A New Straightforward Method for Lipophilicity (logP) Measurement using 19F NMR Spectroscopy
Summary
A novel and straightforward variation of the shake-flask method was developed for accurate lipophilicity measurement of fluorinated compounds by 19F NMR spectroscopy.
Abstract
Fluorination has become an effective tool to optimize physicochemical properties of bioactive compounds. One of the applications of fluorine introduction is to modulate the lipophilicity of the compound. In our group, we are interested in the study of the impact of fluorination on lipophilicity of aliphatic fluorohydrins and fluorinated carbohydrates. These are not UV-active, resulting in a challenging lipophilicity determination. Here, we present a straightforward method for the measurement of lipophilicity of fluorinated compounds by 19F NMR spectroscopy. This method requires no UV-activity. Accurate solute mass, solvent and aliquot volume are also not required to be measured. Using this method, we measured the lipophilicities of a large number of fluorinated alkanols and carbohydrates.
Introduction
Lipophilicity is a key physicochemical parameter of drug molecules which influences the properties of drug candidates in many aspects, including drug solubility, bioavailability, and toxicity1. Lipophilicity is measured as the logarithm (logP) of the ratio of compound concentrations after partitioning between n-octanol and water. Optimal lipophilicity ranges have been proposed based on statistical data of orally administered drugs, of which the Lipinski's "rule of 5'' is the most famous example2,3. Indeed, controlling lipophilicity has shown to be essential for improving the prospect of drug candidates. Increasing drug binding affinity by elevated lipophilicity has been identified as one of the main problems in drug discovery projects during the past few decades, leading to increased attrition rates3. Therefore, it has been suggested that successful drug development is associated with keeping the molecular lipophilicity of the drug candidates within optimal boundaries during the affinity optimization process3,4. In that regard, new concepts (such as lipophilic efficiency indices) have been introduced5,6.
It is thus of great importance to accurately measure lipophilicity during the drug development process. Besides, the availability of straightforward methods for lipophilicity measurement is in demand as fundamental research aims to identify solutions for logP modulation. Currently, numerous established methods are accessible for lipophilicity determination1. The standard 'shake-flask (SF)' method7and its variations are commonly employed to measure logP values directly, which in most cases depend on UV-Vis spectroscopy for quantification. The main disadvantage of this classic SF method is its labor-intensive nature. In addition, the formation of emulsions may occur, especially for highly lipophilic compounds8,9.Several methods were developed to circumvent such issues, such as by using flow injection analysis, dialysis tubing, etc.9,10.However, none of those methods are straightforward or easily applicable in non-specialized laboratories.
There are also many indirect methods available for use, such as potentiometric titration11, electrophoretic methods12,13, RP-HPLC-based chromatographic methods, mass-spectrometry-based methods14, etc. These are indirect methods, as the logP values are obtained by calibration curves. Among these methods, the RP-HPLC method has been widely used because it is user-friendly and time-saving. Nevertheless, its accuracy depends on the training set used to establish the calibration curve, and the estimated lipophilicity depends on the partition system used13,15.
There are a number of 1H NMR-based methods reported in the literature for lipophilicity determination. Mo et al. developed a method for logP measurement using 1H NMR without deuterated solvents. Water and octanol, as partition solvents, were used as references for the quantification of solute concentration in each phase16. Herth and co-workers also reported an approach, by which the partition experiment occurred directly in an NMR tube, where the NMR data of the bottom D2O aqueous layer were collected before and after the extraction with 1-octanol, to obtain the distribution coefficient17. In addition, Soulsby et al. exploited 1H NMR as an analysis tool, determining the amplitude of signals by using complete reduction to amplitude-frequency table software. The ratio of the amplitudes in both layers led to the measured partition coefficient18. These methods are relatively simple to use but often require the calibration of selective pulses and power levels or the use of shaped gradient pulses to ensure appropriate solvent suppression and signal selectivity.
Calculated logP (clogP) values for compounds can also be obtained. Several calculation methods and commercially available software are available. Such clogP values are commonly used in the pharmaceutical industry when evaluating large numbers of drug molecules. However, large errors from clogP values are not uncommon19,20.
The requirements of UV-activity for concentration analysis and the establishment of calibration curves for logP calculation impede research progress in this field. In particular, this is the case for non-UV-active aliphatic compounds. Fluorinated aliphatic moieties have become increasingly attractive for drug design in recent years, and their influence on overall lipophilicity of the compound is a research topic in our group21. In addition, 19F is a highly sensitive NMR-active nucleus, making 19F NMR a useful tool for analyzing fluorinated compounds. It also has a larger chemical shift range compared to that of 1H. Therefore, it is worthwhile to develop a straightforward method for logP determination of non-UV-active fluorinated compounds by 19F NMR spectroscopy. Hence, the overall goal of this method is to achieve convenient lipophilicity determination of fluorinated compounds.
The key principle of our 19F NMR-based method is to add a fluorinated reference compound in the partition experiment (Figure 1)21. Compound X and reference compound (ref) are partitioned between water and n-octanol. After equilibrating, an aliquot from each phase is taken into an NMR tube, and 19F NMR experiments are run on both NMR samples. The intensity of the fluorine peaks is proportional to compound concentration (C) and the number of fluorine atoms (n) of the compounds. Between compound X and ref, integral ratios can be obtained for both phases. The ratio in n-octanol layer is defined as ρoct, and ρaq for water layer (eq. 1). The ratio of ρ values equals the ratio of partition coefficients (P) of compound X and ref (eq. 2). This leads to the final equation (eq. 4) for logP measurement of compound X. Therefore, in order to determine the logP value of an unknown compound X, only integration ratios (ρoct and ρaq) in both layers are needed to be measured by 19F NMR.
Protocol
1. Partitioning
- Add 4,4,4-trifluorobutan-1-ol (compound X, ca. 6.0 mg) and 2,2,2-trifluoroethanol (reference compound, ca. 3.0 mg) to a 10 mL pear-shaped flask, dissolve in n-octanol (HPLC grade, ca. 2 mL), and add water (HPLC grade, ca. 2 mL).
Note: This experiment is run in triplicate. Compound solubility in water and n-octanol must be checked. The amount of the compound used for partition must be carefully considered to avoid oversaturation of the compound in any layer. The mass ratio between compound X and reference compound ref must also be considered to avoid that the integral ratios of a given NMR sample are outside a 10/1 to 1/10 range. For example, if there is a difference of <2 logP units between compound X and ref, optimal mass ratio can assure that integration ratios in water and 1-octanol NMR samples are within a 10/1 to 1/10 range. In contrast, if an integration ratio of 50/1 in one layer is obtained, there will be more likely relatively larger errors in the integration for the peak with lower concentration. The equation below can be used to predict optimal compound mass ratio:
mX / mref = {(cPX / Pref)-0.5 * (MX/ Mref) * [(1 + cPX) / (1 + Pref)]} / (NX / Nref)
m, mass; M, molecular mass; N, number of F atoms; P, partition coefficients; cP, calculated partition coefficients. - Place the flasks inside a temperature-controlled receptacle above a stirplate, and connect to a recirculating chiller. Stir the biphasic mixture at 25 °C for 2 h, with stirring speed set at 600 rpm.
- Equilibrate the mixture at 25 °C overnight (ca. 16 h), to allow for complete phase separation.
Note: In some cases, the formation of a foam between the n-octanol and water boundary can be observed. In this case, the mixture was transferred into a 4 mL glass vial and centrifuged till the disappearance of the foam. The biphasic mixture was then left to equilibrate again at 25 °C overnight.
2. NMR Sample Preparation
- Fix the flask to a retort stand with a clamp.
- Take an aliquot of ca. 0.70-0.85 mL from both water and n-octanol layers, by using 1 mL disposable plastic syringes with long needles.
- For taking the water aliquot, draw ca. 0.02 mL of air into the syringe before putting the needle into the mixture. While moving the needle through the upper n-octanol layer into water layer, gently push out the air to prevent n-octanol solution from entering the needle.
- Remove the long needle from the mixture. Discard a small amount of water sample, leaving ca. 0.6 mL of sample left in the syringe. Carefully wipe the needle with dry tissue, and inject ca. 0.5 mL of water sample into a clean NMR tube. Quickly close the NMR tube with a cap.
- For the n-octanol sample, remove the long needle from the n-octanol layer. Discard a small amount of n-octanol sample, leaving ca. 0.6 mL of sample left in the syringe. Carefully wipe the needle with dry tissue and inject ca. 0.5 mL of n-octanol sample into a clean NMR tube. Quickly close the NMR tube with a cap.
- Visually inspect both n-octanol and water samples for any contamination (e.g., small droplets of n-octanol in water sample or small droplets of water in n-octanol sample).
Note: If there is any contamination, the aliquot sample needs to be re-prepared from the biphasic mixture. As the measurement is done in triplicate, six NMR tubes are obtained. - To each NMR tube, add 0.1 mL of a deuterated NMR solvent that is miscible with both n-octanol and water (e.g., acetone-d6) to enable signal lock during NMR acquisition.
- For compounds with low boiling points (e.g., <120 °C), seal the NMR tubes using a blow torch, and, after cooling, invert the tube to check for any leaks. Carefully invert the sealed or non-sealed NMR tubes 20 times to obtain a homogenous solution for 19F NMR experiments.
3. NMR Experiments
- Run, using standard NMR parameter settings (NS 64, D1 1 s, SW 300 ppm, O1P -100 ppm), 19F{1H} NMR experiments to identify chemical shifts of 4,4,4-trifluorobutan-1-ol (compound X) and 2,2,2-trifluoroethanol (reference compound) in both n-octanol and water NMR samples.
- Measure the spin-lattice relaxation time (T1) for diagnostic fluorine nuclei by using an inversion-recovery sequence22. Gauge the level of appropriate pulse delay time (D1, set as ≥ 5*T1) from the obtained T1 values for accurate quantitative NMR integration.
Note: This is very time-consuming, but a D1 of 60 s for the water phase sample, and of 30 s for the octanol phase sample, are conservative settings which will safely fulfill the D1 ≥ 5*T1 criterium. - Run 19F{1H} NMR experiments again with adjusted parameter settings as follows: a) use D1 ≥ 5*T1; b) center the frequency offset point (O1P) between the two diagnostic fluorine signals so both nuclei can be equally excited; c) Set the spectral width (SW) as 300 ppm, but reduce if a better SNR ratio if needed; d) Set the number of transients (NS) as 64 but increase if higher SNR is required.
Note: Non-decoupled 19F NMR experiments can be also used for NMR data acquisition. However, proton-decoupled 19F NMR experiments are preferred here as it simplifies the fluorine signals by removing proton-fluorine couplings which also increases signal-to-noise ratio. We use inverse-gated decoupling to obtain a decoupled spectrum without nOe (nuclear Overhauser effect) enhancements23. For quantitative integration, a signal-to-noise ratio (≥300) is desired.24
4. Data Processing
- Process the obtained data using ACD/NMR Processor Academic Edition or other custom NMR processing software.
- Open the NMR data file, then open the pdata folder, followed by folder 1. Delete the 1r file.
- Return to the NMR data file and drag the fid file into the ACD/NMR Processor window.
- Click the WFunctions button, select Exponential, set LB value as 2, and click the OK button.
- Click the Zero Filling button, increase the Points Count to 4 times of its Original Points Count by clicking a small button next to the number, and click the OK button.
- Click the Fourier Tr. button.
- Click the Phase button, then click the Mouse Ph. button, click and hold the left mouse button, move the mouse forward or backward till the major peak of the spectrum is properly phased.
- Click and hold the right mouse button, move the mouse forward or backward until the other peak(s) of the spectrum is properly phased. Then unclick the Mouse Ph. button, zoom into the spectral area with the fluorine peaks, click Fine Tuning, perform the phase correction if needed as described earlier till all peaks are correctly phased, and then click the Tick button.
- Click the Baseline button, then the Options button. Select Spectrum Averaging for Automatic Models, adjust the number of points for Box Half Width if needed (in particular for spectrum with low S/R ratio), click OK | Auto, and then click the Tick button.
- Click Integration, integrate the diagnostic fluorine peaks, and click the Tick button.
Note: If the integral curve is not parallel to the baseline, click the Bias Corr. button, and adjust the tilt and slope until the curve is parallel to the baseline.
- Obtain the integration ratios from n-octanol and water NMR samples and use in the logP calculation equation (Figure 1, eq. 4) to obtain the logP value of of 4,4,4-trifluorobutan-1-ol (compound X).
Representative Results
Two sets of data as control experiments are shown in Figure 221. Using 2,2,2-trifluoroethanol as reference compound, logP values were obtained for 2-fluoroethanol and 3,3,3,2,2-pentafluoropropanol as -0.75 and +1.20, respectively (Figure 2A). Subsequently, the lipophilicity of 2-fluoroethanol was determined again but with 3,3,3,2,2-pentafluoropropanol as the reference (using its previous experimentally measured logP value +1.20). The measured logP value was -0.76, which only had a difference of 0.01 logP units when compared with the value measured using 2,2,2-trifluoroethanol as reference.
Likewise, for cis-2,3-difluoro-1,4-butanediol, the difference in measured logP values by using 2-fluoroethanol and its trans isomer is also very small (0.01 logP units, Figure 2B). This demonstrated that the selection of reference compound does not have impact on the logP measurement. In addition, a rather small standard deviation (<0.01) indicated good reproducibility of our method.
Using our method, a series of compounds with known logP values was measured as shown in Table 1. The difference between literature data and the values measured using our method is shown in the last column of the Table. Overall, the experimentally obtained logP values (at 25 °C) have good to excellent accordance with the literature values, which further validated our method.
Additional selected examples21 were shown in Figure 3. All these non-UV-active aliphatic compounds (from fluorinated carbohydrates to fluorohydrins) can be easily measured with our method.
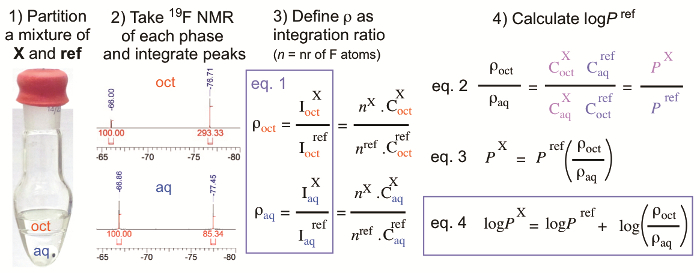
Figure 1: Principle of the logP determination method. This figure has been reproduced with permission from Wiley-VCH Verlag GmbH & Co. KGaA.21. This shake-flask method is based on 19F NMR spectroscopy. A reference compound is used for partition experiment. Aliquots for both n-octanol and water phase were taken for NMR experiment. Integration ratios between reference compound and the compound to be measured are obtained for the determination of logP value. Detailed mathematical deduction of equations, which leads to the final equation for measurement, are also given. Please click here to view a larger version of this figure.
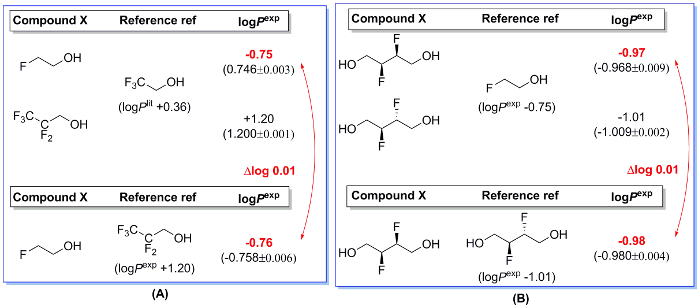
Figure 2: Examples for internal validation21. Two sets of control experiments, using two different reference compounds to measure logP value of one compound, were conducted. The logP difference between those experiments is negligible. Standard deviation (<0.01) from experiments run in triplicate shows good reproducibility of the method. Please click here to view a larger version of this figure.
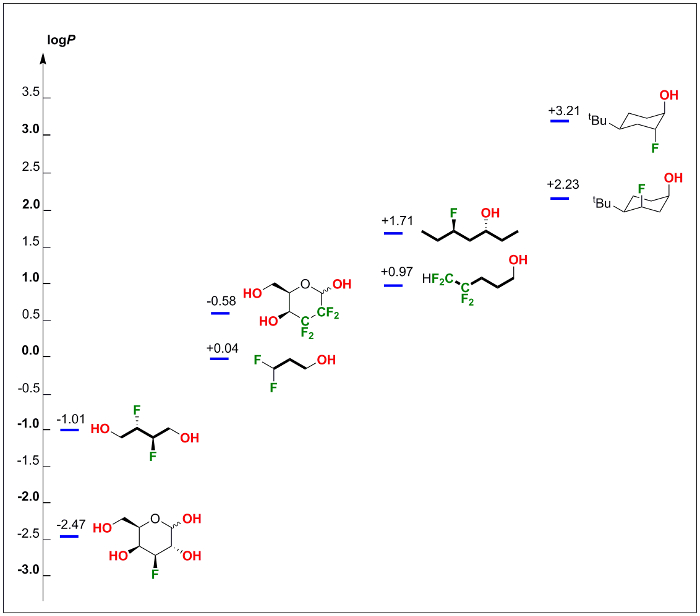
Figure 3: Further selected examples of logP measurement using our method. Applying this method, logP values for 8 fluorinated compounds (such as fluorinated carbohydrates, acyclic alkanols and conformationally restricted fluorohydrins) were obtained. Please click here to view a larger version of this figure.
Table 1: Comparison between literature data and the experimental logP values using our method21. logP values for 14 fluorinated compounds (with known logP data) were measured using this new method. The reference compounds used for each measurement were also tabulated. Comparision (logP) between literature values and logP results from our method demonstrated good accuracy of this method. a2,2,2-Trifluoroethanol (TFE), 2-Fluoroethanol (FE); bAveraged logP value from at least three experiments; cExperimentally measured logP value by our method (-0.75) was used as reference. Please click here to download this file.
Discussion
The protocol described in the paper is a straightforward method for logP measurement of fluorinated compounds. This method is applicable to fluorinated compounds with a logP value from -3 to 3. For more hydrophilic (logP < -3) or lipophilic compounds (logP > 3), this method can still be used but will require much longer NMR experiment time as extended number of transients are needed to obtain a good signal-to-noise ratio. Hence, this is a limitation of the method. There is no requirement for the frequency of NMR spectrometer, as long as the conditions (NMR parameter settings and sufficient SNR) for quantitative integration are met. As for any shake flask method, it is critical to avoid oversaturation and contamination during the layer sampling.
Compared with previous shake-flask method and its variations, there are several advantages in our method with respect to existing methods. 1) Measurements of solute mass, volume of partition solvents and aliquots for NMR sample are not necessary. 2) The compound for measurement can be impure provided that the fluorine chemical shifts of the impurities are different from that of the measured compound. 3) Because of the intrinsic compensation effect when working with the ratio of a ratio, systematic errors are eliminated. 4) This method is applicable to non-UV-active fluorinated compounds. 5) This method is easy to use with open-access NMR facilities as no special NMR settings are needed (such as solvent suppression, applying a small excitation angle, etc.).
Currently, we are using this method to measure the lipophilicities of fluorinated carbohydrates, fluorohydrins and fluorinated amides, in order to investigate the influence of fluorination on lipophilicity and to identify fluorinated moieties with lipophilicity-lowering effect. Method development for logP measurement of more lipophilic compounds (logP >3) and for fluorinated amines is ongoing in our group.
It can be pointed out that 19F NMR can also be used for critical micelle concentration (CMC) determination30.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This research is funded as part of EPSRC grants EP/K016938/1 and EP/P019943/1 (ZW, HRF) and of an EPSRC/AstraZeneca CASE conversion award (BFJ). The University of Southampton is thanked for additional support. The EPSRC is further thanked for a core capability grant EP/K039466/1.
Materials
| NMR (400 MHz) with Bruker 5 mm SEF probe | Bruker | n/a | AVIIIHD400 |
| NMR (400 MHz) with Bruker 5 mm SMART probe | Bruker | n/a | |
| DrySyn Snowstorm reactor | Asynt | ADS13-S | |
| recirculating chiller | Asynt | n/a | model:Grant-LTC2 |
| magnetic stirplate | Asynt | ADS-HP-NT | |
| ACD/NMR processor software | ACD/Labs | n/a | ACD/NMR processor academic edition or ACD/Spectrus processor 2015 |
Riferimenti
- Arnott, J. A., Planey, S. L. The influence of lipophilicity in drug discovery and design. Expert Opinion on Drug Discovery. 7 (10), 863-875 (2012).
- Lipinski, C. A., Lombardo, F., Dominy, B. W., Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 23 (1), 3-25 (1997).
- Leeson, P. D., Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nature Reviews Drug Discovery. 6, 881 (2007).
- Perola, E. An Analysis of the Binding Efficiencies of Drugs and Their Leads in Successful Drug Discovery Programs. Journal of Medicinal Chemistry. 53 (7), 2986-2997 (2010).
- Tarcsay, A., Nyiri, K., Keseru, G. M. Impact of Lipophilic Efficiency on Compound Quality. Journal of Medicinal Chemistry. 55 (3), 1252-1260 (2012).
- Tarcsay, &. #. 1. 9. 3. ;., Keserű, G. M. Contributions of Molecular Properties to Drug Promiscuity. Journal of Medicinal Chemistry. 56 (5), 1789-1795 (2013).
- . . OECD Guidelines for Testing of Chemicals. , (1992).
- Tsang, S. C., Yu, C. H., Gao, X., Tam, K. Y. Preparation of nanomagnetic absorbent for partition coefficient measurement. International Journal of Pharmaceutics. 327 (1), 139-144 (2006).
- Andersson, J. T., Schräder, W. A Method for Measuring 1-Octanol−Water Partition Coefficients. Analytical Chemistry. 71 (16), 3610-3614 (1999).
- Danielsson, L. -. G., Yu-Hui, Z. Mechanized determination of n-octanol/water partition constants using liquid-liquid segmented flow extraction. Journal of Pharmaceutical and Biomedical Analysis. 12 (12), 1475-1481 (1994).
- Scherrer, R. A., Donovan, S. F. Automated Potentiometric Titrations in KCl/Water-Saturated Octanol: Method for Quantifying Factors Influencing Ion-Pair Partitioning. Analytical Chemistry. 81 (7), 2768-2778 (2009).
- Poole, S. K., Poole, C. F. Separation methods for estimating octanol-water partition coefficients. Journal of Chromatography B. 797 (1), 3-19 (2003).
- Ishihama, Y., Oda, Y., Uchikawa, K., Asakawa, N. Evaluation of Solute Hydrophobicity by Microemulsion Electrokinetic Chromatography. Analytical Chemistry. 67 (9), 1588-1595 (1995).
- Jorabchi, K., Smith, L. M. Single Droplet Separations and Surface Partition Coefficient Measurements Using Laser Ablation Mass Spectrometry. Analytical Chemistry. 81 (23), 9682-9688 (2009).
- Kaliszan, R. Quantitative structure-retention relationships. Analytical Chemistry. 64 (11), 619A-631A (1992).
- Mo, H., Balko, K. M., Colby, D. A. A practical deuterium-free NMR method for the rapid determination of 1-octanol/water partition coefficients of pharmaceutical agents. Bioorganic & Medicinal Chemistry Letters. 20 (22), 6712-6715 (2010).
- Stéen, E. J. L., et al. Development of a simple proton nuclear magnetic resonance-based procedure to estimate the approximate distribution coefficient at physiological pH (logD7.4): Evaluation and comparison to existing practices. Bioorganic & Medicinal Chemistry Letters. 27 (2), 319-322 (2017).
- Soulsby, D., Chica, J. A. M. Determination of partition coefficients using 1H NMR spectroscopy and time domain complete reduction to amplitude-frequency table (CRAFT) analysis. Magnetic Resonance in Chemistry. 55 (8), 724-729 (2017).
- Tetko, I. V., Poda, G. I., Ostermann, C., Mannhold, R. Accurate In Silico log Predictions: One Can’t Embrace the Unembraceable. QSAR & Combinatorial Science. 28 (8), 845-849 (2009).
- Waring, M. J. Lipophilicity in drug discovery. Expert Opinion on Drug Discovery. 5 (3), 235-248 (2010).
- Linclau, B., et al. Investigating the Influence of (Deoxy)fluorination on the Lipophilicity of Non-UV-Active Fluorinated Alkanols and Carbohydrates by a New log P Determination Method. Angewandte Chemie International Edition. 55 (2), 674-678 (2016).
- Derome, A. E. . Modern NMR Techniques for Chemistry Research. , (1997).
- Claridge, T. . High-Resolution NMR Techniques in Organic Chemistry. , (1999).
- Zhang, F. -. F., et al. Quantitative analysis of sitagliptin using the 19F-NMR method: a universal technique for fluorinated compound detection. Analyst. 140 (1), 280-286 (2015).
- Muller, N. When is a trifluoromethyl group more lipophilic than a methyl group? partition coefficients and selected chemical shifts of aliphatic alcohols and trifluoroalcohols. Journal of Pharmaceutical Sciences. 75 (10), 987-991 (1986).
- Hansch, C., Leo, A. . Substituent constants for correlation analysis in chemistry and biology. , (1979).
- Dillingham, E. O., Mast, R. W., Bass, G. E., Autian, J. Toxicity of Methyl- and Halogen-Substituted Alcohols in Tissue Culture Relative to Structure-Activity Models and Acute Toxicity in Mice. Journal of Pharmaceutical Sciences. 62 (1), 22-30 (1973).
- Leo, A., Hansch, C., Elkins, D. Partition coefficients and their uses. Chemical Reviews. 71 (6), 525-616 (1971).
- Fujita, T., Iwasa, J., Hansch, C. A New Substituent Constant, π, Derived from Partition Coefficients. Journal of the American Chemical Society. 86 (23), 5175-5180 (1964).
- Zhong-Xing, J., Xin, L., Eun-Kee, J., Bruce, Y. Y. Symmetry-Guided Design and Fluorous Synthesis of a Stable and Rapidly Excreted Imaging Tracer for 19F MRI. Angewandte Chemie International Edition. 48 (26), 4755-4758 (2009).

