Assessment of Long-term Depression Induction in Adult Cerebellar Slices
Summary
In some gene-manipulated animals, using a single protocol may fail to induce LTD in cerebellar Purkinje cells, and there may be a discrepancy between LTD and motor learning. Multiple protocols are necessary to assess LTD-induction in gene-manipulated animals. Standard protocols are shown.
Abstract
Synaptic plasticity provides a mechanism for learning and memory. For cerebellar motor learning, long-term depression (LTD) of synaptic transmissions from parallel fibers (PF) to Purkinje cells (PC) is considered the basis for motor learning, and deficiencies of both LTD and motor learning are observed in various gene-manipulated animals. Common motor learning sets, such as adaptation of the optokinetic reflex (OKR), the vestibular-ocular reflex (VOR), and rotarod test were used for evaluation of motor learning ability. However, results obtained from the GluA2-carboxy terminus modified knock-in mice demonstrated normal adaptation of the VOR and the OKR, despite lacking PF-LTD. In that report, induction of LTD was only attempted using one type of stimulation protocol at room temperature. Thus, conditions to induce cerebellar LTD were explored in the same knock-in mutants using various protocols at near physiological temperature. Finally, we found stimulation protocols, by which LTD could be induced in these gene-manipulated mice. In this study, a set of protocols are proposed to evaluate LTD-induction, which will more accurately allow examination of the causal relationship between LTD and motor learning. In conclusion, experimental conditions are crucial when evaluating LTD in gene-manipulated mice.
Introduction
The synaptic organization of the elaborated neuronal networks of the cerebellar cortex, composed of PCs, molecular layer interneurons (basket and stellate cells), Golgi cells, PFs from granule cells, mossy fibers and climbing fibers (CFs), have been elucidated in terms of excitation/inhibition and divergence/convergence, and the well-organized circuitry diagram has suggested that the cerebellum is a “neuronal machine”1, though there was previously no idea about purpose of this “machine”. Later Marr proposed that the PFs input to PCs constitute a triple layer associative learning network2. He also suggested that each CF conveys a cerebral instruction for elemental movement2. He assumed that simultaneous activation of PFs and CF would enhance PF-PC synapse activity, and cause long-term potentiation (LTP) of the PF-PC synapse. On the other hand, Albus assumed that synchronous activation of PFs and CF resulted in LTD at the PF-PC synapses3. Both the above studies interpret the cerebellum as a unique memory device, the incorporation of which into the cerebellar cortical network leads to the formation of the Marr–Albus model learning machine model.
Following these theoretical predictions, two lines of evidence suggest the presence of synaptic plasticity in the cerebellum. The first line of evidence was suggested by the anatomical organization of the flocculus; here MF pathways of vestibular organ origin and CF pathways of retinal origin converge on the PCs4. This unique convergence pattern suggests that a synaptic plasticity occurring in the flocculus causes the remarkable adaptability of the vestibulo-ocular reflex. Second, the recording of the PCs response in the flocculus and the lesioning of the flocculus also supported the above hypothesis5,6,7. Furthermore, the PC discharge pattern during adaptation of a monkey’s hand movement8 supported the synaptic plasticity hypothesis, especially Albus’s LTD-hypothesis3.
To determine the nature of the synaptic plasticity directly, repeated conjunctive stimulation (Cjs) of a bundle of PFs and the CF that specifically innervates the PC in vivo was shown to induce LTD for the transmission efficacy of the PF–PC synapses9,10,11. In the subsequent in vitro explorations using a cerebellar slice12 and cultured PCs, conjunction of co-cultured granule cell stimulation and olive cell stimulation13 or conjunction of iontophoretically applied glutamate and somatic depolarization14,15 caused LTD. The signal transduction mechanism underlying the LTD-induction was also intensively investigated using in vitro preparations16,17.
Adaptations of the VOR and the OKR were often used for quantitative evaluation of gene-manipulation effects on cerebellar motor learning, because the vestibule-cerebellar cortex was proven to be the essential origin in the adaptive learning of the VOR18,19,20 and the OKR19,21 The correlation between failure of LTD-induction and impairment of behavioral motor learning has been taken as evidence that LTD plays an essential role in motor learning mechanisms22. These views are collectively referred to as the LTD hypothesis of motor learning, or Marr-Albus-Ito hypothesis23,24,25,26.
Adaptive learning of eye movement was measured using similar protocols, while various experimental conditions were used to induce LTD in slice preparation27,28,29,30,31. Recently, Schonewille et al.26 reported that some gene-manipulated mice demonstrated normal motor learning, but the cerebellar slices did not show LTD, and thereby concluded that LTD was not essential for motor learning. However, the induction of LTD was only attempted using one type of protocol at room temperature. Hence, we used several types of LTD-inducing protocols under recording conditions at around 30 °C, and we confirmed that the LTD was reliably induced in the gene-manipulated mice by using these protocols at near physiological temperatures32.
However, there remain some questions regarding the basic properties of conjunctive stimulation. The first is the relationship between the complex spike’s shape and the amplitude of LTD. Second, in conjunction with PF-stimulation and somatic depolarization, whether the number of stimuli used were necessary or not was elusive. In the present study, these questions were investigated using wild type (WT) mice.
Protocol
All experimental procedures were approved by the RIKEN committee on the care and use of animals in experiments. Mice were kept in the animal facility of the RIKEN Center for Brain Science under well-controlled temperature (23–25 °C) and humidity (45%–65%) conditions. Both male and female WT mice (C57BL/6, 3–6 months) were used.
1. Preparation of Solutions Used in the Experiments
NOTE: All solutions should be made in ultrapure water free of metals (resistivity > 18.2 MΩ) and other impurities (total organic carbon (TOC) < 5.0 ppb). Working artificial cerebrospinal fluid (ACSF) for slice-cutting and recording are made freshly on the day of experiment from a 10 times (x10) stock of ACSF. Bubble the solutions with 5% CO2/ 95% O2 gas mixture before use. The pH of ACSF is adjusted to 7.4 ± 0.1, and osmolarity is adjusted 315 ± 5 mOsm/kg by adding ultrapure water.
- Prepare 10x stock of ACSF containing 1250 mM NaCl, 30 mM KCl, 12.5 mM NaH2PO4, and 260 mM NaHCO3. This solution can be stored at 4 °C.
- Prepare working ACSF containing 125 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM Mg2SO4, 1.25 mM NaH2PO4, 26 mM NaHCO3 and 20 mM glucose.
- First, add 1 mL of 2 M CaCl2 solution followed by 1 mL of 1 M Mg2SO4 solution into around 800 mL of ultrapure water, to avoid precipitation. Then add 100 mL of 10x ACSF and glucose. Finally, make up to a total volume of 1,000 mL by adding ultrapure water.
- Prepare 3.3% agar for brain handling. Dissolve 1 g of agar in 30 mL of 0.9% NaCl solution, and heat in a microwave until just boiling. Stir to mix, then pour it into a sterile 4 cm x 10 cm plastic box and allow to solidify. Store the agar plate (~8 mm thickness) in a refrigerator.
- Prepare the internal solution.
- Prepare the K+-based internal solution containing 60 mM KCl, 60 mM K-gluconate, 0.3 mM EGTA, 4 mM MgCl2, 4 mM ATP, 0.4 mM GTP and 30 mM HEPES (pH 7.2).
NOTE: Low concentration (0.3 mM) of EGTA, a slow Ca2+-chelator, is added to chelate possibly contaminated Ca2+ in pure water, but this low concentration of EGTA in the internal solution never blocks the induction of LTD (Figure 3, Figure 4, Figure 5) during whole-cell recording. Measured osmotic pressure is 285 mOsm/kg. - Prepare the Cs+-based internal solution containing 60 mM CsCl, 46 mM D-gluconate, 27 mM tetraethylammonium chloride (TEA-Cl), 0.3 mM EGTA, 4 mM MgCl2, 4 mM ATP, 0.4 mM GTP and 30 mM HEPES (pH 7.2, adjusted using CsOH).
NOTE: Cs+ blocks voltage-dependent K-channels, and improves space-clamp conditions at remote dendrites by increasing the length-constant. Measured osmotic pressure is 285 mOsm/kg. - Prepare 200 µL aliquots of the solutions and store at -30 °C.
- Prepare the K+-based internal solution containing 60 mM KCl, 60 mM K-gluconate, 0.3 mM EGTA, 4 mM MgCl2, 4 mM ATP, 0.4 mM GTP and 30 mM HEPES (pH 7.2).
2. Brain Dissection and Trimming
- Chill and oxygenate two 50 mL beakers of ACSF on ice until the temperature is lower than 4 °C. Add 50 µL of tetrodotoxin (TTX, 1 mM) into one of the ice-cold beakers of ACSF and reserve it for slices cutting. To obtain mouse cerebellar slice reserving LTD-inducing ability, the addition of TTX to the normal ACSF is necessary.
- Cool down the metal specimen tray by filling an ice-bath area of the slicing chamber with ice.
- Pour 1 mL of isoflurane into an anesthetizing jar (~1000 mL) then place a mouse in it for 30–45 s. Ensure that the mouse is deeply anesthetized by confirming its inability to respond to mechanical stimulation.
- Decapitate the mouse using surgical scissors. Hold the head and cut the superficial skin along the midline using an ophthalmological scissor. Pull the skin by holding with fingers to widely expose the skull’s surface.
- Cut the skull horizontally along a line from the major spinocerebellar hole just above the ear and eye using an ophthalmological scissor. Cut the skull along a line above both eyes and remove to isolate the skull.
- Cut the brain at the middle of cerebrum by using a scalpel, then isolate the caudal part of the brain including the cerebellum from the skull. Immerse it into an ice-cold beaker of ACSF. Usually, the total time from decapitation to immersion of the brain block into the pre-chilled beaker of ACSF should be less than 60 s.
- Position of bubbling tubing should be adjusted so as not to stir the brain block in the beaker. Mechanical damage might cause swelling of the slice during recording. Leave it for at least 7 min and allow the brain to cool down.
- To trim the brain block, cut a rectangular agar piece (2 cm x 2 cm) from a large agar plate (4 cm x 10 cm, stored at 4 °C) and put it on a filter paper to absorb the excess liquid.
- Turn the agar piece upside-down on the filter paper, then place the agar piece on a filter paper on a pre-chilled metal specimen tray (16 cm x 20 cm). Pick up the brain block using a spatula and absorb excessive liquid around it with a piece of filter paper.
- Mount the brain block onto the agar block using glue (medical cyanoacrylate instant adhesive). Make sure to attach the bottom (ventral side) of the brain block to the agar.
- Cut out the right hemisphere with a blade. Be sure that the side of the cutting plane is as parallel as possible to the dendritic plane of the PC because this side is attached to the surface of the specimen tray. Cut and remove the other side of the hemisphere. Then, cut the brain between the superior and inferior colliculi, and cut off the spinal cord.
- Glue the right side of trimmed cerebellum with the agar block onto the pre-chilled specimen tray. Spread excess glue around the cerebellum with the flat part of a spatula, to prevent excess glue from attaching to the cerebellar surface. Tilt the metal tray and pour ACSF in order to fix the glue and wash away the excess glue.
3. Brain Slicing
- Orient the sample such that the dorsal side of the cerebellum is on the front side. Pour ice-cold cutting ACSF, containing 1 µM TTX, sufficient to immerse the cerebellum completely. Place a gas tube into the ACSF and start bubbling with O2/CO2 gas mixture.
- Remove the arachnoid mater using a fine tweezer under binoculars. Cut the cerebellar peduncle with a blade, and remove the brainstem and agar block. Rotate the tray 180°, so that the dorsal surface of the cerebellum faces a razorblade.
- Set the blade, and adjust the first cutting location. Set the vibratome slicing parameters to the following: amplitude to 5.5, frequency to 85 Hz, speed to 3–4, and slice thickness to 300 µm.
- Transfer the cerebellar slice on a nylon-net into an acrylic incubator and immerse the slice completely into the oxygenated ACSF. The incubator should be placed in a water-bath that maintains a temperature at 26 °C.
- Store the slices for at least 1 h to allow recovery from the damage during slicing.
4. Whole-cell Patch-clamp Recording
NOTE: A patch-clamp recording requires following equipment: an upright microscope with infrared differential interference contrast (IR-DIC) optics, a patch-clamp amplifier, data digitizer, digital stimulator, isolator, computer, software for data-acquisition and analysis, motorized manipulator, microscope platform, vibration isolation table, Faraday cage, solution heating system, peristaltic pumps and electrode puller.
- Add picrotoxin (0.1 mM) to ACSF and resolve it using ultra-sonication for 3 min.
- Perfuse a recording chamber with picrotoxin-containing, O2-CO2-saturated ACSF at rate of 2 mL/min. Maintain the temperature of the recording chamber at around 30 °C.
- Make a recording electrode by pulling a borosilicate glass capillary with filament (outer diameter = 1.5 mm) using a puller with 4 steps. The tip-diameter should be around 1 µm.
- Make a stimulating electrode by pulling the same capillary using the puller with 2 steps, then break to produce a fine tip by striking the tip against an iron block under a binocular microscope. The final diameter should be 3–5 µm.
- Transfer the cerebellar slice to the recording chamber and fix it with a Pt-weight with nylon threads. Fill a stimulating electrode with ACSF.
- For stimulation of the PFs, place the stimulating electrode on the surface of the molecular layer, around 50 µm away from the Purkinje cell layer.
- For stimulation of the CF, place the stimulating electrode at the bottom of the Purkinje cell layer (steps 5.3, 5.4).
- Filter K+-based or Cs+-based internal solution with a 0.45 µm filter. Use a micro-loader to fill a recording electrode with 8 µL of internal solution.
- Apply a weak positive pressure to the recording electrode before immersing it into the ACSF. Its resistance should be 2–4 MΩ and the liquid junctional potential should be corrected.
- Approach the healthy, bright cell body of the PC with the recording electrode. Push the surface of Purkinje cell slightly, stop applying positive pressure, next apply negative pressure until forming a giga-ohm seal. Then establish the whole-cell configuration using negative pressure.
- Hold the membrane potential at -70 mV, and apply -2 mV pulse (duration, 100 ms) at 0.1 Hz to monitor input resistance, series resistance and input capacitance, continuously. Do not use the series resistance compensation. Discard data when the series resistance varies by more than 15%.
5. Induction of LTD
- Stimulate the molecular layer with a pulse (duration, 0.1 ms). Identify the PF-excitatory postsynaptic currents (EPSC) by applying a double pulse stimulus (interspike interval (ISI) of 50 ms). The PF-EPSC should show paired-pulse facilitation and gradual increase in amplitude relative to increase in stimulation intensity.
- Record the test response of the PF-EPSC by applying a single pulse at 0.1 Hz. Adjust the intensity of the stimulus so that the evoked EPSC amplitude is around 200 pA. Avoid contamination of current through the voltage-dependent ionic channel.
- Stimulate the CF at the bottom of the Purkinje cell layer, and identify the EPSC elicited by the CF activation (by applying a double pulse stimulus). The CF-EPSC should show paired-pulse depression and an all-or-none manner according to the increase in stimulation intensity. For LTD induction, a single stimulus should be used.
- LTD-inducing protocol 1
- Using an electrode containing K+-based internal solution under current-clamp conditions, apply a single PF-stimulus and a single CF-stimulus simultaneously at 1 Hz for 5 min (300 pulses) (Figure 1A).
- LTD-inducing protocol 2
- Using an electrode-containing K+-based internal solution under current-clamp conditions, apply double PF-stimuli (ISI of 50 ms) and single CF-stimulus as the second PF-stimulus is coincident with CF-stimuli at 1 Hz for 5 min (Figure 1B).
- LTD-inducing protocol 3
- Using an electrode containing Cs+-based internal solution under voltage-clamp conditions, apply a double PF-stimulus (ISI of 50 ms) and a single depolarizing voltage-step (-70 to 0 mV, 50 ms) to the soma at 1 Hz for 3 min, so that the second PF-stimulus is equivalent to the beginning of the depolarizing voltage step (Figure 1C).
- LTD-inducing protocol-4
- Using an electrode containing Cs+-based internal solution under voltage-clamp conditions, apply the PF-stimuli (5x at 100 Hz) and a single depolarizing voltage-step (-70 to 0 mV, 50 ms) to the soma at 0.5 Hz for 3 min, simultaneously (Figure 1D).
Representative Results
Four protocols were used in this study to induce cerebellar LTD. In the first two protocols (protocol 1 and 2), the conjunction of the PF-stimulation and the CF-stimulation was applied under current-clamp conditions. In the other two protocols (protocol 3 and 4), somatic depolarization was substituted for the CF-stimulation under voltage-clamp conditions. Voltage-traces or current-traces during conjunctive stimulation were compared (Figure 2).
Conjunction of 1 PF-stimulation and 1 CF-stimulation under current-clamp conditions (protocol-1) were conventionally used for slice preparation26,27. The shape of the complex spike elicited by the Cj was similar to that elicited by the CF-stimulation alone, with the first steep spikelet followed by 2 to 3 spikelets (Figure 2A). A similarly shaped complex spike was observed during stimulation with protocol 2, namely, 1 PF-stimulation was followed 50 ms later by a conjunctive second PF- and CF-stimulation (Figure 2B). Under voltage-clamp conditions using a Cs+-based internal solution, conjunction of a 2 PF stimulation and somatic depolarization were applied (protocol 3) (Figure 2C). The first PF-stimulation was followed 50 ms later by a concomitant application of a second PF-stimulation and somatic depolarization. An inward current was elicited upon somatic depolarization from -70 to 0 mV. A tail current was also evoked after repolarization. Sometimes, repetitive generation of an inward current was observed, which would reflect the Ca-spike activity at the remote dendritic region where the membrane potential was not clamped sufficiently, in spite of using a Cs+-based internal solution (Figure 2C). Finally, 5 PF-stimuli at 100 Hz were given simultaneously with the somatic depolarization under voltage-clamp conditions (protocol 4). Again, repetitive generation of inward currents were elicited during depolarization and a tail current was elicited after the repolarization. Timing of the repetitive generation of the inward current was not synchronized with the PF-stimuli (Figure 2D). Sometimes, repetitive generation of the inward current continued after repolarization.
As for the LTD induced by protocols-1 and -2, reduction of the EPSC-amplitude measured during the 25-min after the onset of Cj was scattered over a relatively wide range32. Compared to the stable shape of the PF-EPSP, the shape of complex spike was quite variable from cell to cell. Because spikelets in a complex spike reflected the Ca2+-channel activation33, the shape of a complex spike, such as amplitude or steepness of spikelets, affecting the LTD amplitude was examined with the complex spike elicited by protocol 1. Because the shape of the complex spikes elicited by protocol 2 were contaminated with the PF-EPSP, we did not analyze these data. First, the sum of all spikelets (1–4)-amplitude was correlated with the amplitude of the LTD (-ΔEPSC %) (Figure 3A,B). The correlation coefficient (r) was 0.28, but was not statistically significant (p > 0.5). Because spikelets 2–4 contained more of the Ca2+-component34, the sum of the spikelet (2–4)-amplitude was correlated with the LTD-amplitude. The correlation seemed to be stronger (r = 0.67), but still not statistically significant (p > 0.1) (Figure 3C). Next, the maximum value of dVm/dt (maximum rate of rise [MRR]) of each spikelet was calculated (Figure 3D), because the product of membrane capacitance (Cm) and dVm/dt roughly reflects the membrane currents35. Correlation between the product of the Cm and the sum of MRRs of spikelets (1–4) and the LTD-amplitude was examined (Figure 3E), and r was 0.18 (p > 0.9). The correlation between the product of the Cm and the sum of the MRR of spikelets (2–4) showed a slightly stronger r (0.36) but it was not significant (p > 0 .6) (Figure 3F).
Under voltage clamp conditions, protocol 3 with 180 Cjs efficiently induced LTD (Figure 4B)32. However, whether a smaller number of stimuli can effectively induce LTD remains unknown. Thus, 60 Cjs were applied at 1 Hz for 1 min. Around 10 min after Cj, the EPSC amplitude was suppressed, however, it recovered at 15 min after Cj-onset. This suggests that 60 times Cjs at 1 Hz was insufficient to induce LTD (Figure 4A). Furthermore, repetition of somatic depolarization alone (180 times) did not induce LTD (Figure 4B)32.
The protocol 4 was originally used by Steinberg et al.30 for young mice (P14–21). LTD was reportedly induced by 30 Cjs at 0.5 Hz at RT in the wild type cerebellum. However, when 30 Cjs were applied to the adult mice cerebellar slice (3–6 month) at around 30 °C, no LTD was induced (Figure 5A). In contrast, when 90 Cjs were applied, the usual amplitude of LTD was observed (Figure 5B)32. Again, somatic depolarization alone (90 times at 0.5 Hz) did not induce LTD (Figure 5B).
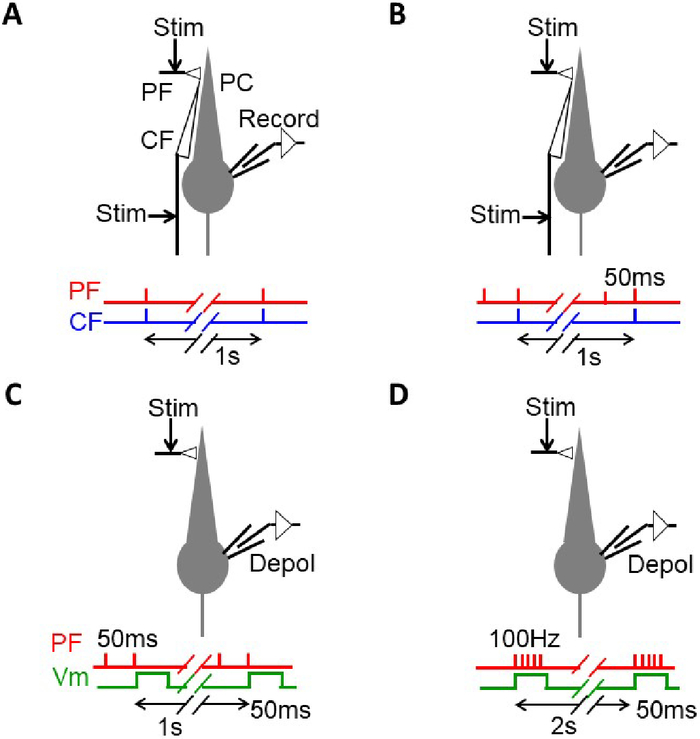
Figure 1: Schematic illustration of protocols to induce LTD in PF-PC synapse. (A) Protocol 1 Cj. 1 PF and 1 CF stimuli are applied simultaneously 300 times at 1 Hz (5 min) under current-clamp conditions. Electrode for whole-cell recording contains K+-based internal solution. (B) Protocol 2 Cj. 2 PF and 1 CF stimuli are applied simultaneously 300 times at 1 Hz (5 min) under current-clamp condition. Electrode contains K+-based internal solution. (C) Protocol 3 Cj. 2 PF and somatic depolarization (-70 to 0 mV, 50 ms) are applied 180 times at 1 Hz (3 min) under voltage-clamp condition, so that the second PF stimulus is applied simultaneously with the beginning of the somatic depolarization. Electrode contains Cs+-based internal solution. (D). Protocol 4 Cj. 5 PF at 100 Hz and somatic depolarization are applied 90 times at 0.5 Hz (3 min) under voltage-clamp condition, simultaneously. Electrode contains Cs+-based internal solution. Please click here to view a larger version of this figure.
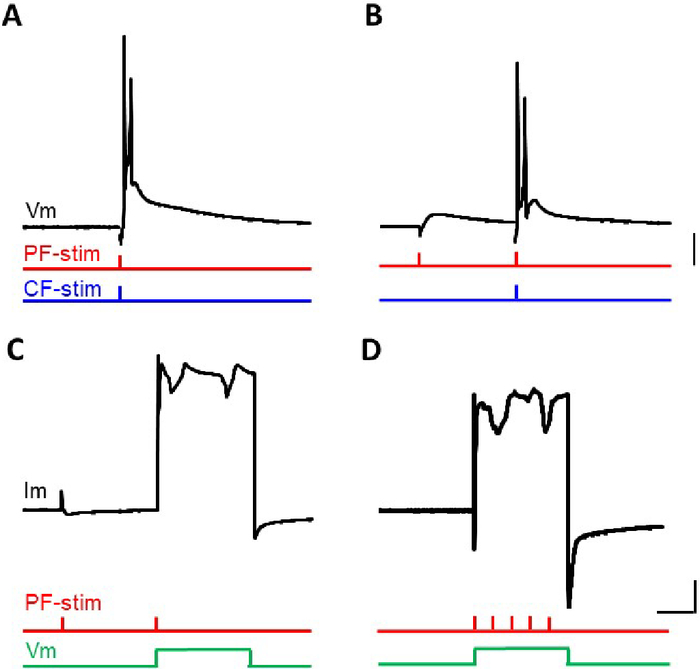
Figure 2: Voltage or current traces of the PC during conjunction stimulation. (A) Membrane potential trace elicited by protocol 1 Cj. (B) Membrane potential trace elicited by protocol 2 Cj. (C) Membrane current trace elicited by protocol 3 Cj. (D) Membrane current trace elicited by protocol 4 Cj. Vertical bars = 10 mV (A and B), 1 nA (C and D). Horizontal bar = 20 ms. Please click here to view a larger version of this figure.
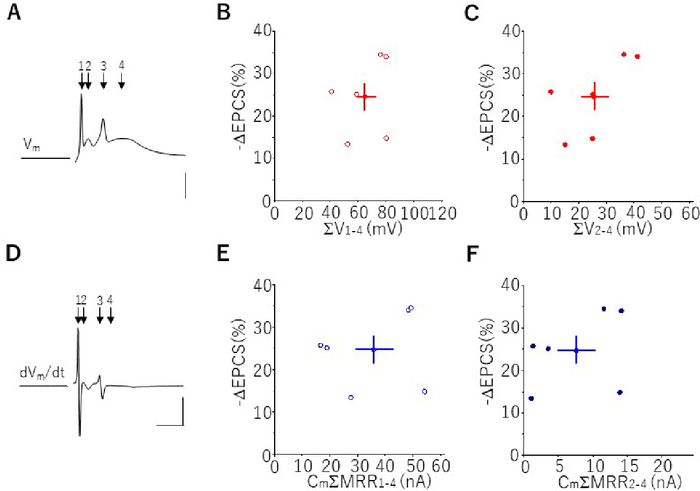
Figure 3: Relationship between spikelet of a complex spike and LTD-amplitude. (A) Representative trace of a complex spike elicited by protocol 1. Arrows indicate peaks of spikelets (1–4). Scale bar = 20 mV. (B) Relationship between the sum of the amplitude of spikelets (1–4) and LTD-amplitude (-ΔEPSC %) (r = 0.28, p > 0.5). (C) Relationship between the sum of the amplitude of spikelets (2–4) and LTD-amplitude (r = 0.67, p > 0.1). (D) Representative trace of differentiated complex spikes shown in A. Arrows indicate peaks of dVm/dt of spikelets. Scale bars = 5 ms, 50 V/s. (E) Relationship between products of the Cm and the sum of the MRR of spikelets (1–4) and amplitude of LTD (-ΔEPSC %) (r = 0.18, p > 0.7). (F) Relationship between the product of the Cm and the sum of the MRR of spikelets (2–4) and amplitude of LTD (r = 0.36, p > 0.4). Please click here to view a larger version of this figure.
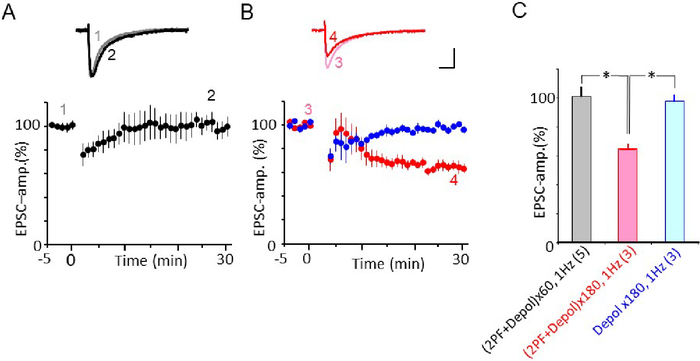
Figure 4: Effect of number of repetitions on LTD-induction using protocol-3 Cj. (A) Failure of LTD induction by protocol-3 Cj, repetition was 60 at 1 Hz. Mean PF-EPSC amplitude recorded before and after protocol-3 Cj (black column at the bottom). PF-EPSC amplitude was normalized by those recorded before Cj. Filled symbol indicate the mean EPSC amplitude. Error bars denote SEM. Inset: superimposed PF-EPSC traces (top) were recorded before (marked 1) and 25–29 min after Cj-stim onset (marked 2). Each trace represents the average of 6 records. Scale bars = 100 pA, 10 ms. (B) Red symbol: LTD induced by protocol 3 Cj, repetition was 180 times at 1 Hz. Blue symbol: no conjunction stimulation but somatic depolarization was applied 180 times at 1 Hz. LTD was not induced. Inset: superimposed PF-EPSC traces (top) were recorded before (marked 3) and 25–29 min after Cj-stim onset (marked 4). Each trace represents the average of 6 records. Scale bars = 100 pA, 10 ms. Data shown in B is the same used in Figure 3B of Yamaguchi et al.32. (C). Summary plot of mean PF-EPSC amplitude recorded during 25–29 min after onset of Cj. Depol: depolarization. Numerical character in parentheses represents number of cells. x60 = 60 times, x180 = 180 times. Please click here to view a larger version of this figure.
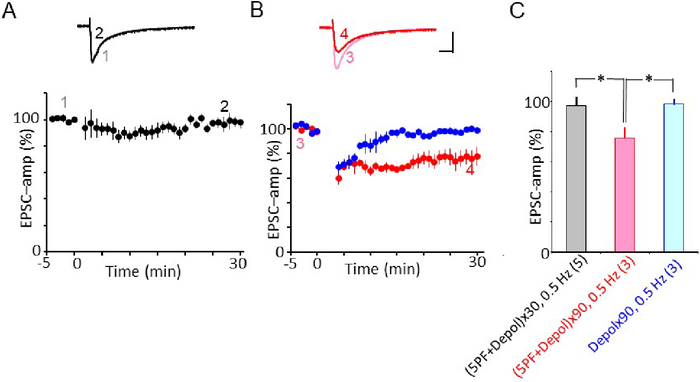
Figure 5: Effect of number of repetitions on LTD-induction using protocol 4 Cj. (A) Failure of LTD induction by protocol 4 Cj, repetition was 30 times at 0.5 Hz. Mean PF-EPSC amplitude recorded before and after protocol-4 Cj (black column at thr bottom). Filled symbol indicate the mean EPSC amplitude. Error bars denote SEM. Inset: superimposed PF-EPSC traces (top) were recorded before (marked 1) and 25–29 min after Cj-stim onset (marked 2). Each trace represents the average of 6 records. Scale bars = 100 pA, 10 ms. (B) Red symbol: LTD induced by protocol 4 Cj., blue symbol: no conjunction stimulation but somatic depolarization was applied 180 times at 0.5 Hz. LTD was not induced. Inset: superimposed PF-EPSC traces (top) were recorded before (marked 3) and 25–29 min after Cj-stim onset (marked 4). Scale bars = 100 pA, 10 ms. Data shown in B is the same used in Figure 4B of Yamaguchi et al.32. (C). Summary plot of mean PF-EPSC amplitude recorded during 25–29 min after onset of Cj. Depol: depolarization. Numerical character in parentheses represents number of cells. x30 = 30 times, x90 = 90 times. Please click here to view a larger version of this figure.
Discussion
Differences among the four protocols
In LTD-inducing protocols 1 and 2, Cjs 300 times at 1 Hz is sufficient to induce cerebellar LTD. Stimulation frequency of the CF seemed to be in a physiological range, because the complex spike firing rate in alert adult mice (P60) was reported to be 1.25 Hz36. However, the CF stimulation alone did not cause long-term plasticity in the PF-CF synapse, as used in protocols 1 and 2 (Figure 4, Figure 5), though CF-stimulation alone at higher frequency induced LTD24. The shape of the complex spike, elicited in protocol 1, also had no significant relationship with the LTD-amplitude (Figure 3). Perhaps the shape of the complex spike reflects the averaged Ca2+-concentration, but did not represent the local Ca2+-concentration in a branchlet where LTD was induced.
Regarding the PF stimulation, although 1 PF stimulation was conventionally used to induce cerebellar LTD26,27, the granule cell tended to fire in burst mode in vivo37. Therefore, multiple activations of the PF would be better than a single PF stimulation to mimic the physiological firing pattern, and the mGluR1 activation depended on the number and frequency of PF firing38. Thus, protocol-2 would activate PKC more intensively than protocol-1 did. In some mutated GluA2 knock-in mice (K882A), only protocol-2 was sufficient to induce LTD as compared with protocol 132, suggesting that a higher concentration of activated PKC was required to induce LTD for this particular GluA2 mutation.
Multiple stimulations of the PF would increase the incidence of LTD-induction, but activation of a voltage dependent Ca2+-channel via CF-stimulation or somatic depolarization was still required. In order to increase activation of a voltage-dependent Ca2+-channel at the PC dendrite, where the PF was stimulated, the cell body of the PC was depolarized under voltage-clamp conditions30. When using a Cs+-based internal solution, input resistance increased markedly32, suggesting an increase in the cable constant. Consequently, the number of activated Ca2+-channels in the distal area of the PC dendrite would be increased. In some GluA2 mutant mice (Δ7), 2PF stimulation + somatic depolarization (protocol 3) or 5 PF stimulation + somatic depolarization (protocol 4) could induce LTD. Conversely, no LTD phenomenon was observed by protocol 1 or 2 stimulation. It may be that somatic depolarization under voltage-clamp conditions with Cs+-internal solution activated voltage-dependent Ca2+-channels occurs more effectively than activation by the CF-stimulation under current-clamp conditions with K+-based internal solution, and this might cause a non-additive increase in [Ca2+]di39 and a subsequent robust PKC-activation required for LTD.
Possible compensatory mechanism for LTD in gene manipulated animals
Theoretical study assumes an all-or-none type activation of PKC upon LTD induction40, but in experimental results using some gene-manipulated animals such as GluA2 knock-in mice demonstrated that activation of PKC varied depending on LTD induction protocols32. Among 4 different protocols, the most effective induction protocol to activate PKC were protocols 3 and 4, followed by protocol 2 and the weakest was protocol 1. In gene-manipulated animals, compensatory mechanisms might be causing LTD32. Such compensatory mechanisms might have lower sensitivity to activated PKC. If so, multiple sets of LTD-inducing protocols, including one which can activate PKC stronger than conventional protocols, is necessary to evaluate the LTD induction ability in gene manipulated animals. Though normal induction of LTD with Cs+-based internal solution is reported in cultured PCs15 or PCs in slices30, a Cs+-based internal solution is not physiological. However, activation of a voltage-dependent Ca2+-channel at a remote dendrite is difficult using somatic depolarization in the slice, because of the possible mechanical damage during preparation and recording which may cause a decrease in the length-constant. Thus, to ensure activation of Ca2+-channels in the PF-stimulating dendritic region, it is necessary to use a protocol that increases the length-constant by using a Cs+-internal solution.
Other experimental conditions
Other factors should also be taken into consideration when synaptic plasticity and animal behavior are examined in parallel such as matching of the animal age and recording temperature in vitro, because signal transduction rate constants and receptor trafficking are highly temperature sensitive. Actually, motor learning in vivo reduced the number of surface expressed AMPA-type glutamate receptors41 and the size of asynchronous mini-EPSC at the PF-PC synapse42. Ideally, whole-cell patch clamp recording should be done at 37 °C, however, a stable long-term recording is difficult at 37 °C. Hence, all electrophysiological recordings in this study were conducted at around 30 °C. Though this temperature is lower than physiological temperature, it still would provide more favorable conditions to compare synaptic properties analyzed in vitro with behavioral learning ability. This difference in recording temperatures might be another factor to cause these results to differ from previous report26. In addition, assessment of constancy of passive membrane properties32 and intrinsic excitability43,44 is also important in study of the synaptic plasticity.
In conclusion, to investigate the causal relationship between synaptic plasticity and animal behavior in gene manipulated animals, assessment of synaptic plasticity in vitro would require careful control of experimental conditions such as using temperature regulation and several types of protocols.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank A. Oba for her technical assistance. This research was partially supported by Grant-in-Aid for Scientific Research (C) 17K01982 to K.Y.
Materials
| Amplifier | Molecular Devices-Axon | Multiclamp 700B | |
| Borosilicate glass capillary | Sutter | BF150-110-10 | |
| Digitizer | Molecular Devices-Axon | Digidata1322A | |
| Electrode puller | Sutter | Model P-97 | |
| Isoflurane | FUJIFILM Wako Pure Chemical | 26675-46-7 | |
| Isolator | A.M.P.I. | ISOflex | |
| Linear slicer | Dosaka EM | PRO7N | |
| Microscope | NIKON | Eclipse E600FN | |
| Peristaltic pump | Gilson | MP1 Single Channel Pump | |
| Picrotoxin | Sigma-Aldrich | P1675 | |
| Pure water maker | Merck-Millipore | MilliQ 7000 | |
| Software for experiment | Molecular probe-Axon | pClamp 10 | |
| Software for statistics | KyensLab | KyPlot 5.0 | |
| Stimulator | WPI | DS8000 | |
| Temperature controller | Warner | TC-324B | |
| Tetrodotoxin | Tocris | 1078 |
Riferimenti
- Eccles, J. C., Ito, M., Szentagothai, J. . The Cerebellum as a Neuronal Machine. , (1967).
- Marr, D. A theory of cerebellar cortex. Journal of Physiology. 202 (2), 437-470 (1969).
- Albus, J. S. Theory of cerebellar function. Mathematical Biosciences. 10 (1), 25-61 (1971).
- Maekawa, K., Simpson, J. I. Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. Journal of Neurophysiology. 36 (4), 649-666 (1973).
- Ito, M., Shiida, T., Yagi, N., Yamamoto, M. Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Research. 65 (1), 170-174 (1974).
- Ghelarducci, B., Ito, M., Yagi, N. Impulse discharge from flocculus Purkinje cells of alert rabbits during visual stimulation combined with horizontal head rotation. Brain Research. 87 (1), 66-72 (1975).
- Robinson, D. A. Adaptive gain control of vestibulo-ocular reflex by the cerebellum. Journal of Neurophysiology. 39 (5), 954-969 (1976).
- Gilbert, P. F. C., Thach, W. T. Purkinje cell activity during motor learning. Brain Research. 128 (2), 309-328 (1977).
- Ito, M., Sakurai, M., Tongroach, P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. Journal of Physiology. 324, 113-134 (1982).
- Ito, M., Kano, M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neuroscience Letters. 33 (3), 253-258 (1982).
- Ekerot, C. F., Kano, M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Research. 342 (2), 357-360 (1985).
- Sakurai, M. Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. Journal of Physiology. 394, 462-480 (1987).
- Hirano, T. Depression and potentiation of the synaptic transmission between a granule cell and a Purkinje cell in rat cerebellar culture. Neuroscience Letters. 119 (2), 141-144 (1990).
- Linden, D. J. A long-term depression of AMPA currents in cultured cerebellar purkinje neurons. Neuron. 7 (1), 81-89 (1991).
- Linden, D. J., Connor, J. A. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science. 254 (5038), 1656-1659 (1991).
- Ito, M. Cerebellar long-term depression: characterization, signal transduction and functional roles. Physiological Reviews. 81 (3), 1143-1195 (2001).
- Ito, M. The molecular organization of cerebellar long-term depression. Nature Reviews Neuroscience. 3, 896-902 (2002).
- Ito, M., Jastreboff, P. J., Miyashita, Y. Specific effects of unilateral lesions in the flocculus upon eye movements in albino rabbits. Experimental Brain Research. 45 (1-2), 233-242 (1982).
- Nagao, S. Effects of vestibulocerebellar lesion upon dynamic characteristics and adaptation of vestibulo-ocular and optokinetic responses in pigmented rabbits. Experimental Brain Research. 53 (1), 36-46 (1983).
- Watanabe, E. Neuronal events correlated with long-term adaptation of the horizontal vestibulo-ocular reflex in the primate flocculus. Brain Research. 297 (1), 169-174 (1984).
- van Neerven, J., Pompeiano, O., Collewijn, H. Effects of GABAergic and noradrenergic injections into the cerebellar flocculus on vestibulo-ocular reflexes in the rabbit. Progress in Brain Research. 88, 485-497 (1991).
- Ito, M. Mechanism of motor learning in the cerebellum. Brain Research. 886, 237-245 (2000).
- De Schutter, E. Cerebellar long-term depression might normalize excitation of Purkinje cells: a hypothesis. Trends in Neurosciences. 18 (7), 291-295 (1995).
- Hansel, C., Linden, D. J. Long-term depression of the cerebellar climbing fiber-Purkinje neuron synapse. Neuron. 26 (2), 473-482 (2000).
- Safo, P., Regehr, W. G. Timing dependence of the induction of cerebellar LTD. Neuropharmacology. 54 (1), 213-218 (2007).
- Schonewille, M., et al. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 70 (1), 43-500 (2011).
- Karachot, L., Kado, T. R., Ito, M. Stimulus parameters for induction of long-term depression in in vitro rat Purkinje cells. Neuroscience Research. 21 (2), 161-168 (1994).
- Hartell, N. A. Induction of cerebellar long-term depression requires activation of glutamate metabotropic receptors. Neuroreport. 5, 913-916 (1994).
- Aiba, A., et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 79, 377-388 (1994).
- Steinberg, J. P., et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 46 (6), 845-860 (2006).
- Koekkoek, S. K., et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 47 (3), 339-352 (2005).
- Yamaguchi, K., Itohara, S., Ito, M. Reassessment of long-term depression in cerebellar Purkinje cells in mice carrying mutated GluA2 C terminus. Proceedings of the National Academy of Sciences of the United States of America. 113 (36), 10192-10197 (2016).
- De Schutter, E., Bower, J. M. An active membrane model of the cerebellar Purkinje cell II. Simulation of synaptic responses. Journal of Neurophysiology. 71 (1), 401-419 (1994).
- Swensen, A. M., Bean, B. Ionic mechanisms of burst firing in dissociated Purkinje neurons. Journal of Neuroscience. 23 (29), 9650-9663 (2003).
- Fukuda, J., Kameyama, M., Yamaguchi, K. Breakdown of cytoskeletal filaments selectively reduces Na and Ca spikes in cultured mammal neurones. Nature. 294 (5836), 82-85 (1981).
- Arancillo, M., White, J. J., Lin, T., Stay, T. L., Silltoe, R. V. In vivo analysis of Purkinje cell firing properties during postnatal mouse development. Journal of Neurophysiology. 113, 578-591 (2015).
- Ishikawa, T., Shimuta, M., Häusser, M. Multimodal sensory integration in single cerebellar granule cell in vivo. eLife. 4, e12916 (2015).
- Tempia, F., Minlaci, M. C., Anchisi, D., Strata, P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. Journal of Neurophysiology. 80, 520-528 (1998).
- Wang, S. S., Denk, W., Häusser, M. Coincidence detection in single dendritic spines mediated by calcium release. Nature Neuroscience. 3, 1266-1273 (2000).
- Kuroda, S., Schweighofer, N., Kawato, M. Exploration of signal transduction pathways in cerebellar long-term depression by kinetic simulation. Journal of Neuroscience. 21 (15), 5693-5702 (2001).
- Wang, W., et al. Distinct cerebellar engrams in short-term and long-term motor learning. Proceedings of the National Academy of Sciences of the United States of America. 111 (1), E188-E193 (2014).
- Inoshita, T., Hirano, T. Occurrence of long-term depression in the cerebellar flocculus during adaptation of optokinetic response. eLife. 27, 36209 (2018).
- Belmeguenai, A., et al. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. Journal of Neuroscience. 30 (41), 13630-13643 (2010).
- Ohtsuki, G., Piochon, C., Adelman, J. P., Hansel, C. SK2 channel modulation contributes to compartment specific dendritic plasticity in cerebellar Purkinje cells. Neuron. 75, 108-120 (2012).

