1 μM of Nile Red.
- Make a 1% agarose solution in hot water and pour into a plastic Petri dish. Ensure the agarose solution is approximately 1/8 inch thick and allow the solution to cool undisturbed on a flat surface. After the agarose sets, use a spatula to cut and transfer 1 cm x 1 cm squares to a glass slide.
- To collect the sheets in the same plane, spot 1 μL of sheet solution on the piece of agarose. Allow the agarose to absorb the buffer for 2 minutes, leaving the sheets at the surface. Image within 15 minutes, otherwise the agarose will begin to deform due to dehydration.
- To image sheets in solution, load 15 μL inside a 20 mm diameter 0.12 mm gasket on a glass slide. Cover with a coverslip. If the sheets are simply sandwiched between a glass slide and coverslip without a gasket, many sheets will shear and the seemingly minor evaporation will cause the sheets to constantly move.
- Image sheets under epifluorescence illumination (e.g. an Olympus IX81 inverted microscope fitted with an Andor iXonEM+ EMCCD spectra with a Texas red filter).
6. Scanning Electron Microscopy (SEM) of Nanosheets
- Plasma etch of silicone substrate (optional): The silicon chips are plasma etched to aid in the adsorption of sheets. Place the silicon chips in the vacuum chamber of a plasma cleaner (e.g. Harrick Plasma Cleaner/Sterilizer PDC-32G). Pump down to 200 mTorr and set the RF coil to 18W (high setting for PDC-32G). Etch for 2 minutes.
- Drop 20 μL of peptoid sheet solution on a plasma-treated silicon substrate. Allow to sit for 3 minutes. Remove excess solution with tip of Kim-wipe. Pipette 20 μL of water onto the surface and remove excess solution again to remove buffer and salts. Repeat 4x.
- Alternatively, dialyze the peptoid sheet solutions against water to remove buffer and salt. Drop 20 μL of dialyzed sheet solution on plasma-treated silicon substrates. Air dry the sample.
- Image sheets with SEM (e.g. Zeiss Gemini Ultra-55 Analytical Scanning Electron Microscope) with an in-lens detector and at beam energies between 1 kV and 5 kV (Fig. 3).
7. Safety Notes:
- Dimethylformamide and Dichloromethane are reasonably suspected carcinogens.
- N,N’-Diisopropylcarbodiimide, 4-methylpiperdine and bromoacetic acid are hazardous to the skin, eyes, and respiratory tract. They should be used in the hood with care. It may be toxic if inhaled or absorbed through the skin, and exposure may result in sensitization. Empty containers retain product residue (liquid/vapor) and should be thoroughly rinsed before removing them from the hood.
- TFA is a strong acid, and is extremely destructive to the upper respiratory tract, eyes, and skin. TFA is also volatile-keep concentrated solutions of TFA in the hood at all times to avoid respiratory damage. Use proper PPE, and caution when handling solutions of TFA. Change gloves promptly if they come in contact with TFA, and immediately clean up any spills.
8. Representative Results:
This section describes the synthesis, characterization, and purification of a sequence-specific 36-mer peptoid chain that folds into a highly ordered nanosheet3 (Fig. 1).
The block-charge peptoid H-[Nae-Npe]9-[Nce-Npe]9-NH2 was synthesized on 100 mg of Rink amide resin. A 2 M amine solution was used for all displacement reactions, which were carried out for 60 minutes for residue 1-18 and 120 minutes for residue 19-36. t-Butyl beta-alanine HCl was converted to the free base (see discussion) whereas phenethylamine and boc-ethylenediamine were both used directly. The resin was cleaved with 95% TFA, 2.5% triispropylsilane, 2.5% water for 2 hours. TFA was evaporated and the resulting viscous oil (~180 mg) was re-dissolved in 6 mL acetonitrile:water 1:1 (v/v). Product purity (Fig. 4) and presence of the product mass was confirmed by from analytical RP-HPLC (30-80% acetonitrile in water gradient, both containing 0.1% (v/v) TFA, at 1 mL/min over 30 minutes at 60 °C with a C18, 5 μm, 50 X 2 mm column) and MALDI (Fig. 5).
Purification with reverse phase HPLC on a Vydac C18 column (10 μm, 22 mm x 250 mm) proceeded, using a gradient of 30-60% acetonitrile in water with 0.1% TFA over 60 minutes at 10 mL/min. The column was loaded with 60 mg of crude product for each chromatographic run. The purified fractions were combined based on purity from analytical RP-HPLC (Fig. 4) and lyophilized to yield ˜80 mg of a fluffy white powder.
Purified block-charge peptoid molecular weight was confirmed by MALDI. 1 μL of 100 μM purified peptoid in acetonitrile:water 1:1 (v/v) was mixed with 1 μL of matrix (5 mg/mL α-cyano-4-hydroxycinnamic acid in acetonitrile:water 1:1 v/v and 0.1% TFA) and 1 μL was spotted on the MALDI plate. After the sample air-dried, it was placed in the Applied Biosystem/MDS SCIEX 4800 MALDI TOF/TOF Analyzer. The acquisition and processing modes were linear low mass. The calculated weight was entered in the targeted mass to automatically adjust for the delay time. The laser intensity was set to 3400. The observed mass, 4981.2, matches closely to the calculated mass of 4981.74.
The lyophilized purified powder was dissolved in DMSO to make a 2 mM stock solution, which can be stored at 4 °C. Sheets were prepared by aforementioned protocol and imaged with fluorescence optical microscopy and SEM (Fig. 2 and 3). A variety of shapes with feature sizes ranging up to 300 μm are observed, and notably, straight edges are prominent.
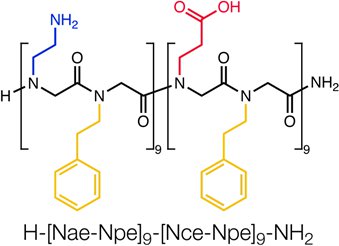
Figure 1. Sequence of the block-charge peptoid H-[Nae-Npe]9-[Nce-Npe]9-NH2. A single-chain, block charge, amphiphilic polypeptoid 36-mer self-assembles into highly-ordered, two-dimensional nanosheets3. The calculated molecular weight is 4981.74.
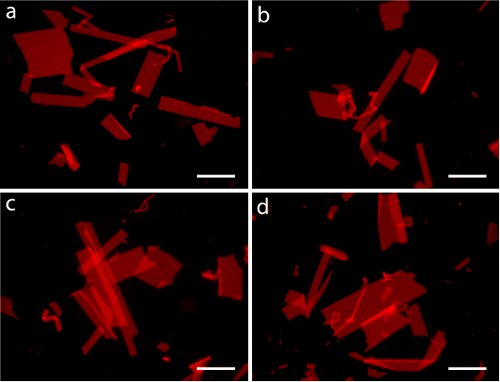
Figure 2. Fluorescence microscopy images of peptoid nanosheets. Sheets were formed from a 20 μM peptoid solution in 10 mM Tris, 100 mM NaCl, pH 8.0. The sheets were imaged on agarose with 1μM Nile Red. Scale bars are 100 μm.

Figure 3. Scanning electron microscopy images of peptoid nanosheets. Sheets were formed from a 20 μM peptoid solution in 10 mM Tris, 100 mM NaCl, pH 8.0. Scale bars are 5 μm.
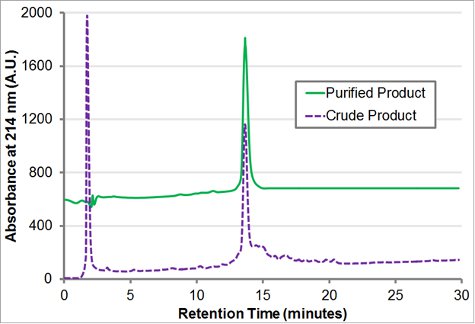
Figure 4. Analytical reverse phase HPLC trace of H-[Nae-Npe]9-[Nce-Npe]9-NH2. The crude and purified analytical HPLC trace (30-80% gradient at 1 mL/min over 30 minutes at 60°C with a C18, 5 μm, 50 x 2 mm column) of the crude and purified block-charge peptoid H-[Nae-Npe]9-[Nce-Npe]9-NH2 is shown.
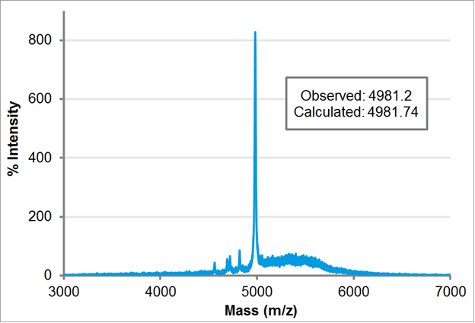
Figure 5. MALDI-TOF mass spectroscopy trace of H-[Nae-Npe]9-[Nce-Npe]9-NH2. The observed mass, 4981.2, is in close agreement to the calculated mass, 4981.74.
Discussion
Applications and Significance
This protocol describes a simple and efficient method of peptoid synthesis and the aqueous self-assembly of the peptoids into nanosheets. Most laboratories are easily capable of synthesizing peptoids because inexpensive materials, basic expertise and straightforward techniques are utilized4. Likewise, the self-assembly of ultra-thin, highly-ordered nanosheets merely requires repeated tilting a vial of a dilute aqueous peptoid solution2. Peptoids are promising materials for biomedical and nanoscience research because they are robust and synthetically flexible yet sequence-specific and highly tunable5. Peptoids have demonstrated biological activity (therapeutics6,7, diagnostics8, intracellular delivery9-10) and folding into hierarchical nanostructures3, 11-14. Because of their modular synthesis, combinatorial peptoid libraries15-19 can be readily synthesized and screened for a broad series of activities or properties. In particular, the nanosheets serve as a potential platform for two-dimensional display scaffolds, membrane mimetics, biological sensors, protein mimetics and device fabrication. With the practically inexhaustible different sequences possible, the realm of peptoid research is quickly expanding.
Variables in solid-phase submonomer synthesis of polypeptoids
Because of the ability to choose from an incredibly large and diverse alphabet of monomers20, the submonomer method needs occasional modifications for cases where increasing the coupling efficiency of each step will improve the overall product yield. Incorporation of unprotected heterocyclic side chains requires the use of chloroacetic acid instead of bromoacetic acid21. Extended displacement times and higher amine concentrations are usually employed after about 20 couplings for long peptoid sequences or less nucleophilic amines. Heating the reaction vessel to 35 °C, by using a water-jacketed reaction vessel, helps to drive the reaction. For highly-volatile amines such as isopropylamine, care must be taken to avoid evaporation.
Amines in the form of an HCl salt, such as t-butyl beta-alanine HCl, need to be free-based before being introduced in the displacement reaction. This can be achieved by dissolving or suspending the amine in DCM (˜5g amine/25 mL DCM), and neutralizing with an equimolar solution of aqueous sodium hydroxide in a separatory funnel. The DCM layer is collected and the aqueous layer is washed with additional DCM. The combined DCM layers are dried over sodium sulfate and filtered into a pre-weighed round bottom flask. Remove solvent by rotary evaporation to yield an oil, and record the product weight.
During the cleavage step, TFA cleavage cocktail and cleavage time is dependent on the number and variety of protecting groups used. Guidelines for cleavage cocktails are similar to traditional peptide deprotection cleavages1. Generally, 10 minute incubations are required for sequences without protecting groups or sequences with few highly acid labile protecting groups (e.g. BOC, trityl). Two hour incubations are recommended for sequences with more difficult protecting groups (e.g. t-butyl ester, Mtr, Pbf) or sequences with many protecting groups to ensure full deprotection of each chain. Crude peptoid products will generally dissolve in acetonitrile:water 1:1 (v/v), but higher acetonitrile proportions are common with side chains with a high overall hydrophobicity.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Byoung-Chul Lee, Philip Choi and Samuel Ho for valuable assistance. This work was carried out at the Molecular Foundry at Lawrence Berkeley National Laboratory, which is supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DE-AC02-05CH11231 and the Defense Threat Reduction Agency under Contract No: IACRO-B0845281.
Materials
| Name of the reagent | Company | Catalogue number | Comments |
| Dimethylformamide | EMD | EM-DX1726P-1 | 99+% |
| N-methylpyrrolidinone | BDH | BDH1141-4LP | 99% |
| Bromoacetic Acid | Acros Organics | 200000-106 | 99% |
| 4-Methylpiperidine | Sigma Aldrich | M73206 | 96% |
| N,N’-diisopropylcarbodiimide | Chem-Impex | 001100 | 99.5% |
| Dichloromethane | EMD | EMD-DX0835 | ACS grade |
| Acetonitrile | EMD | EM-AX0145P-1 | 99.8% |
| Trifluoroacetic acid | Sigma Aldrich | T6508 | 99% |
| Triisopropylsilane | Sigma Aldrich | 233781-10G | For TFA cleavage |
| 1,2-Dichloroethane | JT Baker | JTH076-33 | For siliconization of glass reaction vessels |
| Phenethylamine | Sigma Aldrich | 407267-100ML | >99.5% Hydrophobic side-chain amine |
| Boc-ethylenediamine | CNH Technologies | C-1112 | Cationic side-chain amine |
| t-Butyl beta-alanine HCl | Chem-Impex International | 04407 | Anionic side-chain amine |
| α-Cyano-4-hydroxycinnamic acid | Sigma Aldrich | C8982-10X10MG | For MALDI matrix |
| Nile Red | Sigma Aldrich | 19123-10MG | For fluorescence Imaging |
| Dichlorodimethylsilane | Sigma Aldrich | 80430-500G-F | For siliconization of glass reaction vessels |
| Disposable PP fritted cartridge | Applied Separations | 2416 | 6 mL polypropylene cartridge with 20 mm PE frit |
| Disposable 3 way luer adapter | Cole Parmer | 31200-80 | Stopcock for disposable manual synthesis reaction vessel |
| Luer Lock ring | Cole Parmer | 45503-19 | ¼” fitting for disposable manual synthesis reaction vessel |
| Fittings Luer | Cole Parmer | 45500-20 | ¼” fitting for disposable manual synthesis reaction vessel |
| Disposable PP pipets | VWR | 16001-194 | For TFA transfers |
| Luer lock plastic syringe | National Scientific | S7515-5 | 6 mL syringes |
| 1 dram glass vial | VWR | 66011-041 | With phenolic molded screw cap with polyvinyl-faced pulp liner |
| 20 mL scintillation vial | VWR | 66022-060 | With attached PP cap and pulp foil liner |
| Secure-Seal adhesive spacer | Invitrogen | S-24736 | For fluorescence imaging |
| Glass slides | Electron Microscopy Sciences | 63411 | For fluorescence imaging |
| Cover slip | VWR | 48366-067 | For fluorescence imaging |
| 4” Silicon wafer | Ted Pella | 16007 | Pre-dice in 5×7 mm chips |
| 0.45 filter | VWR, Acrodisc | 28143-924 | For HPLC. PTFE membrane |
| Agarose | BD | 212272 | For fluorescence imaging |
| SPE Vacuum Manifold | Sigma Aldrich | 57044 | Example of SPE vacuum manifold |
| Fritted glass vessel | Ace glass | 6402-12 | Porosity C frit |
| Plasma Cleaner/Sterilizer | Harrick Plasma | PDC-32G | Example of plasma cleaner to prepare silicon chips for SEM |
Riferimenti
- King, D. S., Fields, C. G., Fields, G. B. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int. J. Pept. Pro. Res. 36, 255-266 (1990).
- Sanii, B., Kudirka, R., Cho, A., Venkateswaran, N., Oliver, G. K., Olson, A. M., Tran, H., Harada, R. M., Tan, L., Zuckermann, R. N. Shaken, not stirred: Collapsing a peptoid monolayer to produce free-floating, stable nanosheets. J. Am. Chem. Soc. , (2011).
- Kudirka, R., Tran, H., Sanii, B., Nam, K. T., Choi, P. H., Venkateswaran, N., Chen, R., Whitelam, S., Zuckermann, R. N. Folding of a single-chain, information-rich polypeptoid sequence into a highly-ordered nanosheet. Bioploymers: Peptide Science. 96, 586-595 (2011).
- Utku, Y., Rohatgi, A., Yoo, B., Zuckermann, R., Pohl, N., Kirshenbaum, K. Rapid multistep synthesis of a bioactive peptidomimetic oligomer for the undergraduate laboratory. J. Chem. Ed. 87, 637-639 (2010).
- Fowler, S. A., Blackwell, H. E. Structure-function relationships in peptoids: Recent advances toward deciphering the structural requirements for biological function. Org. Biomol. Chem. 7, 1508-1524 (2009).
- Zuckermann, R. N., Kodadek, T. Peptoids as potential therapeutics. Curr. Op. Mol. Ther. 11, 299-307 (2009).
- Chongsiriwatana, N. P., Patch, J. A., Czyzewski, A. M., Dohm, M. T., Ivankin, A., Gidalevitz, D., Zuckermann, R. N., Barron, A. E. Peptoids that mimic the structure, function and mechanism of helical antimicrobial peptides. , 105-2794 (2008).
- Yam, A. Y., Wang, X., Gao, C., Connolly, M. D., Zuckermann, R. N., Bleua, T., Halla, J., Fedynyshyn, J., Allauzen, S., Peretz, D., Salisbury, C. M. A Universal method for detection of amyloidogenic misfolded proteins. Biochem. 50, 4322-4329 (2011).
- Huang, C. -. Y., Uno, T., Murphy, J. E., Lee, S., Hamer, J. D., Escobedo, J. A., Cohen, F. E., Radhakrishnan, R., Dwarki, V., Zuckermann, R. N. Lipitoids – novel cationic lipids for cellular delivery of plasmid DNA in vitro. Chem. Biol. 5, 345-354 (1998).
- Schroeder, T., Niemeier, N., Afonin, S., Ulrich, A. S., Krug, H. F., Bräse, S. Peptoidic amino- and guanidinium-carrier systems: Targeted drug delivery into the cell cytosol or the nucleus. J. Med. Chem. 51, 376-379 (2008).
- Murnen, H. K., Rosales, A. M., Jaworski, J. N., Segalman, R. A., Zuckermann, R. N. Hierarchical self-assembly of a biomimetic diblock copolypeptoid into homochiral super helices. J. Am. Chem. Soc. 132, 16112-16119 (2010).
- Nam, K. T., Shelby, S. A., Marciel, A. B., Choi, P. H., Chen, R., Tan, L., Chu, T. K. Free-floating ultra-thin two-dimensional crystals from sequence-specific peptoid polymers. Nature. Mater. 9, 454-460 (2010).
- Burkoth, T. S., Beausoleil, E., Kaur, S., Tang, D., Cohen, F. E., Zuckermann, R. N. Toward the synthesis of artificial proteins: The Discovery of an amphiphilic helical peptoid assembly. Chemistry & Biology. 9, 647-654 (2002).
- Murphy, J. E., Uno, T., Hamer, J. D., Cohen, F. E., Dwarki, V., Zuckermann, R. N. A Combinatorial approach to the discovery of efficient cationic peptoid reagents for gene delivery. Proc. Natl. Acad. Sci. U. S. A. 95, 1517-1522 (1998).
- Mora, P. u. i. g., Masip, I. s. a. b. e. l., Cortés, N. u. r. i. a., Marquina, R. e. g. i. n. a., Merino, R. a. m. &. #. 2. 4. 3. ;. n., Merino, J. e. s. &. #. 2. 5. 0. ;. s., Carbonell, T. e. r. e. s. a., Mingarro, I. s. m. a. e. l., Messeguer, A. n. g. e. l., Pérez-Payá, E. n. r. i. q. u. e. Identification from a positional scanning peptoid library of in vivo active compounds that neutralize bacterial endotoxins. J. Med. Chem. 48, 1265-1268 (2005).
- Zuckermann, R. N. Discovery of nanomolar ligands for 7-transmembrane G-protein coupled receptors from a diverse (N-substituted)glycine peptoid Library. J. Med. Chem. 37, 2678-2685 (1994).
- Alluri, P., Liu, B., Yu, P., Xiao, X., Kodadek, T. Isolation and characterization of coactivator-binding peptoids from a combinatorial library. Moleular Biosystems. 2, 568-579 (2006).
- Figliozzi, G. M., Goldsmith, R., Ng, S., Banville, S. C., Zuckermann, R. N. Synthesis of N-(substituted)glycine peptoid libraries. Methods Enzymol. 267, 437-447 (1996).
- Culf, A. S., Ouellette, R. J. Solid-phase synthesis of N-substituted glycine oligomers (α peptoids) and derivatives. Molecules. 15, 5282-5335 (2010).
- Burkoth, T. S., Fafarman, A. T., Charych, D. H., Connolly, M. D., Zuckermann, R. N. Incorporation of unprotected heterocyclic side chains into peptoid oligomers via solid-phase submonomer synthesis. J. Am. Chem. Soc. 125, 8841-8845 (2003).

