Remotely Supervised Transcranial Direct Current Stimulation: An Update on Safety and Tolerability
Summary
This manuscript provides an updated remote supervision protocol that enables participation in transcranial direct current stimulation (tDCS) clinical trials while receiving treatment sessions from home. The protocol has been successfully piloted in both patients with multiple sclerosis and Parkinson's disease.
Abstract
The remotely supervised tDCS (RS-tDCS) protocol enables participation from home through guided and monitored self-administration of tDCS treatment while maintaining clinical standards. The current consensus regarding the efficacy of tDCS is that multiple treatment sessions are needed to observe targeted behavioral reductions in symptom burden. However, the requirement for patients to travel to clinic daily for stimulation sessions presents a major obstacle for potential participants, due to work or family obligations or limited ability to travel. This study presents a protocol that directly overcomes these obstacles by eliminating the need to travel to clinic for daily sessions.
This is an updated protocol for remotely supervised self-administration of tDCS for daily treatment sessions paired with a program of computer-based cognitive training for use in clinical trials. Participants only need to attend clinic twice, for a baseline and study-end visit. At baseline, participants are trained and provided with a study stimulation device, and a small laptop computer. Participants then complete the remainder of their stimulation sessions at home while they are monitored via videoconferencing software.
Participants complete computerized cognitive remediation during stimulation sessions, which may serve a therapeutic role or as a "placeholder" for other computer-based activity. Computers are enabled for real-time monitoring and remote control by study staff.
Outcome measures that assess feasibility and tolerance are administered remotely with the aid of visual analogue scales that are presented onscreen. Following completion of all RS-tDCS sessions, participants return to clinic for a study end visit in which all study equipment is returned.
Results support the safety, feasibility, and scalability of the RS-tDCS protocol for use in clinical trials. Across 46 patients, 748 RS-tDCS sessions have been completed. This protocol serves as a model for use in future clinical trials involving tDCS.
Introduction
Transcranial direct current stimulation (tDCS) is a type of noninvasive brain stimulation with a wide range of potential therapeutic uses. A mild electrical current (typically ≤2.5 mA) is directed through electrodes placed on the scalp to influence brain activity by altering neuronal polarization1. tDCS is typically paired with a rehabilitation strategy in efforts to increase training outcomes. Other popular forms of neuromodulation, such as repetitive transcranial magnetic stimulation, are used to similar ends, but lack key advantages of tDCS such as its portability, simple applicability, and relative inexpensiveness1,2.
Multiple tDCS sessions are required for cumulative clinical benefit1,3. Behavioral effects, such as reductions in fatigue or depressive symptoms, rely upon repeated, consecutive sessions. For instance, studies have only observed such treatment effects after twenty or more sessions4,5.
Typically, tDCS is administered in clinic by a trained clinician or study personnel familiar with the facets and operations of the device and stimulation method. This is costly to both the patient and clinician as significant time, clinic space, and travel are required. As a solution to the need for daily, in-clinic tDCS, we have developed remotely supervised tDCS (RS-tDCS)6. This protocol enables at-home tDCS sessions to be completed through controlled supervision and guidance as provided by study personnel via a study laptop and has the benefit of only requiring two in-clinic visits (baseline and study-end visits) by the participant.
The patient populations chosen to pilot the methodology of this protocol include patients with multiple sclerosis (MS) and Parkinson's disease (PD). Both diseases impose distinct deficits on the patient, such as symptoms of fatigue in patients with MS and dyskinesia in patients with PD. tDCS presents a unique opportunity to improve symptoms of fatigue7 and cognitive dysfunction8,9,10 as well as to promote motor learning and control11,12. Participants representing a spectrum of disease severity were included in this study with steps taken, as permitted by RS-tDCS, to accommodate their respective disability.
Among these populations, people with MS or PD may have distinct obstacles that prevent them from conveniently reaching the clinic. Motor impairments such as confinement to a wheelchair or cognitive deficits that result in loss of autonomy can limit their inclusion in clinical trials or other cognitive studies. Additionally, familial and professional obligations reduce the time available to attend clinic, limiting availability for cognitive remediation trials confined to the clinic13. These patient populations, due to their diverse range of impairment, serve as model populations to test the limits and feasibility of RS-tDCS.
The RS-tDCS protocol marks a major step in the tDCS field, since it studies the use of stimulation, as it will be administered in a patient's care at home. The protocol strengthens the rate of recruitment, trial completion rate, relieves patient burden, and minimizes clinical costs. Herein we report the specific details of the protocol as well as preliminary findings regarding the feasibility, safety, and tolerability of the device when remotely administered.
Protocol
All procedures and device protocols have been approved for human subjects by institutional review boards at both Stony Brook University and New York University Langone Medical Center.
1. Participant Recruitment and Screening
- Recruit potential participants via referrals from IRB approved study physicians.
- Contact participants and perform a pre-screening to confirm basic eligibility before the baseline study visit (see Supplemental File 1).
- Assess cognitive competency via remote administration of a cognitive functioning test, (e.g. the symbol digit modalities test or SDMT)14. Exclude participants who score below three standard deviations of the healthy normative mean in their age group due to concerns regarding their cognitive capacity to comply with the study protocol.
- Briefly assess the potential participant's medical history over the phone. Assess inclusion and exclusion criteria. Exclusion criteria can be broad based on a recent consensus safety paper1. Assess the following portion of the inclusion criteria, including that patients must be 18 years or older and have the following: the understanding and ability to give consent, adequate home facilities to store equipment and perform RS-tDCS sessions, clearance for all study procedures by a study physician, and stable internet access at home.
NOTE: Allot sufficient time for the baseline visit so that the study physician may clear the potential participant. At least 3 hours are typically needed to complete all baseline procedures, questionnaires, and neuropsychological tests in the current iteration of this study protocol. - Rate each patient's disease related disability (e.g., the Expanded Disability Status Scale (EDSS)15 score for MS patients only). Participants with severe disability (e.g. an EDSS score above 6.5) will complete daily tDCS sessions with the aid of a healthcare proxy. The healthcare proxy will complete, on behalf of the participant, the device and headstrap preparation steps that require more advanced ambulation.
- In sham-control studies, randomize the participant to either the active or sham condition, as dictated by the blocked stratification table. The table stratifies participants based on the cognitive functioning and disability ratings collected during pre-screening16. Any study using sham should be double-blinded and have an un-blinded study technician, who will not supervise the participant's daily sessions, assign the condition, and prepare study devices for participants and study personnel.
2. Baseline Study Visit
- Administer neuropsychological assessments and self-report questionnaires.
- Choose relevant neuropsychological assessments (such as the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS)17, based on the respective patient population. Administer measures and assess the motor and cognitive abilities of participants.
- Administer self-report questionnaires relevant to symptoms that are specific to the patients' disease. For example, the Unified Parkinson's Disease Rating Scale (UPDRS)18 is a widely used clinical self-assessment questionnaire measuring Parkinson's specific symptoms. Likewise, the Multiple Sclerosis Neuropsychology Questionnaire (MSNQ) is designed and validated to measure neuropsychological competence in daily living specifically in patients with multiple sclerosis19,20. Administer universal symptom rating inventories (such as the Patient-Reported Outcomes Measurement Information System (PROMIS)21 and the Positive and Negative Affect Schedule (PANAS)22) to allow for comparison between patient populations.
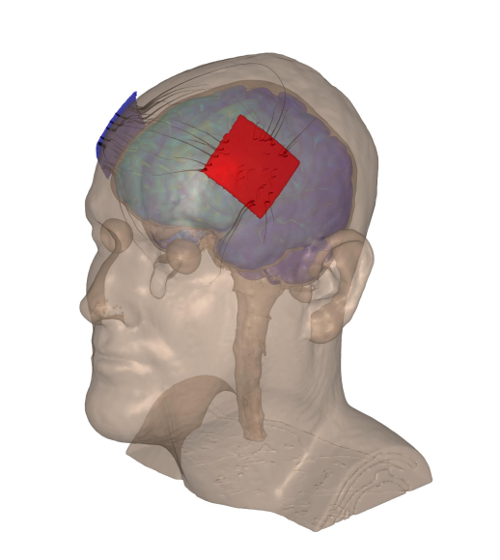
Figure 1: Left anodal DLPFC montage used in both studies. The red and blue patches are modeled representations of the left anode and right cathode, respectively, placed upon a scalp. Please click here to view a larger version of this figure.
- Instruct and train participants to self-administer tDCS.
- Show participants an instructive tDCS training video that details the entire step-by-step process of administering the stimulation.
- Ask participants to prepare the headstrap with guidance from study personnel. The headstrap holds the sponge electrodes in position throughout the duration of the stimulation session.
- Have participants attach the sponges, which are pre-moistened with 5-mL of saline solution, to the cable electrodes.
- Instruct participants to place the headstrap upon their head, taking care to align the nasion marker on the front of the headstrap with the bridge of their nose.
- Have participants pull the back the headstrap towards the posterior end of their heads so the back of the headstrap rests over their inion. The Dorsolateral Prefrontal Cortex (DLPFC), left anodal tDCS montage is used23,24,25,26,27.
- Confirm that the participant has achieved optimal or moderate contact quality before continuing.
NOTE: The device dynamically adjusts its voltage depending on the entire resistive path between the anodal and cathodal electrodes (including skin and skull impedance) to deliver consistent amperage. The device will not unlock to release the stimulation if the contact quality is poor or the impedance is too high. - If a participant has a poor or moderate contact quality then suggest methods to improve contact quality, such as adding saline to the sponges, adjusting headstrap placement, or brushing hair away from electrode sites. The participant can monitor their own contact quality by looking at the device's interface screen.
- Assess the participant's aptitude to complete study procedures to determine whether they understand and can replicate the procedures competently at home.
- Participants complete a tolerability test lasting 90 seconds to determine whether they are able to comfortably tolerate 2.0 mA of direct current. If a participant finds they are unable to handle 2.0 mA, lessen the amperage to 1.5 mA, and further to 1.0 mA if deemed necessary. If a participant finds 1.0 mA intolerable, they must be discontinued from the study.
- Conducting the first tDCS session in-clinic.
- Ask the participant, after preparing the headstrap and prior to beginning the stimulation session, to report the duration of sleep from the previous night as well as any pain experienced due to the stimulation, disease specific pain, fatigue, and mood before the session with the aid of visual analogue scales.
- Confirm that the contact quality is still optimal or moderate. Provide the device unlock code to begin the participant's stimulation session.
- Begin stimulation and deliver a tolerable amount of stimulation (as detailed above) for an intended twenty minutes. The stimulation's length depends upon the parameters set by the study; for all studies presented herein, the stimulation length was twenty minutes. Should the participant be randomized to the sham condition, the participant receives only one minute of stimulation at the beginning of the session and at the end of the session, during which the amperage ramps up to the target tolerable amount of stimulation during the first thirty seconds and then ramps back down to 0 mA in the final thirty seconds, in order to convince participants that they are receiving stimulation. Aside from the first and last minute, sham participants receive no stimulation for 18 minutes.
- Participants complete cognitive remediation with guidance from the study technician. The cognitive remediation may be swapped out for a different remediation strategy depending on the intended goals of the study. Similarly, the length of the remediation strategy may be modified.
NOTE: Stimulation can be halted at any time by pressing "zero" on the device which immediately begins to abort stimulation by ramping down to 0 mA. Study technicians instruct participants to abort the session and end stimulation when any pain is reported above a 7 on a 1-10 visual analogue scale or if any other emergency situation occurs. - After ten minutes, ask the participant whether they are experiencing any pain from the stimulation. Study technicians monitor placement through the videoconferencing software to determine that electrode placement does not shift and remains acceptable during the stimulation session.
- After an additional ten minutes, the pre-programmed tDCS device powers down and stimulation ceases. The device makes a loud beeping noise to notify the participant that the stimulation has ended. Should the participant have the sham condition, then the device ramps up once again during the final minute of the twenty-minute session to provide thirty seconds of the target stimulation current and then ramps down to 0 mA during the final thirty seconds of the session.
- Ask the participant once more to report their pain due to the device, disease specific pain, fatigue, and mood following the session, as well as any adverse events that may have been experienced during the course of the session.
- Plan a regular time for the remaining study sessions to ensure daily consistency for standardization purposes. Additionally for PD patients, coordinate session times so that stimulation takes place within 1-3 hours of their last dose of PD medication – a time window that study-approved physicians determined would provide maximum benefit in accordance with previous evidence showing greater improvement among Parkinson's patients in cognitive and motor dimensions (as measured by the Unified Parkinson's Disease Rating Scale, or UPDRS) when tDCS was delivered during participants' "on" drug state vs. "off" drug state11.
3. At-home tDCS Sessions
- Participants complete computer preparation prior to study sessions.
- Participants connect to the internet in their homes prior to any further study procedures. Participants without stable internet in their homes are excluded from all study procedures.
- Connect to a participant's computer with the use of remote desktop software28 and engage in HIPAA-compliant video conferencing with the participant29.
- Study technicians administer study outcome measures wherein participants report the amount of sleep they received the previous night and rate pain due to the device, disease specific pain, fatigue, and mood before the stimulation session with the aid of visual analogue scales. Ask the participant to report any adverse events that may have occurred following the previous day's session (see supplemental files 2 and 3).
- Participants remotely activate the stimulation.
- Participants prepare the headstrap by attaching the pre-moistened sponges to the electrodes on the inside of the frontal headstrap. The sponge electrodes easily snap into the headstrap to minimize preparation.
- Participants place the headstrap upon their heads as study technicians monitor placement. Participants report the contact quality, which is tested by the device and assesses the sponge placement and sponge saturation measured by the tDCS device. As previously described, moderate and poor contact quality is remedied by suggestions from study personnel such as adding additional saline solution or checking sponge placement.
- Verbally state the stimulation code that unlocks the device to the participant and allow administration of the stimulation for twenty minutes.
- Should a participant have an EDSS score above 6.5, the healthcare proxy completes the above steps in lieu of the participant.
- Participants complete computerized cognitive remediation during the twenty-minute stimulation period. The remediation includes cognitive training tasks that specifically target working memory systems. A previous publication of ours details the cognitive results concerning our first pilot study8.
- After 10 min of stimulation, ask participants to report any pain they may be experiencing.
- Participants complete post-session procedures.
- Participants take off the headstrap and dispose of study sponges.
- Ask participants to report any pain due to the device, disease specific pain, fatigue, and mood after the session as well any adverse events that they may have experienced during the session. Record any adverse events that occurred along with their intensity and duration.
- Plan the session for the following day.
4. Post-stimulation Visit
- Administer neuropsychological assessments and self-report questionnaires.
- Schedule the final study visit as soon as possible after the final stimulation session.
- Administer study outcome measures that were completed during the baseline visit a second time to assess whether patients experienced benefit as a result of the stimulation17.
- Obtain and sanitize all study equipment.
- Offer participants who were assigned the sham condition the option to complete an additional ten active, open-label, RS-tDCS sessions. This open label study period is modeled after our first tDCS pilot study6.
5. One Month Follow-up Survey
- Reach out to participants about a month after their study end to ask if they would remotely complete an online survey asking whether they believe benefits received from the stimulation persisted.
Representative Results
One pilot study has been completed using the RS-tDCS protocol at Stony Brook University and a second is currently ongoing at NYULMC. Respective institutional review boards approved all study procedures at both sites.
Since the presented studies were both intended to pilot the RS-tDCS methodology, participants were not selected on the basis on symptoms but instead using broad eligibility criteria for both patients with MS and PD. Similarly, patients who could not tolerate the target amperage of the stimulation were given an option of lower amperage (detailed further below).
Study 1:
MS participants were recruited through the Lourie Center for Pediatric MS at Stony Brook between the dates of March 2015 and February 2016. This trial was an open-label RS-tDCS study to pilot the feasibility of the protocol in MS. All participants knowingly received 20 minutes x 1.5 mA (or 1.0 mA if 1.5 mA was not initially tolerated) open-label tDCS applied to the DLPFC (left anodal)23,24,25,26,27. During the stimulation, cognitive training games30 targeting attention, information speed, and working memory were completed. The first session was completed at the end of the baseline visit in clinic and the remaining sessions were completed at-home, daily, five days per week (M-F) over the course of two weeks, for a total of nine sessions at home. In total, 26 participants were recruited for this study.
Study 2:
MS Arm – Participants with MS were recruited through the NYULMC's MS Comprehensive Care Center between the dates of January 2016 and October 2016. This arm of the study was a randomized, double-blinded, sham-controlled trial using 2.0 mA (or 1.5 mA if 2.0 mA was not initially tolerated) of RS-tDCS applied to the DLPFC (left anodal) for twenty minutes daily. The first stimulation session was completed at the end of the baseline visit in clinic while the remaining nineteen at-home sessions were completed over the following four weeks (M-F). This study is ongoing and we report the findings of 20 MS participants that have been recruited and completed the study.
PD Arm – Participants with PD were recruited through the NYULMC's Fresco Institute for Parkinson's and Movement Disorders between the dates of June 2016 and December 2016. This arm's focus was to pilot the RS-tDCS protocol in patients with PD, similar to the pilot study conducted with MS. The study was open-label, and all participants knowingly received 2.0 or 1.5 milliamps of tDCS applied to the DLPFC (left anodal). Similar to Study 1, the first session was completed at the end of the baseline clinic visit and the remaining nine sessions were completed at the participant's home remotely (M-F). This study is ongoing and we report the completed findings from 6 PD participants that have been recruited.
To assess the feasibility and tolerability of the RS-tDCS protocol, we measured the percentage of completed sessions, adverse event rates, and the average intensity of the most common adverse events.
A total of 748 RS-tDCS sessions have been successfully completed across 46 participants in approximately a year. This supports the feasibility of the RS-tDCS protocol. In Study 1, 2 participants discontinued tDCS: one participant discontinued due to personal obligations and the other participant discontinued due to study stop criteria of uncomfortable sensations of skin burning greater than a score of 7 (albeit without physical burns). In Study 2, 2 participants were discontinued: one was discontinued due to an abnormal adverse event of "tongue tingling" and the other was discontinued due to a pain rating of 7 (on the 1-10 analog scale) from headaches. From the PD cohort, no patients have been discontinued. In total, 4 patients were discontinued from the study and none of them were discontinued due to an inability to complete the RS-tDCS sessions.
The numbers of adverse events per stimulation type were tallied and their rates of occurrence were calculated. The stimulation types have been placed into four different categories: 1.5 mA open-label, 2.0 mA blinded, 2.0 mA open-label, and the sham condition. The rationale for division into these categories is that participants may have differing interpretations of their sensations depending on what they expected during stimulation. As seen in Figure 1, the three most common adverse events were sensations of skin tingling, itching, and burning (no participants received physical burns).
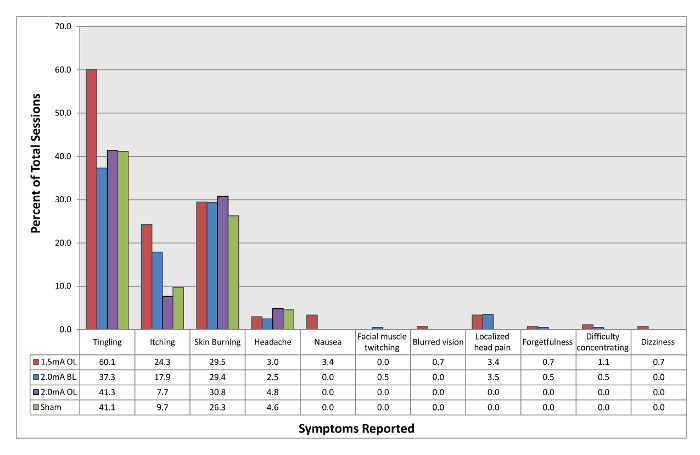
Figure 2: Rates of adverse events experienced with tDCS. 1.5 mA OL refers to sessions in which participants knowingly received 1.5 mA of open-label tDCS. 2.0 mA BL refers to sessions in which participants were blinded to the stimulation they were receiving which was 2.0 mA tDCS. 2.0 mA OL refers to sessions in which participants knowingly received 2.0 mA of open-label tDCS. Sham refers to sessions in which participants were blinded to the stimulation they were receiving but only received 60 s of stimulation at the beginning and end of the 20 min session in order to simulate active tDCS. Please click here to view a larger version of this figure.
The average intensity of the most common adverse events has been calculated. As shown in Table 1, the average intensity of the most common adverse events did not exceed a score of 3 (on a 1-10 visual analog scale, 1 being mild and 10 being extreme) for any of the adverse events in any of the stimulation conditions.
| Session Condition | Total Sessions | Tingling (SD, n) | Itching (SD, n) | Burning Sens. (SD, n) |
| 2.0 mA Blinded | 201 | 1.6 (0.8, 75) | 2.2 (0.9, 36) | 2.5 (1.3, 59) |
| 2.0 mA Open-Label | 104 | 1.9 (1.2, 43) | 1.8 (1.1, 8) | 2.0 (1.4, 32) |
| 1.5 mA Open-Label | 268 | 2.4 (2.2, 161) | 2.0 (1.6, 65) | 2.9 (2.0, 79) |
| Sham | 175 | 1.9 (1.2, 72) | 1.7 (0.9, 17) | 1.6 (1.2, 46) |
Table 1: Average intensity of commonly experienced adverse events on a visual analog scale (1-10, mild-intense).
The stimulation also shows promise for symptom management as can be seen in Figure 3. Cohen's d values were calculated for change in mood, fatigue, and pain from baseline to study end for MS patients in studies 1 and 2. PD patients were not included in this analysis due to the small cohort completed to date (n=6). An effect size analysis was employed due to the small sample sizes in each study to identify signals suggesting efficacy. The active sessions in Studies 1 and 2 showed far greater average effect sizes for improvement. On average, participants who received active tDCS reported greater positive effects, and had less negative effects, fatigue, and pain by study end compared to the sham tDCS group.
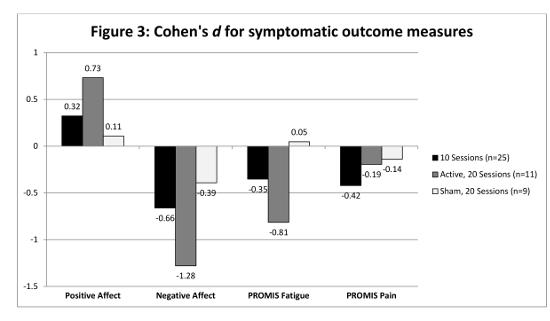
Figure 3: Cohen's d for symptomatic outcome measures. Positive effects were shown by the participants receiving 20 active sessions of rtDCS while the participants in sham group had negligible effects. Please click here to view a larger version of this figure.
Discussion
This study employed the DLPFC left anodal montage24, but this could easily be interchanged for another montage and the effects of stimulation may change accordingly. Depending on the location of stimulation, the brain effects and side effects experienced may change1. Stimulation type, such as intended inhibition instead of excitement may influence effects as well. Similarly, the form of remediation paired with stimulation may influence the results of the study31. Future experiments need to be conducted with different remediation strategies paired with RS-tDCS to identify its specific affects.
While the RS-tDCS protocol focuses on providing tDCS to patients in their homes, there may still be a place for stimulation provided in a clinical setting. For example, more complex montages, such as those used by HD-tDCS, may not be feasible at home even with proper training32. The RS-tDCS protocol provides a detailed procedure to ensure clinical trial standards of treatment sessions and dosing control while delivering the stimulation at home using a tele-rehabilitation protocol. Uniform electrode preparation and placement as well as simplified procedures, including the incorporation of simpler sponge electrode techniques, ensure consistency across participants. The RS-tDCS protocol allows for the completion of stimulation sessions by cognitively and physically impaired individuals who otherwise would have great difficulty reaching the clinic daily.
All troubleshooting can be immediately addressed by the study personnel who are live video-conferencing with participants during at-home stimulation sessions. In the case that the study laptop is malfunctioning, a simple restart of the computer can resolve technical issues. In the case that study equipment malfunctions from a laptop or the tDCS device are not resolved following technical support, then study personnel should arrange for the delivery of new, properly functioning equipment.
The protocol is inherently reliant upon internet access, which is the method's greatest limitation. Currently, those without internet cannot be enrolled in any trial using RS-tDCS. The internet utilized in conjunction with our protocol enables the remote supervision in our RS-tDCS protocol.
RS-tDCS remains one of the few recognized and feasible protocols for at-home delivery of tDCS33. The large volume of sessions completed with the protocol (748 in little over a year), speaks to the effectiveness of the protocol, as other centers have reported smaller and underpowered studies. The RS-tDCS protocol has been effective in providing tDCS directly to patients with a wide range of disabilities. By enabling clinical trials with the RS-tDCS protocol, rapid recruitment and swift trial completion are possible.
The RS-tDCS protocol is generalizable to other neurologic conditions. As demonstrated here, we have already generalized our protocol to PD and plan to demonstrate the applicability of the protocol to other conditions. Both the parameters of stimulation and the remediation strategy can be adjusted to target specific treatment outcomes.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank collaborators at City College of New York and Soterix Medical for their assistance and support.
Materials
| tDCS Mini-CT device | Soterix | Provides direct current stimulation | |
| EASYstrap with conducting cables | Soterix | Sponges attach to the strap and the strap lays on the head | |
| EASYpad sponges | Soterix | Premoistened sponges with 5mL of saline. Snaps into electodes. | |
| Stream laptop | Hewlett Packard | Used for videoconferencing and cognitive remediation. | |
| Device Charger | Soterix | Recharges the Mini-CT. | |
| TeamViewer Software | Teamviewer | Remote desktop software that enable remote control of participant's computers. | |
| Vsee Software | VSee Lab, Inc. | Enables HIPAA compliant video-conferencing | |
| Lumosity | Lumos Labs | Online platform used for cognitive remediation |
Riferimenti
- Bikson, M., et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. , (2016).
- Bashir, S., Yoo, W. K. Cheap Technology Like Transcrinal Direct Current Stimulation (tDCS) Could Help in Stroke Rehabilitation in South Asia. Basic Clin Neurosci. 4 (3), 188-189 (2013).
- Boggio, P. S., et al. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 25 (2), 123-129 (2007).
- Brunoni, A. R., et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 5 (3), 175-195 (2012).
- Kalu, U. G., Sexton, C. E., Loo, C. K., Ebmeier, K. P. Transcranial direct current stimulation in the treatment of major depression: a meta-analysis. Psychol Med. 42 (9), 1791-1800 (2012).
- Kasschau, M., et al. A Protocol for the Use of Remotely-Supervised Transcranial Direct Current Stimulation (tDCS) in Multiple Sclerosis (MS). J Vis Exp. (106), e53542 (2015).
- Ferrucci, R., et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation. 34 (1), 121-127 (2014).
- Charvet, L., et al. Remotely Supervised Transcranial Direct Current Stimulation Increases the Benefit of At-Home Cognitive Training in Multiple Sclerosis. Neuromodulation. , (2017).
- Mattioli, F., Bellomi, F., Stampatori, C., Capra, R., Miniussi, C. Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Mult Scler. , (2015).
- Manenti, R., et al. Mild cognitive impairment in Parkinson’s disease is improved by transcranial direct current stimulation combined with physical therapy. Mov Disord. 31 (5), 715-724 (2016).
- Benninger, D. H., et al. Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 81 (10), 1105-1111 (2010).
- Fregni, F., et al. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord. 21 (10), 1693-1702 (2006).
- Larocca, N. G. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 4 (3), 189-201 (2011).
- Smith, A. . The Symbol Digit Modalities Test (SDMT) Symbol Digit Modalities Test: Manual. , (1982).
- Kurtzke, J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 33 (11), 1444-1452 (1983).
- Suresh, K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 4 (1), 8-11 (2011).
- Langdon, D. W., et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler. 18 (6), 891-898 (2012).
- Martinez-Martin, P., et al. Unified Parkinson’s Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord. 9 (1), 76-83 (1994).
- Krupp, L. B., LaRocca, N. G., Muir-Nash, J., Steinberg, A. D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 46 (10), 1121-1123 (1989).
- O’Brien, A., et al. Relationship of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) to functional, emotional, and neuropsychological outcomes. Arch Clin Neuropsychol. 22 (8), 933-948 (2007).
- Christodoulou, C., Junghaenel, D. U., DeWalt, D. A., Rothrock, N., Stone, A. A. Cognitive interviewing in the evaluation of fatigue items: results from the patient-reported outcomes measurement information system (PROMIS). Qual Life Res. 17 (10), 1239-1246 (2008).
- Watson, D., Clark, L. A., Tellegen, A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 54 (6), 1063-1070 (1988).
- Forogh, B., et al. Repeated sessions of transcranial direct current stimulation evaluation on fatigue and daytime sleepiness in Parkinson’s disease. Neurol Sci. 38 (2), 249-254 (2017).
- Seibt, O., Brunoni, A. R., Huang, Y., Bikson, M. The Pursuit of DLPFC: Non-neuronavigated Methods to Target the Left Dorsolateral Pre-frontal Cortex With Symmetric Bicephalic Transcranial Direct Current Stimulation (tDCS). Brain Stimul. , (2015).
- Nord, C. L., Lally, N., Charpentier, C. J. Harnessing electric potential: DLPFC tDCS induces widespread brain perfusion changes. Front Syst Neurosci. 7, 99 (2013).
- Eddy, C. M., Shapiro, K., Clouter, A., Hansen, P. C., Rickards, H. E. Transcranial direct current stimulation can enhance working memory in Huntington’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 77, 75-82 (2017).
- Lefaucheur, J. P., et al. The treatment of fatigue by non-invasive brain stimulation. Neurophysiol Clin. , (2017).
- . TeamViewer-the All-In-One Software for Remote Support and Online Meetings Available from: https://www.teamviewer.com/en/index.aspx (2015)
- Flachenecker, P. Clinical implications of neuroplasticity – the role of rehabilitation in multiple sclerosis. Front Neurol. 6, 36 (2015).
- Mattioli, F., Bellomi, F., Stampatori, C., Capra, R., Miniussi, C. Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Mult Scler. 22 (2), 222-230 (2016).
- Borckardt, J. J., et al. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. J Pain. 13 (2), 112-120 (2012).
- Kasschau, M., et al. Transcranial Direct Current Stimulation Is Feasible for Remotely Supervised Home Delivery in Multiple Sclerosis. Neuromodulation. , (2016).

