Using a Cell-Tracer Injection to Investigate the Origin of Neointima-Forming Cells in a Rat Saccular Side Wall Model
Summary
We performed a one-point, lipophilic cell-tracer injection to track endothelial cells, followed by an arteriotomy and suturing of sidewall aneurysms on the abdominal rat aorta. Neointima formation seemed dependent on the parent artery in decellularized aneurysms and was promoted by the recruitment from aneurysm wall cells in vital cell-rich walls.
Abstract
Microsurgical clipping creates a subsequent barrier of blood flow into intracranial aneurysms, whereas endovascular treatment relies on neointima and thrombus formation. The source of endothelial cells covering the endoluminal layer of the neointima remains unclear. Therefore, the aim of the present study was to investigate the origin of neointima-forming cells after cell-tracer injection in the already well-established Helsinki rat microsurgical sidewall aneurysm model.
Sidewall aneurysms were created by suturing decellularized or vital arterial pouches end-to-side to the aorta in male Lewis rats. Before arteriotomy with aneurysm suture, a cell-tracer injection containing CM-Dil dye was performed into the clamped aorta to label endothelial cells in the adjacent vessel and track their proliferation during follow-up (FU). Treatment followed by coiling (n = 16) or stenting (n = 15). At FU (7 days or 21 days), all rats underwent fluorescence angiography, followed by aneurysm harvesting and macroscopic and histological evaluation with immunohistological cell counts for specific regions of interest.
None of the 31 aneurysms had ruptured upon follow-up. Four animals died prematurely. Macroscopically residual perfusion was observed in 75.0% coiled and 7.0% of stented rats. The amount of cell-tracer-positive cells was significantly elevated in decellularized stented compared to coiled aneurysms with respect to thrombus on day 7 (p = 0.01) and neointima on day 21 (p = 0.04). No significant differences were found in thrombus or neointima in vital aneurysms.
These findings confirm worse healing patterns in coiled compared to stented aneurysms. Neointima formation seems particularly dependent on the parent artery in decellularized aneurysms, whereas it is supported by the recruitment from aneurysm wall cells in vital cell-rich walls. In terms of translation, stent treatment might be more appropriate for highly degenerated aneurysms, whereas coiling alone might be adequate for aneurysms with mostly healthy vessel walls.
Introduction
Subarachnoid hemorrhage caused by the rupture of an intracranial aneurysm (IA) is a devastating neurosurgical condition associated with high morbidity and mortality1,2,3,4. In addition to microsurgical clipping, which provides direct endothelium-to-endothelium contact, endovascular devices have gained increasing importance over the past decades for treating ruptured and incidentally discovered IAs. The healing response in endovascularly treated IAs mainly depends on neointima formation and thrombus organization. Both are synergic processes, depending on cell migration from the adjacent vessel and the aneurysm wall.5 To date, the origin of endothelial cells in neointima formation of endovascular treated aneurysms remains unclear. There is an ongoing debate in the literature about the source from which neointima-forming cells are recruited.
By using a cell-tracer injection of CM-Dil dye (see the Table of Materials) in the abdominal aorta of rats, we aimed to analyze the role of endothelial cells, originating in the parent artery, in neointima formation at two different FU time points (day 7 and day 21) (Figure 1). An advantage of the model is the direct local cell-tracer incubation in vivo in a parent artery prior to aneurysm suture, allowing FU at later time points. In vivo injection techniques, such as cell-tracer incubation, have not been described in the literature. An advantage of this technique is the direct, one-point, intraoperative, in vivo injection, which makes the model robust and reproducible.
Protocol
Veterinary support was performed according to institutional guidelines. Experiments were approved by the Local Ethics Committee, Switzerland (BE 60/19). The ARRIVE guidelines and 3R principles have been strictly followed6,7. Thirty-one male Lewis rats, 12 weeks old and weighing 492 ± 8 g, were included. House all rats at a room temperature of 23 °C and a 12 h light/dark cycle. Provide free access to water and pellets. Statistical analyses have been performed using the nonparametric Wilcoxon-Mann-Whitney U test. Probability values (p) of ≤ 0.05 and/or ≤ 0.01 were considered significant.
1. Preoperative phase-general preparation and anesthesiological aspects
- Randomize rats into either coil or stent treatment groups (Figure 2) via a web-based randomization system. Now, perform a preoperative clinical examination of all animals planned for surgery next to a quiet, aseptic operating room maintaining a room temperature of 23 ± 3 °C. Analyze the animals' behavior and inspect the mucous membranes and turgor as part of the preoperative clinical examination.
- Record the weight of each animal.
- Prior to surgery, incubate the arterial pouches from donor rats in 0.1% sodium dodecyl sulfate for 10 h at 37 °C to obtain decellularized aneurysms8. Collect these pouches from donor animals a few days before the surgery.
- Prepare the full length of the abdominal aorta with microscissors and forceps and apply 6-0 nonabsorbable ligatures at an interval of 3-4 mm.
- Directly generate vital aneurysms intraoperatively by a previously ligated arterial vessel pouch from the thoracic part of a donor animal9. Perform thoracotomy with scissors and surgical forceps at the indicated FU time point and ligate the vessel pouch at the desired length.
- Directly implant the pouch into the recipient and harvest the aneurysm from the donor animal for further macroscopic analysis and histological processing.
- For anesthesia induction, place all rats in a clean box provided with oxygen (O2) until loss of consciousness after 5-10 min. Anesthetize rats with a subcutaneous (SC) injection of a mixture of fentanyl 0.005 mg/kg, medetomidine 0.15 mg/kg, and midazolam 2 mg/kg.
NOTE: This ensures a surgical plane of at least 45 min. - Check the depth of anesthesia by the absence of the pedal withdrawal reflex.
- Place the rats in a supine position and shave the thoracoabdominal part with an electric shaver.
- Fixate the 4 paws of the rats with tape on a board, covered by a heating pad connected to an autoregulating rectal probe. Insert the rectal probe in the rat's anus to maintain the desired temperature of 37 °C with the help of the heating pad.
- Now, install a sensor on the right hind leg connected to a computerized system for checking vital signs intraoperatively.
- Cover the rat's nose and mouth with a face mask. If requiring prolonged anesthesia, start isoflurane (1.0-2.0% titrated to effect in 100% O2).
- Disinfect the surgical field with povidone-iodine or alternating disinfectants and drape the surgical field in a sterile fashion.
- For perianesthetic care, apply a sterile ophthalmic lubricant to the eyes and cover them with an opaque foil mask to prevent drying and damage from the surgical lamp.
- Throughout surgery, supply oxygen continuously via the face mask, monitor the body temperature, and provide heat using a heating pad, maintaining normothermia.
- Monitor other vital signs continuously (pulse and breath distension, heart and breath rate, and oxygen saturation).
2. Operative phase – cell-tracer injection
NOTE: The detailed surgical approach in the Helsinki rat microsurgical sidewall aneurysm model9 and techniques for coil- and stent-implantation are described elsewhere8,10,11.
- Store the fluorescent lipophilic cell-tracer at ≤ -20 °C all the time, protected from light.
- Perform the surgery by preparing the rat aorta and caval vein, followed by the separation of both, as well as proximal and distal temporary clamping of the aorta.
NOTE: This technique has been described previously9.- Clamp the proximal and distal parts of the aorta with two temporary titan clips.
- Put one microswab with purple padding each under the proximal and distal parts of the aorta for better visualization of the artery.
- Now, protect the abdomen with wet gauze.
- On the day of the operation, dissolve 2 µL of the cell-tracer by pipetting in 1 mL of phosphate-buffered saline (PBS).
- Transfer the mixture to a 1 mL syringe fitted with a 27-1/2 G (0.4 x 13 mm) sterile cannula.
NOTE: Be careful to avoid light exposure while performing steps 2.5 and 2.6. - Turn off the light in the operating room. While looking under a microscope, perform the one-point injection in the middle ventral part of the aorta using micro forceps and carefully inject 1 mL of heparinized 0.9% saline solution.
- Inject the cell-tracer carefully (Video 1) and immediately turn off the operating microscope as well. Again, protect the abdomen with wet gauze.
- Let the dye incubate for at least 15 min. After the incubation period, turn on the microscope and operating room lights.
- Perform the longitudinal arteriotomy and suturing of the aneurysm, as described elsewhere11.
- Use microforceps and microscissors to perform the arteriotomy so that its length averages the diameter of the harvested aneurysm (step 1.3). To ensure the correct length, place the aneurysm beside the aorta before performing arteriotomy. Suture the aneurysm with 8-10 single-stitches using a nonabsorbable 10-0 suture, and carefully remove the temporary clamps-starting distally-under continuous irrigation with heparinized saline. Close the wound in a layered fashion. Of note, use a coil packing density of 1 cm.
NOTE: The technique of coil- or stent-implantation has been described elsewhere8,10.
- Use microforceps and microscissors to perform the arteriotomy so that its length averages the diameter of the harvested aneurysm (step 1.3). To ensure the correct length, place the aneurysm beside the aorta before performing arteriotomy. Suture the aneurysm with 8-10 single-stitches using a nonabsorbable 10-0 suture, and carefully remove the temporary clamps-starting distally-under continuous irrigation with heparinized saline. Close the wound in a layered fashion. Of note, use a coil packing density of 1 cm.
3. Postoperative phase-monitoring and analgetic care
- At the end of the surgery, reverse the anesthesia with an SC injection mixture of buprenorphine 0.05 mg/kg, atipamezol 0.75 mg/kg, and flumazenil 0.2 mg/kg. Let each operated animal recover in a clean cage until fully awake and warm, as needed, with a heating lamp.
- For 3 days, administer 1 mg/kg meloxicam (one injection or oral application per day) and buprenorphine (0.05 mg/kg four times each day) SC. Overnight, provide buprenorphine continuously in the drinking water with the same dosing: 6 mL buprenorphine 0.3 mg/mL, 360 mL drinking water, 10 mL of 5% glucose.
- In the immediate postoperative phase, house each animal in a single cage for protection. Regroup the animals after 24 h.
- If any rat shows distressed or aggressive behavior after SC injection, administer buprenorphine in the drinking water during the day.
- Provide soft feed on the cage floor to support feeding and recovery postoperatively.
- Observe and take care of all the animals according to the wellbeing and pain score sheet.
- Administer rescue analgesia SC (meloxicam 1 mg/kg and 0.05 mg/kg buprenorphine) when needed.
Representative Results
A total of 31 animals were included in the laboratory setting: 27 rats were included in the final statistical analysis; 4 rats died prematurely (12.9% mortality rate). Intraoperatively, breath distension was significantly (p = 0.03) reduced in stent- (12.9 μm ± 0.7) compared to coil-treated (13.5 μm ± 0.6) rats. Fluorescence angiography was performed for every rat at the end of the final FU. Reperfusion was indicated in all 6 coil-treated animals, whereas reperfusion was observed in only 12.5% of the 8 stent-treated animals.
Pooled baseline aneurysm volumes for day 7 and day 21 did not differ significantly (neither for decellularized (p = 0.9) nor vital (p = 0.1) aneurysms) between the coil- or stent-treatment groups (Figure 3). Pooled FU volumes for decellularized aneurysms showed a nonsignificant aneurysm growth in coiled compared to the stented aneurysms (p = 0.28), significantly greater in the vital coiled than the stented group (60.1 mm3 ± 31.1 mm3 vs. 20.5 mm3 ± 20.6 mm3; p = 0.002).
Amounts of cell-tracer-positive cells in the neointima of decellularized aneurysms did not significantly differ between the stent- or coil-treated groups at day 7 FU (p = 0.8) but were significantly higher in stented rats at day 21 FU (Figure 4; p = 0.04). In vital aneurysm-sutured rats, no significant differences were noted at either 7 days (p = 1.0) or 21 days (Figure 5) FU (p = 0.66). In decellularized aneurysms at 7 days FU, significantly more cell-tracer-positive cells remained in the thrombus of the stent-treated compared to the coil-treated group (p = 0.01). This difference was not observed in vital aneurysms at 7 days FU. See Table 1 for the proportion of cell-tracer-positive cells for decellularized, as well as vital coiled and stented aneurysms for day 7 and day 21 FU. Counterstaining for von Willebrand factor (F8) was performed in the endothelial cells of the neointima of each rat (Figure 6).
The average duration of the surgical procedure was 119.1 ± 21.3 min for the coiling group compared to 154.1 ± 30.2 min for the stent group (p = 0.001). The number of stitches for aneurysm sutures also differed significantly (p = 0.000002) for the coil (15.6 ± 2.9 stitches) and stent groups (11.3 ± 1.1).
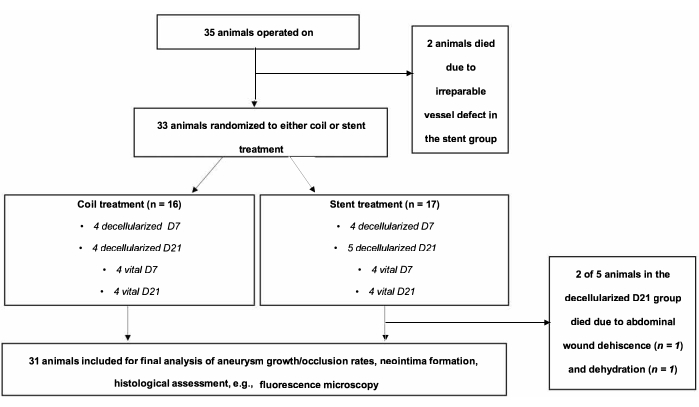
Figure 1: Flow chart of the experimental setting. A total of 35 animals were operated on and randomized to coiling or stenting groups. Two animals of the stent group died in the immediate postoperative course. Please click here to view a larger version of this figure.
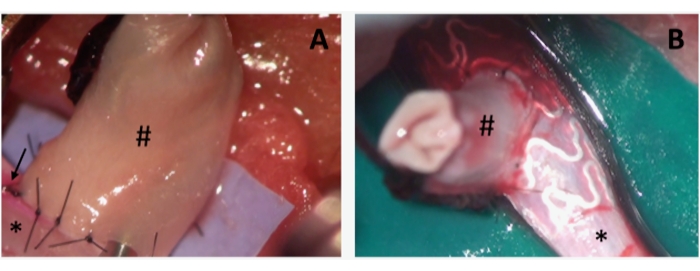
Figure 2: Intraoperative photographs of aneurysms during coil and stent embolization. (A) depicts a sidewall-aneurysm (#), sutured on the abdominal rat aorta (*). Note the coil device introduced in the aneurysm before performing the last single stitch to complete the aneurysm suture. Note the pinkish staining (arrow) on the left side of the arteriotomy, indicating the correct distribution of the cell tracer. (B) The same setting as in A, showing the stent device already in situ. Please click here to view a larger version of this figure.
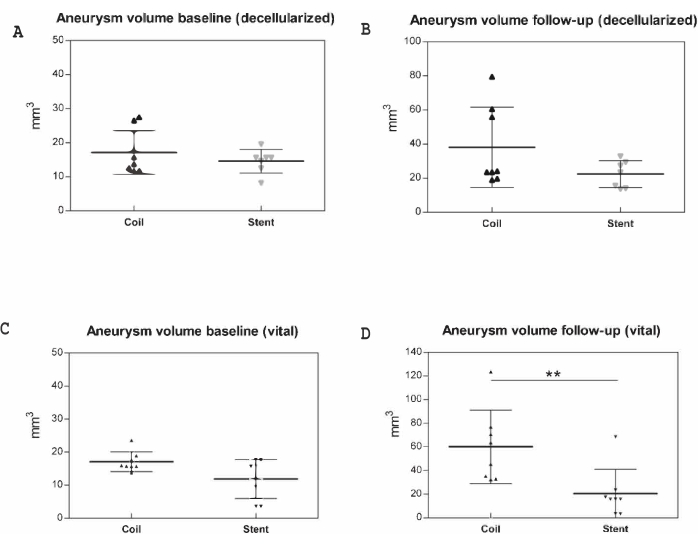
Figure 3: Macroscopic postmortem measurements in 31 animals. Aneurysm volumes (mm3) were documented prior to implantation and at follow-up, represented along the y-axis. (A) Baseline (decellularized), (B) follow-up (decellularized), (C) baseline (vital), (D) follow-up (vital). Data for day 7 and day 21 are pooled. ** p < 0.01. Values are expressed as medians with interquartile ranges. Please click here to view a larger version of this figure.
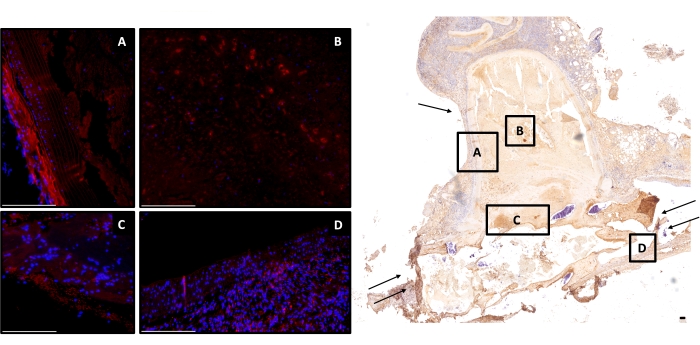
Figure 4: Exemplary image of a stent-treated decellularized aneurysm at day 21. Right, image overview of a monoclonal antiα-SMA, cell-depleted aneurysm (2-fold magnification) is shown; scale bar = 150 μm. Left, counterstained with DAPI; red cells are cell-tracer-positive (A) in the aneurysm wall, (B) in the thrombus, (C) residual stained but faded cell-tracer-positive cells in the neointima, and (D) in the adjacent vessel complex. Scale bars = 100 μm (A–D). Single arrow marks the aneurysm wall, double arrow the parent artery. Abbreviations: DAPI = 4',6-diamidino-2-phenylindole; α-SMA = α-smooth muscle actin. Please click here to view a larger version of this figure.
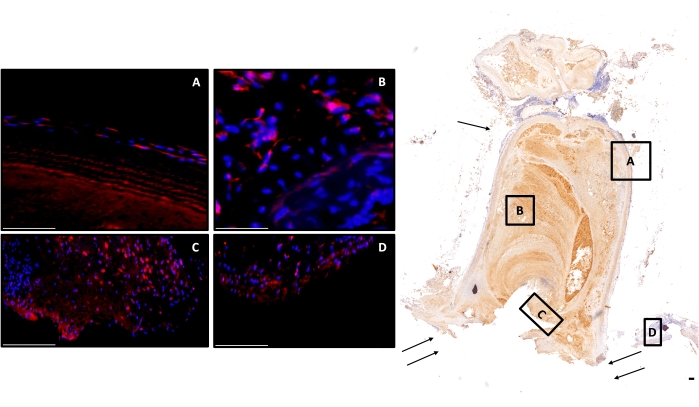
Figure 5: Exemplary image of a coil-treated vital aneurysm at day 21. Right side, image overview of a monoclonal antiα-SMA, cell-rich aneurysm (2-fold magnification) is shown; scale bar = 150 μm. Left side, counterstained with DAPI; red cells are cell-tracer-positive (A) in the aneurysm wall, (B) in the thrombus, (C) multiple positive cells in the neointima, and (D) in the adjacent vessel complex. Scale bars = 100 μm (A–D). Single arrow marks the aneurysm wall, double arrow the parent artery. Abbreviations: DAPI = 4',6-diamidino-2-phenylindole; α-SMA = α-smooth muscle actin. Please click here to view a larger version of this figure.
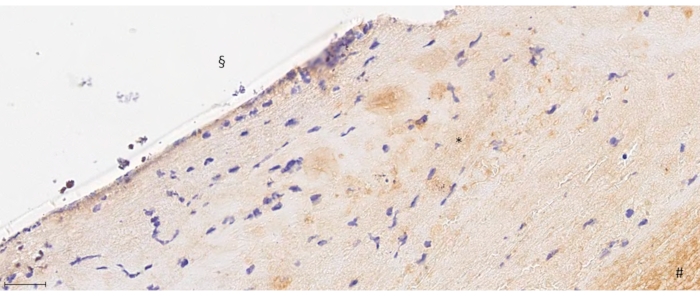
Figure 6: 40-fold magnification from F8 staining. # depicts the thrombus formation, * the neointima, and § the endoluminal side below the aneurysm orifice. Note the endothelial layering shown as purple staining in the endoluminal layer of the neointima. Scale bar = 175 μm. Please click here to view a larger version of this figure.
| DAPI/CM-Dil dye (%) | Coil | Stent | |||
| Day 7 | Day 21 | Day 7 | Day 21 | ||
| Decellularized pouches | Neointima | 68.00% | 7.70% | 72.20% | 34.30% |
| Parent artery | 75.50% | 10.50% | 76.50% | 35.60% | |
| Thrombus | 7.50% | 5.50% | 25.20% | 8.30% | |
| Aneurysm wall | 12.20% | 8.50% | 11.70% | 9% | |
| Vital pouches | Neointima | 56.70% | 11.50% | 58.20% | 15.00% |
| Parent artery | 60.00% | 24.20% | 81.50% | 26.00% | |
| Thrombus | 62.00% | 26.20% | 71.20% | 23.70% | |
| Aneurysm wall | 13.20% | 10.20% | 13.50% | 11.60% |
Table 1: Proportion of cell-tracer positive cells in neointima, parent artery, thrombus, and aneurysm wall. Values are depicted as percentages for decellularized and vital pouches for coil and stent treatment for day 7 and day 21. Abbreviation: DAPI = 4',6-diamidino-2-phenylindole.
Video 1: Cell-tracer injection in the abdominal part of the rat aorta. This technique is performed using a one-point injection into the clamped rat aorta. Please click here to download this Video.
Discussion
This study demonstrates that neointima formation is mediated via endothelial cells originating in the parent artery of the aneurysm complex but is supported by the recruitment of cells derived from the aneurysm wall in vital aneurysms. Nevertheless, the role of circulating progenitor cells in aneurysm healing remains controversial12,13. Overall, 31 male Lewis rats were included in this investigation; only 4 died prematurely (12.9% mortality).
In contrast to surgical clipping, which promotes subsequent endothelium-to-endothelium contact, the success of endovascular treatment relies on delayed biological responses. Newly developed techniques, such as flow-diversion, bioactive endovascular devices, or intraluminal cell-based therapies, are noteworthy with respect to endovascular treatment devices14,15. In this context, evidence shows that treatment success in successful aneurysm eradication is additively associated with the biologic response from the aneurysm wall itself5,16,17.
Recent studies have suggested that thrombus organization and neointima formation are concurrent processes in aneurysm healing after endovascular therapies. Both processes involved in aneurysm healing rely on moving cells from the adjacent vessel of the aneurysm complex and the aneurysm wall itself. Further, both processes are facilitated by the presence of endovascular devices such as coils or stents. As Grüter et al. demonstrated,5 thrombus-organizing cells mainly derive from the adjacent vessel for both types of endovascular treatment approaches. Here, neointima formation in coil-treated aneurysms mainly relies on cell migration from the vessel wall, whereas the adjacent vessel served as the primary donor in stent-treated aneurysms.
A common thread in establishing and expanding research questions using the Helsinki rat microsurgical sidewall aneurysm model could be observed over the years. First, decellularized and, therefore, degenerated aneurysms are more prone to growth and rupture than cell-rich vital aneurysms9. Further, coil treatment showed more success in aneurysm treatment with vital pouches than highly degenerated ones8. Moreover, cell transplantation provided sufficient aneurysm healing in even highly degenerated aneurysms14. Comparing different endovascular devices in this aneurysm model, stent treatment was clearly superior to coil treatment alone11. Thus, appreciating the different cell recruitment modes in coiled and stented aneurysms from the parent artery and aneurysm wall5, the major questions remain whether neointima formation is mainly triggered by endothelial cells from the parent artery, cells from the aneurysm wall, or even circulating progenitor cells. Recent findings on circulating progenitor cells triggering neointima formation are controversial12,13,15,18.
Only male rats were included in this series to avoid the confounding effects of estrogen on aneurysm growth, thrombus formation, and wall inflammation, as reported previously19. In addition to the sophisticated multimodal monitoring with fluorescence angiography20 and vital signs monitoring, we used a specific cell tracer to label the parent artery to differentiate cells derived from circulating cells in the bloodstream from those derived from true migration of neighboring cells. Nevertheless, we cannot exclude a slight fading of the signal intensity of the endothelial cells with time and cell division, although studies have shown a strong signal intensity of myofibroblasts at these time points (day 7 and day 21)14. Last, this aneurysm model used hemodynamics and subsequent biological processes, such as the rate of spontaneous thrombosis or aneurysm healing, which are highly influenced by the side wall constellation of the aneurysm21.
As demonstrated in these findings, it remains obvious that the adjacent vessel of the aneurysm complex serves as an important source of cells in forming a neointima. These findings are strongly in line with the recently published results by Kallmes et al., showing that strut thickness is also a determinant of wall apposition in flow diverters, which is an important driver of effective endothelialization. Here, increasing strut thickness reduces the probability of malapposition, improves contact with the parent artery wall, and, therefore, optimizes cellular reconstruction via struts22. In rats with decellularized aneurysms, a significantly higher amount of cell-tracer-positive endothelial cells was observed in the stented group at day 21 than in the coiled group at the same time point (Table 1).
This finding could be attributed to the fact that stents, applied in a cell-rich region of the parent artery in even highly degenerated aneurysms, serve as guiding structures for cell movements, allowing continuous endothelial lining of the endoluminal layer of the neointima and providing progressive aneurysm healing. Additively, comparing stenting and coiling on day 7 FU, a significantly higher amount of cell-tracer-positive cells was observed in the thrombus of stented animals than in coiled ones. Therefore, a reasonable explanation is that stent struts readily facilitate cell migration from the adjacent vessel in the thrombus. In vital aneurysms comparing coiling versus stenting, neither for neointima after 21 days, nor for thrombus formation on day 7, significant differences in cell-tracer positive cells were observed. In line with a previous finding5, this can be attributed to the support for neointima formation via cell recruitment in healthy vessel walls.
The absence of any significant differences in the amounts of cell-tracer-positive cells in the thrombus after 21 days in decellularized or vital coiled and stented aneurysms is because the neointima was almost completely sealed23. Therefore, even via stents, cell migration into the thrombus is not possible anymore. Critical points to be considered while performing stent implantation include a possible iatrogenic vessel rupture during stent application or critical stenosis formation in the region of the arteriotomy, with potential ischemia development in the lower limbs. To prevent ischemia, choose the arteriotomy site next to the vessel bifurcation for insertion of the stent small enough to avoid iatrogenic stenosis after stent implantation and suturing arteriotomy. Further, before closure, flush this region with heparinized saline to minimize the distal transport of any potential emboli due to the presence of any thrombogenic component.
Materials required for these procedures are typically extremely cost-intensive and rare, and their availability is crucial for young residents in neurosurgery24,25. However, in addition to the wealth of information obtained from this model, practicing this surgery will help enhance surgical skills.
To conclude, the biological healing response of endovascularly treated aneurysms in the Helsinki rat microsurgical sidewall aneurysm model depends on cell migration from the adjacent vessel complex. It is additionally supported by the recruitment of cells from a vital, healthy aneurysm wall. However, in decellularized and, therefore, highly degenerated aneurysms, the parent cell-rich artery is the most important source of cells for the formation of a neointima, which is facilitated by endovascular devices, such as stents, connecting the adjacent cell-rich tissues to the aneurysm orifice. To help translate this finding into clinical settings, highly degenerated aneurysms could be treated via scaffolds placed in cell-rich healthy vessel regions. Coil embolization alone might be sufficient for aneurysms with mostly healthy vessel walls.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors thank Alessandra Bergadano, DVM, PhD, for the dedicated supervision of long-term animal health. This work was supported by the research funds of the Research Council, Kantonsspital Aarau, Aarau, Switzerland, and the Swiss national science foundation SNF (310030_182450).
Materials
| 3-0 resorbable suture | Ethicon Inc., USA | VCP428G | |
| 4-0 non-absorbable suture | B. Braun, Germany | G0762563 | |
| 6-0 non-absorbable suture | B. Braun, Germany | C0766070 | |
| 9-0 non-absorbable suture | B. Braun, Germany | G1111140 | |
| Atipamezol | Arovet AG, Switzerland | ||
| Bandpass filter blue | Thorlabs | FD1B | any other |
| Bandpass filter green | Thorlabs | FGV9 | any other |
| Bipolar forceps | any other | ||
| Bicycle spotlight | any other | ||
| Board (20 x 10 cm) | any other | ||
| Buprenorphine | Indivior, Switzerland | 1014197 | |
| Camera | Sony NEX-5R, Sony, Tokyo, Japan | ||
| Cannula (27-1/2 G) | any other | ||
| Cell count software | Image-J version 1.52n, U.S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/ | ||
| CellTracker CM-Dil dye | ThermoFisher SCIENTIFIC, USA | C7000 | |
| Coil-Device | Styker, Kalamazoo, MI, USA | 2 cm of Target 360 TM Ultra, 2-mm diameter | |
| Desinfection | any other | ||
| Eye-lubricant | any other | ||
| Fentanyl | Sintetica, S.A., Switzerland | 98683 | any generic |
| Flumazenil | Labatec-Pharma, Switerzland | ||
| Fluoresceine | Curatis AG | 5030376 | any generic |
| Fluorescence microscope | Olympus BX51, Hamburg, Germany; Cell Sens Dimension Imaging software v1.8 | ||
| Foil mask | any other | ||
| Glucose (5%) | any other | ||
| Heating pad | Homeothermic Control Unit, Harvard, Edenbridge, England | any other | |
| Isotonic sodium chloride solution (0.9%) | Fresenius KABI | 336769 | any generic |
| Isoflurane | any generic | ||
| Longuettes | any other | ||
| Meloxicam | Boehringer Ingelheim | P7626406 | any generic |
| Medetomidine | Virbac, Switzerland | QN05CM91 | |
| Micro needle holder | any other | ||
| Midazolam | Roche, Switzerland | ||
| Monitoring-system | Starr Life Sciences Corp., 333 Allegheny Ave, Oakmont, PA 15139, United States | ||
| Needle holder | any other | ||
| O2-Face mask | any other | ||
| Operation microscope | OPMI, Carl Zeiss AG, Oberkochen, Germany | any other | |
| Oxygen | any other | ||
| Rectal temperature probe | any other | ||
| Scalpell | Swann-Morton | 210 | any other |
| Small animal shaver | any other | ||
| Smartphone | any other | ||
| Sodium dodecyl sulfate (0.1%) | Sigma-Aldrich | 11667289001 | |
| Soft feed | Emeraid Omnivore | any generic | |
| Soft tissue forceps | any other | ||
| Soft tissue spreader | any other | ||
| Stainless steel sponge bowls | any other | ||
| Stent-Device | Biotroni, Bülach, Switzerland | modified magmaris device, AMS with polymer coating, 6-mm length, 2-mm diameter | |
| Sterile micro swabs | any other | ||
| Straight and curved microforceps | any other | ||
| Straight and curved microscissors | any other | ||
| Straight and curved forceps | any other | ||
| Surgery drape | any other | ||
| Surgical scissors | any other | ||
| Syringes 1 mL, 2 mL, and 5 mL | any other | ||
| Tape | any other | ||
| Vascular clip applicator | B. Braun, Germany | FT495T | |
| Yasargil titan standard clip (2x) | B. Braun Medical AG, Aesculap, Switzerland | FT242T | temporary |
Riferimenti
- Vergouwen, M. D., et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 41 (10), 2391-2395 (2010).
- Macdonald, R. L., et al. Preventing vasospasm improves outcome after aneurysmal subarachnoid hemorrhage: rationale and design of CONSCIOUS-2 and CONSCIOUS-3 trials. Neurocritical Care. 13 (3), 416-424 (2010).
- Wanderer, S., et al. Levosimendan as a therapeutic strategy to prevent neuroinflammation after aneurysmal subarachnoid hemorrhage. Journal of Neurointerventional Surgery. , (2021).
- Wanderer, S., et al. Aspirin treatment prevents inflammation in experimental bifurcation aneurysms in New Zealand White rabbits. Journal of Neurointerventional Surgery. 14 (2), 189-195 (2021).
- Gruter, B. E., et al. Patterns of neointima formation after coil or stent treatment in a rat saccular sidewall aneurysm model. Stroke. 52 (3), 1043-1052 (2021).
- Kilkenny, C., et al. Animal research: reporting in vivo experiments: the ARRIVE guidelines. British Journal of Pharmacology. 160 (7), 1577-1579 (2010).
- Tornqvist, E., et al. Strategic focus on 3R principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS One. 9 (7), 101638 (2014).
- Nevzati, E., et al. Aneurysm wall cellularity affects healing after coil embolization: assessment in a rat saccular aneurysm model. Journal of Neurointerventional Surgery. 12 (6), 621-625 (2020).
- Marbacher, S., et al. The Helsinki rat microsurgical sidewall aneurysm model. Journal of Visualized Experiments: JoVE. (92), e51071 (2014).
- Nevzati, E., et al. Biodegradable magnesium stent treatment of saccular aneurysms in a rt model – introduction of the surgical technique. Journal of Visualized Experiments: JoVE. (128), e56359 (2017).
- Gruter, B. E., et al. Testing bioresorbable stent feasibility in a rat aneurysm model. Journal of Neurointerventional Surgery. 11 (10), 1050-1054 (2019).
- Kadirvel, R., et al. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology. 270 (2), 394-399 (2014).
- Li, Z. F., et al. Endothelial progenitor cells contribute to neointima formation in rabbit elastase-induced aneurysm after flow diverter treatment. CNS Neuroscience & Therapeutics. 19 (5), 352-357 (2013).
- Marbacher, S., et al. Intraluminal cell transplantation prevents growth and rupture in a model of rupture-prone saccular aneurysms. Stroke. 45 (12), 3684-3690 (2014).
- Frosen, J., et al. Contribution of mural and bone marrow-derived neointimal cells to thrombus organization and wall remodeling in a microsurgical murine saccular aneurysm model. Neurosurgery. 58 (5), 936-944 (2006).
- Marbacher, S., Niemela, M., Hernesniemi, J., Frosen, J. Recurrence of endovascularly and microsurgically treated intracranial aneurysms-review of the putative role of aneurysm wall biology. Neurosurgical Review. 42 (1), 49-58 (2019).
- Frosen, J. Smooth muscle cells and the formation, degeneration, and rupture of saccular intracranial aneurysm wall–a review of current pathophysiological knowledge. Translational Stroke Research. 5 (3), 347-356 (2014).
- Fang, X., et al. Bone marrow-derived endothelial progenitor cells are involved in aneurysm repair in rabbits. Journal of Clinical Neuroscience. 19 (9), 1283-1286 (2012).
- Morel, S., et al. Sex-related differences in wall remodeling and intraluminal thrombus resolution in a rat saccular aneurysm model. Journal of Neurosurgery. , 1-14 (2019).
- Gruter, B. E., et al. Fluorescence video angiography for evaluation of dynamic perfusion status in an aneurysm preclinical experimental setting. Operative Neurosurgery. 17 (4), 432-438 (2019).
- Marbacher, S., Strange, F., Frosen, J., Fandino, J. Preclinical extracranial aneurysm models for the study and treatment of brain aneurysms: A systematic review. Journal of Cerebral Blood Flow & Metabolism. 40 (5), 922-938 (2020).
- Ravindran, K., et al. Mechanism of action and biology of flow diverters in the treatment of intracranial aneurysms. Neurosurgery. 86, 13-19 (2020).
- Marbacher, S., et al. Loss of mural cells leads to wall degeneration, aneurysm growth, and eventual rupture in a rat aneurysm model. Stroke. 45 (1), 248-254 (2014).
- Morosanu, C. O., et al. Neurosurgical cadaveric and in vivo large animal training models for cranial and spinal approaches and techniques – systematic review of current literature. Neurologia i Neurochirurgia Polska. 53 (1), 8-17 (2019).
- Wanderer, S., et al. Arterial pouch microsurgical bifurcation aneurysm model in the rabbit. Journal of Visualized Experiments: JoVE. (159), e61157 (2020).

